Abstract
Background
Cage subsidence is a very common complication after lumbar interbody fusion. It may compromise vertebral interbody fusion through progressive spinal deformity and consequently cause compression of neural elements. Clinical relevance remains, however, unclear, with few studies on this subject and even less information regarding its correlation with clinical findings. The aim of this study was to identify risk factors for cage subsidence and clinical evaluation after transforaminal (TLIF) and posterior (PLIF) lumbar interbody fusion.
Methods
A retrospective study in patients submitted to TLIF and PLIF between 2008 and 2017 was conducted.
Results
A total of 165 patients were included (123 TLIF and 42 PLIF). Univariate analysis showed an increased risk of cage subsidence in spondylolisthesis comparing with degenerative disk disease (p = 0.007). A higher preoperative lumbar lordosis angle (p = 0.014) and cage placement in L2-L3 (p = 0.012) were associated with higher risk of subsidence. The posterior cage positioning on vertebral endplate was associated with a higher risk of subsidence (p = 0.028) and significant subsidence (p = 0.005), defined as cage migration > 50% of cage height. PLIF presented a higher risk when comparing with TLIF (p = 0.024). Hounsfield unit (HU) values < 135 (OR6; 95% CI [1.95–34]) and posterior positioning (OR7; 95% CI [1.7–27.3]) were independent risk factors for cage subsidence and significant subsidence, respectively, in multivariate analysis. There was a tendency for significant subsidence in degrees ≥ 2 of Meyerding spondylolisthesis (OR4; 95% CI [0.85–21.5]). Significant cage subsidence was not associated with worse clinical results. Other analyzed factors, such as age (p = 0.008), low bone mineral density (BMD) (p = 0.029) and type of surgery (TLIF) (p = 0.004), were associated with worse results.
Conclusion
The present study shows that lower BMD and posterior cage positioning are relevant risk factors for lumbar cage subsidence. Low BMD is also a predictor of poor clinical results, so it must be properly evaluated and considered, through HU values measurement in CT scan, a feasible and reliable tool in perioperative planning.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lumbar interbody fusion using an interbody cage is an effective treatment for patients with degenerative lumbar spine diseases. It adds an additional fusion interface and may aid in sagittal and coronal balance correction.
Cage subsidence is a very common complication and may compromise interbody fusion. Progressive spinal deformity and compression of neural elements [1] are possible additional consequences of subsidence. Despite being a relevant point, its clinical significance remains unclear as it has been infrequently studied and does not always correlate with clinical findings [2]. To prevent cage subsidence, it must be properly positioned since the supporting capacity on the vertebral platform is not homogeneous [3]. The center of the endplate is the weakest part and the posterolateral region the strongest, according to biomechanical and anatomical studies [1, 4].
Low bone mineral density (BMD) is a factor frequently implicated in early postoperative cage subsidence [5]. Dual-energy x-ray absorptiometry (DXA) is the “gold standard” to assess BMD and to diagnose osteoporosis. However, DXA is rarely performed before spinal fusion due to its additional cost and radiation exposure as a separate examination [6]. Recently, a new technique to assess BMD by using the vertebral body Hounsfield units (HU) from computed tomography (CT), has been described [7] and HU values have been associated with the BMD measured by DXA [8].
The main goal of this study was to evaluate patient and procedure-related risk factors for cage subsidence and its clinical implication after transforaminal and posterior lumbar interbody fusion.
Methods
All adult patients submitted to one or two-level open lumbar fusion with one or two-level transforaminal (TLIF) and posterior (PLIF) lumbar interbody fusion between January 2008 and December 2017 in a single institution were retrospectively reviewed. Patients were operated by a team of four experienced spine surgeons.
The protocol was approved by the institutional review board of the hospital and the study is in agreement with the Declaration of Helsinki.
Data collection
Electronic and paper medical records were retrospectively reviewed for all patients.
Indications for surgery were lumbar degenerative disease (spondylolisthesis and spondylosis/ spinal stenosis). Patients submitted to long spinal fusions (3 or more levels), scoliosis correction were excluded and those who had postoperative surgical site infection, or those who underwent early revision were excluded. Minimum radiographic follow-up was 6 months.
Patient-related analysis included
Demographic data (age and gender), body mass index (BMI), diabetes mellitus, tobacco use, chronic corticosteroid treatment, rheumatoid arthritis, previous lumbar spine fractures or other osteoporotic fractures (distal radius, proximal humerus or femur), primary or revision surgery, preoperative diagnosis (spondylolisthesis or spondylosis/ spinal stenosis).
Preoperative lumbar BMD measurement by DXA was collected from medical records. Based on the World Health Organization (WHO) classification, patients were classified as normal or with decreased lumbar BMD, osteopenia and osteoporosis, defined as a T-score less than -1 and -2.5, respectively [9].
Procedure-related analysis included
Type of lumbar interbody fusion (transforaminal or posterior), cage insertion level, number of levels and surgical complications.
Clinical outcomes were collected by telephone interview. Pain was assessed using Oswestry Disability Index (ODI), visual analog scales (VAS) for back and leg pain, and self-report improvement of back and leg pain, questioning “How is your lower back pain/leg pain after the surgery? Better, worse or equal?”. Patient return to previous work and daily activities and overall satisfaction were also evaluated.
Radiographic analysis
All radiographic measurements were taken independently by two surgeons. Disagreements were solved through discussion.
Preoperative disk height index (average of the anterior and posterior margins of the intervertebral space, normalized with the anteroposterior diameter of the lower vertebral body endplate), segmental lordosis angle (between the superior endplate of the superior vertebra and the inferior endplate of the inferior vertebra of each treated level) and lumbar lordosis angle (between the superior endplate of the L1 vertebral body to the superior margin of the S1 vertebral body) were measured on standing lateral radiographs. Intraoperative disk height index and segmental lordosis angle were also measured.
Hounsfield units were measured using standard picture archiving and communication system software. A region of interest (ROI) was drawn at three points on axial images that are obtained as parallel to the endplates as possible: just inferior to the superior endplate, midvertebral body, and just superior to the inferior endplate. The ROI is drawn encapsulating only cancellous bone and avoiding cortical edges and osseous abnormalities. The software calculates the average HU in the ROI for each image. An average of the three measurements determines the HU for individual vertebral levels [10] (Fig. 1). Patients with lumbar HU values < 135 and < 110 were classified as having lumbar osteopenia or osteoporosis, respectively [11].
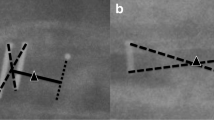
Cage positioning on lateral radiographs, for both TLIF and PLIF, was evaluated according to the positioning of the center of the cage (defined as the midpoint between the anterior and posterior radiomarkers) in the anterior, middle or posterior third of the lower vertebral endplate [12]. PLIF cage was also evaluated in the anteroposterior radiograph according to the symmetrical positioning of the cage in the vertebral endplate—if the cage had crossed the vertebral midline it was classified as misplaced (Fig. 2).
a Cage positioning on lateral radiographs, for both TLIF and PLIF according to the predominant positioning in the anterior, middle or posterior third of the vertebral endplate. b PLIF cage was also evaluated in the anteroposterior radiograph according to the symmetrical positioning of the cage in the vertebral endplate
Patients were divided in two groups: with and without subsidence. Subsidence was defined as any degree of vertical migration on follow-up radiographs compared with immediate postoperative radiographs. Furthermore, patients were also analyzed for significant subsidence defined as cage vertical migration more than 50% of cage height [13]. The vertebral endplate (upper, lower or both) where the cage migration occurred was also recorded.
Regarding bone fusion, static lumbar and dynamic lumbar flexion–extension radiographs were evaluated. A segment was considered to be fused in case of: a visible bridging bone between the endplates of the fused vertebral bodies outside the cage in plain static radiographs, no visible radiolucency around the cage and no visible segment instability in the dynamic flexion–extension radiographs (i.e., ≥ 5° movement on lateral flexion and extension views) [3].
Surgical procedures
A midline skin incision exposed the posterior elements and facet joints. Primarily, bilateral pedicle screws were placed. For PLIF, after bilateral laminotomy (medial to the facet joint), the dural sac was retracted to expose a corridor to the intervertebral disk space. In case of TLIF, following laminotomy, facetectomy was performed to expose the intervertebral disk. For both procedures, the intervertebral disk was excised and the foramina were thoroughly decompressed. The endplate was carefully prepared with dedicated instruments (i.e., rasps and curettes). Autologous bone graft harvested from posterior spinal elements was packed into the intervertebral space and inside the cage. Following cage insertion, rod fixation with axial compression was performed. Cage height and lateral dimension were determined intraoperatively with trials according to the dimensions of the index level.
All PLIF and TLIF cages were made of polyether ether ketone (PEEK). For PLIF, bilateral cages were used in all cases and for TLIF banana-shaped cages were used.
Statistical analysis
Statistical analysis was performed using SPSS 25.0. A univariate analysis was conducted to identify the potential risk factors for cage subsidence and to analyze its clinical outcomes. A Chi-square test and Fisher’s exact test were used to compare categorical parameters and Independent t-tests for continuous variables between groups. Multivariate logistic regression analysis was performed to determine the independent risk factors by systematically pruning the least significant variables out of a multiple logistic regression model that initially included all variables. A significant difference was set as p < 0.05.
Results
Risk factors for cage subsidence
From a total of 203 patients, 34 (17%) patients without appropriate imaging and/ or follow-up were excluded. Two patients (1%) with surgical site infection and another two patients (1%) with early (less than 2 weeks) cage retropulsion causing neurological compression that underwent early revision surgery were also excluded. Ultimately, a total of 165 patients were included: 122 TLIF, 21 (17%) of which, via minimally invasive surgery, and 43 PLIF. Any degree of cage subsidence was identified in 83 (50%) patients and significant subsidence in 36 (22%) patients. Three patients (2%) did not fulfill the fusion criteria: one patient with and two patients without significant subsidence.
A univariate analysis (Table 1) for preoperative patient demographic data showed no significant differences. Spondylolisthesis was associated with an increased risk of cage subsidence (p = 0.007) comparing with degenerative disk disease. However, it was not related to significant subsidence. A higher preoperative lumbar lordosis angle was associated with higher risk of subsidence (p = 0.014), although cage subsidence was not related to this angle obtained intraoperatively, as neither were lumbar segmental lordosis angle nor disk height index. Positioning of the cage in the posterior third of the vertebral endplate was associated with a higher risk of subsidence (p = 0.028) and significant subsidence (p = 0.005) and PLIF presented a higher risk when comparing with TLIF (p = 0.024). Regarding TLIF, no differences in cage subsidence were found between open and minimally invasive. Cage placement in L2–L3 was associated with a higher risk of cage subsidence, compared to other lumbar levels (p = 0.012).
Hounsfield unit values < 135 (Odds Ratio 6; 95% Confidence Interval [1.95–34]) and predominant posterior positioning of the cage on the vertebral endplate (OR 7; 95% CI [1.7–27.3]) were presented as independent risk factors for cage subsidence and significant subsidence, respectively, by performing a multivariate analysis model (Table 2). There was also a tendency for significant subsidence in Meyerding ≥ 2 spondylolisthesis (OR 4; 95% CI [0.85–21.5]).
Clinical outcomes in cage subsidence
Out of the 165 patients included in the cage subsidence primary analysis, only 108 patients answered the telephone questionnaire. The follow-up time was 96 (SD ± 38) months.
The presence of cage significant subsidence was not associated with worse clinical results (Table 3). However, other variables as age and lower BMD seem to correlate with higher ODI (p = 0.008 and p = 0.029, respectively). Higher BMD (p = 0.045) was associated with more return to work ( p = 0.013). Patients undergoing PLIF reported significantly less VAS back pain (p = 0.004) than those undergoing TLIF, and would more likely undergo the same procedure (p = 0.012).
Discussion
While some authors advocate that neither fusion rates nor final clinical outcomes are affected by radiographic subsidence [2, 14], clinical significance of cage subsidence is far from being clarified, with other authors reporting cases of progressive malalignment and neural compression [15] Therefore, understanding the risk factors associated with cage subsidence is essential in order to minimize its occurrence. On the other hand, its clinical repercussion must be thoroughly evaluated.
In this study, the authors found that patients with HU values < 135, meaning osteopenia or osteoporosis, were 6 times more likely to have cage subsidence. This finding is consistent with other studies in the literature, namely in patients submitted to TLIF, whose lower preoperative HU values were associated with cage subsidence, as well as iatrogenic fractures, and overall radiographic complications [16, 17]. Furthermore, studies in human cadaveric models found a correlation between BMD and cage subsidence, concluding that the density of vertebral cancellous bone is more important for the biomechanics of the segment stabilized with a cage, and its eventual clinical success, than the cage material or the applied load [5, 18, 19]. Therefore, the preoperative plan of these patients must take into account the effects of poor bone quality. HU measurement is a simple and feasible way to assess it. Multiple studies have explored the ability of diagnostic CT scan to identify osteopenia and osteoporosis, both based on HU values obtained from the spine [7, 20], with several benefits comparing with DXA [11, 21, 22]. Thus, HU measurement in preoperative CT can be useful for appropriate patient counseling regarding surgical risks, or to identify the need for additional studies, appropriate referral or treatment initiation for osteoporosis.
Here, the authors found that predominant posterior positioning of the cage is 7 times more likely to lead to cage subsidence, comparing with anterior or central positioning. Mapping the structural properties of the lumbosacral vertebral endplates in a human cadaveric model [4] has shown that regional rigidity of lumbar and sacral endplates varies significantly. In general, the lumbar and sacral posterior endplate regions are stronger than the anterior ones and the lumbar lateral regions are stronger than the central ones. However, “in vivo” loads of the lumbosacral spine are complex and biomechanical investigations in cadaveric models do not fully reflect the real loading conditions [1], with the posterior positioning of the cage being more prone to subsidence as seen in patients undergoing PLIF in which central or posterior cage positioning on vertebral endplate were also associated with the highest rates of cage subsidence [3].
Cage positioning in the vertebral body has also been associated with fusion rates, with posterior cage positioning having lower fusion rates [3, 23] than anterior positioning. Here, however, fusion rates were not affected by cage positioning.
The tendency for a higher risk of subsidence in the presence of degrees ≥ 2 of Meyerding spondylolisthesis may be explained by the higher lumbar instability in these patients, compared with patients with degenerative disk disease.
Regarding univariate analysis, higher degrees of preoperative segmental lumbar lordosis were associated with cage subsidence. We argue that as pressure exerted on the posterior region of the vertebral endplates is higher in these patients, in case of posterior positioning of the cage,
the overload in the vertebral endplates will be even greater, leading to cage subsidence.
Higher cage subsidence in L2–L3 level can be explained by less efficient cage positioning and inadequate endplate preparation, due to greater access difficulty compared to other lumbar levels.
We found a greater risk of cage subsidence in PLIF comparing to TLIF. This is in contrast with other studies in the literature where a greater risk of subsidence was found in TLIF, putatively due the potentially need for broader facetectomy and decompression, compared to a more adequate area for fusion axial support provided by the two PLIF cages [24].
In this study, there was no relation between cage subsidence and worse clinical outcomes. Theoretically, cage subsidence could lead to worse clinical outcomes, due to loss of lumbar lordosis, decreased intervertebral height and consequent neurological compression. However, there is insufficient clinical evidence to support these claims and correlations between subsidence and clinical outcomes have reported no significance [2, 25,26,27]. Additionally, patients submitted to PLIF reported lower VAS scores for back pain. The literature shows the divergent results on clinical outcomes, but a recent systematic review with meta-analysis including 990 patients found no clinical difference between procedures [28].
The two patients excluded due to posterior migration of the cage were both submitted to TLIF in L5-S1 for spondylolisthesis grade 2. Both patients had HU greater than 135. The position of the cage was placed in the middle third and in the anterior third, respectively. They were not included in the analysis because the cage was removed prior to the required 6-month follow-up due to neurological compression.
Since there are few studies in the literature about the risk factors for cage subsidence, further investigation must be carried out to reinforce or refute the results discussed on this work.
This study has some limitations. Cage positioning and subsidence were evaluated using X-rays and postoperative CT scans would have been a more reliable method. However, X-rays have often been used for this type of assessment. Another limitation concerns to the non-evaluation of the cage type and size influence on cage subsidence. Studies in the literature show that wider implants with larger footprints provide more resistance to subsidence [13, 29,30,31]. Finally, the retrospective nature of this study, and the lack of standardized preoperative clinical evaluation in these patients, weakened a more valid clinical assessment.
Conclusion
The present study highlights patient and procedure risk factors, as lower BMD and posterior cage positioning, for cage subsidence in lumbar interbody fusion. No clinical differences were found between patients with and without cage subsidence. BMD, which can be evaluated through HU values in CT scans, should be considered in the preoperative planning of patients undergoing spine fusion.
Data availability and material
All the data and materials comply with field standards of the journal.
References
Labrom RD, Tan JS, Reilly CW, Tredwell SJ, Fisher CG, Oxland TR (2005) The effect of interbody cage positioning on lumbosacral vertebral endplate failure in compression. Spine 30(19):E556–E561. https://doi.org/10.1097/01.brs.0000181053.38677.c2
Schiffman M, Brau SA, Henderson R, Gimmestad G (2003) Bilateral implantation of low-profile interbody fusion cages: subsidence, lordosis, and fusion analysis. Spine J 3(5):377–387. https://doi.org/10.1016/s1529-9430(03)00145-1
Abbushi A, Cabraja M, Thomale UW, Woiciechowsky C, Kroppenstedt SN (2009) The influence of cage positioning and cage type on cage migration and fusion rates in patients with monosegmental posterior lumbar interbody fusion and posterior fixation. Eur Spine J 18(11):1621–1628. https://doi.org/10.1007/s00586-009-1036-3
Grant JP, Oxland TR, Dvorak MF (2001) Mapping the structural properties of the lumbosacral vertebral endplates. Spine 26(8):889–896. https://doi.org/10.1097/00007632-200104150-00012
Jost B, Cripton PA, Lund T et al (1998) Compressive strength of interbody cages in the lumbar spine: the effect of cage shape, posterior instrumentation and bone density. Eur Spine J 7(2):132–141. https://doi.org/10.1007/s005860050043
Dipaola CP, Bible JE, Biswas D, Dipaola M, Grauer JN, Rechtine GR (2009) Survey of spine surgeons on attitudes regarding osteoporosis and osteomalacia screening and treatment for fractures, fusion surgery, and pseudoarthrosis. Spine J 9(7):537–544. https://doi.org/10.1016/j.spinee.2009.02.005
Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG (2011) Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am 93(11):1057–1063. https://doi.org/10.2106/JBJS.J.00160
Lee S, Chung CK, Oh SH, Park SB (2013) Correlation between Bone Mineral Density Measured by Dual-Energy X-Ray Absorptiometry and Hounsfield Units Measured by Diagnostic CT in Lumbar Spine. J Korean Neurosurg Soc 54(5):384–389. https://doi.org/10.3340/jkns.2013.54.5.384
Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129.
Choi MK, Kim SM, Lim JK (2016) Diagnostic efficacy of Hounsfield units in spine CT for the assessment of real bone mineral density of degenerative spine: correlation study between T-scores determined by DEXA scan and Hounsfield units from CT. Acta Neurochir 158(7):1421–1427. https://doi.org/10.1007/s00701-016-2821-5
Zaidi Q, Danisa OA, Cheng W (2019) Measurement techniques and utility of hounsfield unit values for assessment of bone quality prior to spinal instrumentation: a review of current literature. Spine 44(4):E239–E244. https://doi.org/10.1097/BRS.0000000000002813
Siu TL, Najafi E, Lin K (2017) A radiographic analysis of cage positioning in lateral transpsoas lumbar interbody fusion. J Orthop 14(1):142–146. https://doi.org/10.1016/j.jor.2016.10.028
Marchi L, Abdala N, Oliveira L, Amaral R, Coutinho E, Pimenta L (2013) Radiographic and clinical evaluation of cage subsidence after stand-alone lateral interbody fusion. J Neurosurg Spine 19(1):110–118. https://doi.org/10.3171/2013.4.SPINE12319
Tokuhashi Y, Ajiro Y, Umezawa N. Subsidence of metal interbody cage after posterior lumbar interbody fusion with pedicle screw fixation. Orthopedics. 2009;32(4)
Park MK, Kim KT, Bang WS et al (2019) Risk factors for cage migration and cage retropulsion following transforaminal lumbar interbody fusion. Spine J 19(3):437–447. https://doi.org/10.1016/j.spinee.2018.08.007
Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J (2017) Vertebral body hounsfield units are associated with cage subsidence after transforaminal lumbar interbody fusion with unilateral pedicle screw fixation. Clin Spine Surg 30(8):E1130–E1136. https://doi.org/10.1097/BSD.0000000000000490
Formby PM, Kang DG, Helgeson MD, Wagner SC (2016) Clinical and radiographic outcomes of transforaminal lumbar interbody fusion in patients with osteoporosis. Global Spine J 6(7):660–664. https://doi.org/10.1055/s-0036-1578804
Lund T, Oxland TR, Jost B et al (1998) Interbody cage stabilisation in the lumbar spine: biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg Br 80(2):351–359. https://doi.org/10.1302/0301-620x.80b2.7693
Polikeit A, Ferguson SJ, Nolte LP, Orr TE (2003) Factors influencing stresses in the lumbar spine after the insertion of intervertebral cages: finite element analysis. Eur Spine J 12(4):413–420. https://doi.org/10.1007/s00586-002-0505-8
Tay WL, Chui CK, Ong SH, Ng AC (2012) Osteoporosis screening using areal bone mineral density estimation from diagnostic CT images. Acad Radiol 19(10):1273–1282. https://doi.org/10.1016/j.acra.2012.05.017
Rand T, Seidl G, Kainberger F et al (1997) Impact of spinal degenerative changes on the evaluation of bone mineral density with dual energy X-ray absorptiometry (DXA). Calcif Tissue Int 60(5):430–433. https://doi.org/10.1007/s002239900258
Franck H, Munz M, Scherrer M (1995) Evaluation of dual-energy X-ray absorptiometry bone mineral measurement–comparison of a single-beam and fan-beam design: the effect of osteophytic calcification on spine bone mineral density. Calcif Tissue Int 56(3):192–195. https://doi.org/10.1007/bf00298608
Kim KS, Yang TK, Lee JC (2005) Radiological changes in the bone fusion site after posterior lumbar interbody fusion using carbon cages impacted with laminar bone chips: follow-up study over more than 4 years. Spine 30(6):655–660. https://doi.org/10.1097/01.brs.0000155421.07796.7f
Lee N, Kim KN, Yi S et al (2017) Comparison of outcomes of anterior, posterior, and transforaminal lumbar interbody fusion surgery at a single lumbar level with degenerative spinal disease. World Neurosurg 101:216–226. https://doi.org/10.1016/j.wneu.2017.01.114
Penta M, Fraser RD. Anterior lumbar interbody fusion. A minimum 10-year follow-up. Spine 1997;22(20):2429–2434. doi:https://doi.org/10.1097/00007632-199710150-00021
Choi JY, Sung KH (2006) Subsidence after anterior lumbar interbody fusion using paired stand-alone rectangular cages. Eur Spine J 15(1):16–22. https://doi.org/10.1007/s00586-004-0817-y
Liljenqvist U, O’Brien JP, Renton P (1998) Simultaneous combined anterior and posterior lumbar fusion with femoral cortical allograft. Eur Spine J 7(2):125–131. https://doi.org/10.1007/s005860050042
de Kunder SL, van Kuijk SMJ, Rijkers K et al (2017) Transforaminal lumbar interbody fusion (TLIF) versus posterior lumbar interbody fusion (PLIF) in lumbar spondylolisthesis: a systematic review and meta-analysis. Spine J 17(11):1712–1721. https://doi.org/10.1016/j.spinee.2017.06.018
Goh JC, Wong HK, Thambyah A, Yu CS (2000) Influence of PLIF cage size on lumbar spine stability. Spine 25(1):35–39. https://doi.org/10.1097/00007632-200001010-00008
Kumar MN, Baklanov A, Chopin D (2001) Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J 10(4):314–319. https://doi.org/10.1007/s005860000239
Kuslich SD, Ulstrom CL, Griffith SL, Ahern JW, Dowdle JD. The Bagby and Kuslich method of lumbar interbody fusion. History, techniques, and 2-year follow-up results of a United States prospective, multicenter trial. Spine 1998;23(11):1267–1278 https://doi.org/10.1097/00007632-199806010-00019
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors cooperated equally for the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Ethical approval
The protocol was approved by the institutional review board of the hospital and the study is in agreement with the Declaration of Helsinki.
Consent to participate
All patients gave informed consent to participate.
Consent for publication
The authors authorize the publication of the present work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amorim-Barbosa, T., Pereira, C., Catelas, D. et al. Risk factors for cage subsidence and clinical outcomes after transforaminal and posterior lumbar interbody fusion. Eur J Orthop Surg Traumatol 32, 1291–1299 (2022). https://doi.org/10.1007/s00590-021-03103-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-021-03103-z