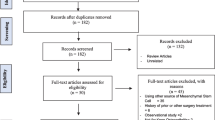
Abstract
Background
Despite being a common cause of quality-of-life impairment, there are no efficacious therapies that could prevent the progression of knee osteoarthritis (KOA). We conducted an open-label trial of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) and hyaluronic acid (HA) for treating KOA.
Methods
This open-label study was conducted from July 2015 to December 2018 at Cipto Mangunkusumo Hospital, Jakarta, Indonesia. Patients diagnosed with KOA were injected three times, comprising of 10 × 106 units of hUC-MSCs in 2-ml secretome implantation and 2-ml hyaluronic acid (HA) injection in the first week, followed with 2-ml HA injection twice in the second and third week.
Results
Twenty-nine subjects (57 knees) were recruited. Seventeen (58.6%) subjects were male, and the mean age was 58.3 ± 9.6 years. Thirty-three (57.9%) knees were classified into Kellgren–Lawrence grade I–II KOA (mild OA). hUC-MSCs significantly decreased pain measured by visual analogue scale in severe KOA from initial to 6th month follow-up [5 ± 2.97 to 3.38 ± 2.44 (p = 0.035)]. The International Knee Documentation Committee score significantly increased at 6th month follow-up (53.26 ± 16.66 to 65.49 ± 13.01, p < 0.001, in subjects with grade I–II and 48.84 ± 18.41 to 61.83 ± 18.83, p = 0.008, in subjects with severe KOA). The Western Ontario and McMaster Universities Osteoarthritis decreased significantly in both groups from initial to 6th month follow-up (from 22.55 ± 15.94 to 13.23 ± 10.29, p = 0.003, and from 27.57 ± 15.99 to 17.92 ± 19.1, p = 0.003, in those with mild and severe KOA, respectively).
Conclusions
hUC-MSCs could be a potentially new regenerative treatment for KOA. The maximum effect of hUC-MSCs was achieved after 6 months of injection.
Level of evidence
Therapeutic level II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Musculoskeletal disorders are the most common cause of severe long-term pain and physical disability affecting hundreds of millions of patients worldwide. Among these diseases, osteoarthritis (OA), defined as a chronic degenerative joint disorder involving articular cartilage and many of its surrounding tissues, is the most common; it is [1,2,3,4,5]. OA is estimated to affect 250 million people worldwide [6], and it mainly affects the weight-bearing joints, such as hips and knees [2]. The knee is the most frequent site for OA, and those presenting with disorder often suffer from progressive loss of function, pain and stiffness, thereby having decreased quality of life of [7].
Despite its consequences, no effective therapy or procedure prevents the progressive destruction of OA [2, 8]. All current treatments, without exception, are more symptomatic than preventive, which further may lead to joint replacement [8,9,10]. Although generally successful, joint replacement is associated with numerous complications and high financial burden [11]. Moreover, it is not as attractive for younger patients because of prosthesis lifespan approximately 15 years [1, 12,13,14,15]. There is, therefore, a crucial need for the development of new treatments for this debilitating condition.
Although intra-articular injection of hyaluronic acid (HA) has been employed in OA treatment, its effect on chondroprotection and the prevention of OA progression of the knee remain controversial [16, 17]. Many preclinical and clinical treatment protocols involving cell-based therapy use HA as a vehicle [18, 19]. The use of HA as a vehicle may have an added benefit of ensuring delivery of MSCs to the articular surface given that is localised to the articular surface after intra-articular infusion [20]. MSCs have been used for cartilage repair. Bone marrow stem cells (BM-MSCs) are the most common source of MSCs and can prompt cartilage regeneration at certain levels [21, 22]. However, collecting BM-MSCs from donors is invasive and inconvenient [23]. In recent years, human umbilical cord MSCs (hUC-MSCs) have received much attention as a potential cell source for tissue engineering and regenerative medicine, because they are easy to obtain and store [24,25,26]. However, the role of hUC-MSCs for treating knee OA remains unclear. Several preclinical studies demonstrated that hUC-MSCs decreased osteogenic genes and inflammatory factors, and promoted chondrocyte proliferation [27,28,29,30,31]. Previously, there were several clinical studies that have investigated the effect of hUC-MSCs in subjects with knee OA [9, 32,33,34]. However, these studies enrolled a few numbers of subjects, with each study consisting of only one to six subjects. Therefore, we try to conduct a larger open-label trial of hUC-MSCs and HA for treating subjects with knee OA.
Methods
This single-arm, open-label study was conducted from July 2015 to December 2018 at Cipto Mangunkusumo General Hospital, Jakarta, Indonesia. We included subjects aged 50 years diagnosed with primary OA of one or both knees based on American College of Rheumatology (AC). Osteoarthritis was diagnosed based on history, physical examination, and radiographic evidence of joint change based on Kellgren–Lawrence grade I–IV 6 months before the initiation of the study.
The study was prospectively registered in ClinicalTrial.Gov (reference NCT03800810). After an IRB approval (no. 659/UN2.F1/ETIK/2015), signed informed consent was obtained in accordance with the latest version of the Helsinki Declaration. Knee OA staging was done in accordance with the Kellgren and Lawrence classification [35] using standard knee X-ray imaging of standing anteroposterior and horizontal lateral projection. Image interpretation and staging were independently done by one radiologist. The inclusion and exclusion criteria are listed in Table 1.
MRI
Knee MRI scans were performed with GE Optima MR450W (wide bore) 1.5 T (GE Healthcare, GE Healthcare, Waukesha, WI). Scans were further analysed using Advance Workstation 4.6 (AW 4.6) and processed to achieve a colourised T2 map using the CartiGram software (GE Healthcare, Waukesha, WI). The T2 mapping sequence was obtained using the following parameters: coronal orientation, 256 × 256 matrix, 16 × 16 cm field of view, 4 mm slice thickness, 1.5 mm slice gap, 62.5 kHz receiver bandwidth, 4 min acquisition time, TR of 1000 ms, TE of 8.3, 16.6, 24.9, 33.2, 41.4, 49.7, 58, 66.3 ms and colour range of 25–75 ms.
MRI scans were performed at baseline, 6 and 12 months. Standard knee MRI imaging protocol was obtained in axial, coronal and sagittal planes, in addition to using a specific cartilage sequence which is T1-weighted FS spoiled 3D gradient echo in axial and sagittal planes. Detailed measurements were taken from each compartment from three points: anterior, central and posterior. The mean thickness was calculated. Identical sequences and measurement sites were done on the follow-up scans.
Treatment
In our study, subjects diagnosed with OA of the knee were injected three times in total. In the first session, the subjects were injected with 10 × 106 units of hUC-MSC in 2 ml secretome and 2 ml hyaluronic acid (HA). In both the second and third week, the subjects were injected with 2 ml HA. The dose was in line with that used in a caprine model of OA in a study conducted by Murphy et al. [36]. Using that dose, the authors found that local delivery of adult MSCs to injured joints stimulates meniscal tissue regeneration. Moreover, such cells also retard the progressive destruction normally seen in OA. hUC-MSCs were isolated by multiple harvest explant method [37], and culture in in-house developed 10% platelet lysate containing xeno-free media as previously described in our study [38]. Subjects were subsequently followed up on the 1st and 3rd, then every 3 months until 1 year. Outcome measures include the International Knee Documentation Committee (IKDC) score, the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the visual analogue score (VAS). T2 map values of the knee cartilage were performed before implantation and then on the 6th and 12th month after the implantation.
Multiple harvest explant method [34]
Umbilical cord from at-term healthy delivery was dissected, and the umbilical artery and vein discarded and minced into small pieces. The pieces were put into wells of 24-well plates, one piece per well, and immersed in a small amount of medium, just to make them wet and to prevent floating of the pieces. The pieces were cultured in an incubator at 37 °C and with 5% CO2. The cultures were observed every day for cell outgrowth and dryness. A small amount of medium was added when necessary, and when cell outgrowth was observed, more medium was added, and cultures were harvested at 80–90% confluence. Harvested cells were recultured to obtain enough cells to be delivered to the patients.
Data analysis
Statistical analyses were performed using SPSS 23 for Mac. Paired t test or Wilcoxon’s test was used to analyse numerical variables between baseline and both 6 and 12 months after implantation.
Results
Patients demographics
A total of 29 subjects were enrolled in this study. Seventeen (58.6%) subjects were male, and the mean age was 58.3 ± 9.6 years. The mean BMI was 27.12 ± 4.4. A total of 57 knees received stem cells implantation. Based on the Kellgren–Lawrence system, 33 (57.8%) knees were classified into grade I–II osteoarthritis, and 24 knees (42.1%) were classified into grade III–IV. Characteristics of the subjects are presented in Table 2.
Clinical outcome
Tables 3 and 4 summarise the distribution of disability indices and knee pain throughout the observation period. Tables 3 and 4 divide the subjects into subjects with Kellgren–Lawrence grade I–II and III–IV (mild and severe OA), respectively. No complication was observed.
VAS between subjects with Kellgren–Lawrence grade I–II and III–IV showed similarity when patients were observed within 6 and 12 months. VAS in both groups showed mean reduction during 6th and 12th month follow-up even though only one period of follow-up reached statistical significance (6th month follow-up in group Kellgren–Lawrence grade III–IV), from 5 ± 2.97 to 3.38 ± 2.44 (p = .035).
Assessment of knee pain with IKDC Knee Forms also showed similar result between both groups, in which the mean IKDC score significantly increased over 6th month follow-up (53.26 ± 16.66 to 65.49 ± 13.01, p < .001, in subjects with grade I–II; and 48.84 ± 18.41 to 61.83 ± 18.83, p = .008, in subjects with grade III–IV). In WOMAC, similarity also found in 6th month follow-up. In this period, mean reduction was found statistically significant in both groups (from 22.55 ± 15.94 to 13.23 ± 10.29, p = .003, and from 27.57 ± 15.99 to 17.92 ± 19.1, p = 0.003, in those with Kellgren–Lawrence grade I–II and III–IV knee OA, respectively).
Radiographic outcome
Magnetic resonance imaging (MRI) quantitative T2 mapping was used to evaluate the result of stem cells treatment in both medial and lateral cartilage of the knees. The result of T2 mapping showed a varied result with no significant differences during 6th and 12th month follow-up (Tables 2, 3). In grade I–II knee OA, quantitative MRI T2 showed tendency to increase after implantation of stem cells (from 43.45 (30.9–361.36), 39,67 (20.5–68) to 43.5 (27.0–70.0), 43.7 (32.8–104) in 6th month follow-up and 48.12 (30.84–481.5), 61.67 (30.84–70.1) during 12th month follow-up for medial and lateral knee, respectively). On the other hand, quantitative MRI T2 for grade III–IV knee OA showed decreasing value during 6th and 12th month follow-up, but for medial cartilage, T2 mapping increased again during 12th month follow-up.
Discussion
Osteoarthritis is a degenerative joint disease characterised by progressive destruction of articular cartilage, which results in limitations of joint motion [3, 39, 40]. However, in this study, we did not measure the limitation of joint movement at baseline and follow-up. This was the limitation of our study. Current treatment options for OA include the use of anti-inflammatory drugs and lubricating supplements, as well as surgeries, such as drilling, microfracture and mosaicplasty. However, these modalities only improve the symptoms transiently [41]. Despite advances in treatment, most therapies do not stop or even reduce the progression of the disease and are only symptomatic [42]. Cell-based therapy may hold promise for the repair of articular cartilage defects as transplantation of autologous chondrocytes was effective in treating cartilage defects in the knee [41]. As this approach is limited by the number of healthy chondrocytes in patients, stem cells have emerged as an alternative [20].
Mesenchymal stem cells are an attractive source of cells because of their chondrogenic potential and easy access for isolation and future expansion [1]. MSCs are multipotent, adult stem cells that show clinical promise as therapeutic agents in regenerative medicine [8, 43,44,45,46]. MSCs can be easily isolated from many anatomic locations, including from whole marrow aspirate, muscle biopsy, adipose liposuction aspirate and from other tissues [43]. For orthopaedic uses, these sources have been compared by many researchers for their ability to heal bone and cartilage, with differences being noted [47]. Due to a painless collection procedure and self-renewal properties, the human UC provides a promising source of MSCs, although the use of UC-derived stem cells in other diseases [48,49,50] and the effect of human UC stem cell for OA treatment have not been reported in the literature. Several studies reported that chondrocytes promoted chondrogenic differentiation of human umbilical cord blood-derived MSCs [51,52,53,54].
UC-MSCs can be isolated from various areas within the umbilical cord, including the subendothelial layer, perivascular zone, Wharton’s jelly, umbilical cord lining and the whole umbilical cord [55]. UC-MSCs also meet the minimum criteria of plastic adherence, immunological profile and differentiation potential [56]. The many advantages of using UC-MSCs in tissue engineering and regenerative medicine are the avoidance of ethical issues, a painless harvesting process, few embryonic features, high cell proliferation, wide differentiation potential [57], hypoimmunogenicity [25] and nontumorigenicity [58].
In the present study, we found that the subjects had significantly improved IKDC and WOMAC scores. However, there was no significant MRI improvement at both 6th and 12th month follow-up. Therefore, it is now known whether improvement was from HA or hUC-MSCs. This is in line with previous studies; a meta-analysis of the 11 trials with 558 patients using MSCs found a significant improvement in various clinical scores [59]. They also found a significant improvement in clinical symptoms and cartilage morphology [59]. However, one published work using allogeneic bone marrow-derived MSCs in advanced knee OA in humans showed clinical improvement but no significant MRI improvement [60].
The mechanism of how UC-MSCs improve OA symptoms remains unclear. Whether MSCs stimulate the proliferation and differentiation of resident progenitor cells or they differentiate into chondrocytes remains to be clarified [61]. Rabbit and goat models of osteoarthritis suggest that the repair occurs through paracrine effects by stimulation of endogenous repair mechanisms [36]. Van Lent, in his editorial, suggested that the main effect of MSC transplantation in the joint is the suppression of the synovial inflammation [62]. Wang et al. [27] found that human umbilical cord stem cell decreased the osteogenic genes (COX2, COL10A1 and MMP13) and production of some inflammatory factors (TNF-α, IL-1β, IL-6, IL-10). This indicates that human umbilical cord stem cell attenuated inflammatory response of OA. Zhu et al. [28] reported that human umbilical cord blood MSC transplantation suppresses inflammatory responses during the early stage of focal cerebral ischaemia in rabbits, an ischaemia-induced increase in IL-1β, IL-6 and TNF-α levels in the serum and peri-ischaemic brain tissues within 6-h MCAO-reperfusion were markedly suppressed human umbilical cord stem cell transplantation. In another study, human UC-MSCs were reported to decrease expression of MDA, GSSG, TNF-α, IFN-γ, TGF-β, IL-1, IL-2, IL-6, collagen type 1 mRNA and MMPs [29].
Further experiments demonstrated that human UC-MSC significantly promoted chondrocyte proliferation that may be explained by that human umbilical cord stem cell containing many soluble factors, such as G-CSF, PDGF-BB and bFGF [30, 31]. In addition, human umbilical cord stem cell may inhibit apoptosis and then promoted proliferation. Zhang et al. [63] found that human umbilical cord stem cell promoted proliferation and inhibited apoptosis of skin cells after head stress in vitro by secreting exosomes.
In this study, we also noted that the greatest improvement in knee function was observed after 6th month follow-up. This finding is in line with previous studies [9, 32,33,34], which found that the maximum results were achieved at 6th month follow-up but decline afterwards. Davatchi et al. [9] conducted an open-label trial in four subjects with moderate-to-severe OA of both knees. They noticed that at 6 months the subjects had considerable improvement particularly on major parameters such as walking time, climbing stairs, gelling pain, VAS and range of motion. However, at 2-year follow-up, the results were started to decline [9]. Centeno, in 2008, was experimenting with one human subject [32, 33]. It showed good results at 6 months with no side effects. In 2012, Emadedin [34] reported the results of six patients with knee OA, who had an MSC transplantation. The outcomes were good at 6 months; however, the effects declined at 12 months. However, compared to the baseline, the outcomes were much better at 12 months [34].
The reason behind the declining efficacy of MSC after 6 months remains unclear. Perhaps, it may be due to the continuous degradation of the cartilage. Probably, after a while, the transplanted MSCs lose their characteristics and become like the original resident MSCs with chaotic function. If that is what happened, the transplanted MSCs did not have the time to repair the cartilage significantly. However, this is just a presumption and has to be verified scientifically. Feng et al. [64] used an intra-articular injection of allogeneic adipose-derived MSCs combined with HA in a sheep model, and they found that it could efficiently block OA progression and promote cartilage regeneration and allogeneic adipose-derived MSCs combined with HA lasted for 14 weeks after intra-articular injection. Davatchi et al. [9] found that although after a while the improvement started to decline, the transplanted knee joint in their study declined slower than the contralateral nontransplanted knee. This suggests that the repair effect of MSCs probably has not entirely disappeared. However, the lasting effect up to 6 months may also be due to the effect of HA. To get a more lasting effect, repeated MSC injection might be needed [65]. In a systematic review of 18 RCTs regarding the effect of HA in knee OA, O’Hanlon et al. [66] found that the functional outcomes of the subjects were improved by intra-articular HA injection. However, the durability of the effects could be measured beyond 6 months. On the other hand, Feng et al. [64] administered adipose-derived mesenchymal stem cells (AD-MSCs) combined with HA in sheep models with OA. They found that the levels of inflammatory factors (such as TNF-α, IL-6) in synovial fluid of AD-MSCs + HA-treated group were significantly lower than those of saline and HA-alone groups. All of these findings suggest that further RCTs comparing the effect of MSC and HA in longer follow-ups are required to investigate the exact reason behind the declining efficacy of MSC after 6 months.
The present study demonstrates that UC-MSCs could be a potential new treatment for OA. However, our study was limited by its open-label, nonrandomised design as well as the absence of a placebo arm. We hope that further clinical studies with better study design and larger sample size will be conducted in the future to investigate the safety and efficacy of this cell-based therapy for OA.
Conclusions
The combination of hUC-MSCs and HA implantation could be a potentially new regenerative modality for osteoarthritis of the knee. The most significant effect was achieved after 6 months of injection. However, incorporation of hUC-MSC as a treatment option in knee OA needs more evidence. Further randomised clinical studies are required to investigate the safety and efficacy of these cells for treating osteoarthritis of the knee.
References
Morille M, Toupet K, Montero-Menei CN et al (2016) PLGA-based microcarriers induce mesenchymal stem cell chondrogenesis and stimulate cartilage repair in osteoarthritis. Biomaterials. https://doi.org/10.1016/j.biomaterials.2016.02.022
Zhen G, Wen C, Jia X et al (2013) Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med 19:704–712. https://doi.org/10.1038/nm.3143
Wang Y, Yuan M, Guo Q-Y et al (2015) Mesenchymal stem cells for treating articular cartilage defects and osteoarthritis. Cell Transplant 24:1661–1678. https://doi.org/10.3727/096368914X683485
Li G, Yin J, Gao J et al (2013) Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther 15:223. https://doi.org/10.1186/ar4405
Dulay GS, Cooper C, Dennison EM (2015) Knee pain, knee injury, knee osteoarthritis and work. Best Pract Res Clin Rheumatol 29:454–461. https://doi.org/10.1016/j.berh.2015.05.005
Mamidi MK, Das AK, Zakaria Z, Bhonde R (2016) Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthr Cartil 24:1307–1361. https://doi.org/10.1016/j.joca.2016.03.003
Hussain SM, Neilly DW, Baliga S et al (2016) Knee osteoarthritis: a review of management options. Scott Med J 61:7–16. https://doi.org/10.1177/0036933015619588
Barry F, Murphy M (2013) Mesenchymal stem cells in joint disease and repair. Nat Rev Rheumatol 9:584–594
Davatchi F, Sadeghi Abdollahi B, Mohyeddin M, Nikbin B (2016) Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis 19:219–225. https://doi.org/10.1111/1756-185X.12670
Buckwalter JA (2000) Advancing the science and art of orthopaedics: lessons from history. J Bone Jt Surg Ser A 82-A:1782–1803. https://doi.org/10.2106/00004623-200012000-00012
Kurtz S, Ong K, Lau E et al (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Jt Surg Ser A 89:780–785. https://doi.org/10.2106/JBJS.F.00222
Derar H, Shahinpoor M (2015) Recent patents and designs on hip replacement prostheses. Open Biomed Eng J. https://doi.org/10.2174/1874120701509010092
Gallo J, Kamínek P, Tichá V et al (2002) Particle disease. A comprehensive theory of periprosthetic osteolysis: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 146:21–28. https://doi.org/10.5507/bp.2002.004
Sargeant A, Goswami T (2006) Hip implants: paper V. Mater Des, Physiological Eff. https://doi.org/10.1016/j.matdes.2004.10.028
Srimongkol S (2012) A review of mathematical modeling in total hip replacement. Int Math Forum 7:2561–2569
Lo GH, LaValley M, McAlindon T, Felson DT (2003) Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. J Am Med Assoc 290:3115–3321. https://doi.org/10.1001/jama.290.23.3115
Gallagher B, Tjoumakaris FP, Harwood MI et al (2015) Chondroprotection and the prevention of osteoarthritis progression of the knee: a systematic review of treatment agents. Am J Sports Med 43:734–744. https://doi.org/10.1177/0363546514533777
Deng M-W, Wei S-J, Yew T-L et al (2015) Cell therapy with G-CSF-mobilized stem cells in a rat osteoarthritis model. Cell Transplant 24:1085–1096. https://doi.org/10.3727/096368914X680091
Saw KY, Anz A, Merican S et al (2011) Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthrosc J Arthrosc Relat Surg 27:493–506. https://doi.org/10.1016/j.arthro.2010.11.054
Chiang ER, Ma HL, Wang JP et al (2016) Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PLoS ONE 11:e0149835. https://doi.org/10.1371/journal.pone.0149835
Liu Q, Niu J, Huang J et al (2015) Knee osteoarthritis and all-cause mortality: the Wuchuan Osteoarthritis Study. Osteoarthr Cartil 23:1154–1157. https://doi.org/10.1016/j.joca.2015.03.021
Gore M, Tai K-S, Sadosky A et al (2011) Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ 14:497–507. https://doi.org/10.3111/13696998.2011.594347
Liu TM, Martina M, Hutmacher DW et al (2006) Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 25:750–760. https://doi.org/10.1634/stemcells.2006-0394
Ding D-C, Chang Y-H, Shyu W-C, Lin S-Z (2015) Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant 24:339–347. https://doi.org/10.3727/096368915X686841
Ding DC, Chou HL, Chang YH et al (2016) Characterization of HLA-G and related immunosuppressive effects in human umbilical cord stroma-derived stem cells. Cell Transplant 25:217–228. https://doi.org/10.3727/096368915X688182
Ding DC, Shyu WC, Lin SZ et al (2012) Human umbilical cord mesenchymal stem cells support nontumorigenic expansion of human embryonic stem cells. Cell Transplant 21:1515–1527. https://doi.org/10.3727/096368912X647199
Wang H, Yan X, Jiang Y et al (2018) The human umbilical cord stem cells improve the viability of OA degenerated chondrocytes. Mol Med Rep 17:4474–4482. https://doi.org/10.3892/mmr.2018.8413
Zhu Y, Guan YM, Huang HL, Wang QS (2014) Human umbilical cord blood mesenchymal stem cell transplantation suppresses inflammatory responses and neuronal apoptosis during early stage of focal cerebral ischemia in rabbits. Acta Pharmacol Sin 35:585–591. https://doi.org/10.1038/aps.2014.9
Min F, Gao F, Li Q, Liu Z (2015) Therapeutic effect of human umbilical cord mesenchymal stem cells modified by angiotensin-converting enzyme 2 gene on bleomycin-induced lung fibrosis injury. Mol Med Rep 11:2387–2396. https://doi.org/10.3892/mmr.2014.3025
Amable PR, Teixeira MVT, Carias RBV et al (2014) Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res Ther 5:53. https://doi.org/10.1186/scrt442
Chen J, Liu Z, Hong MM et al (2014) Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells. PLoS ONE 16:e115316. https://doi.org/10.1371/journal.pone.0115316
Centeno CJ, Busse D, Kisiday J et al (2008) Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells, platelet lysate and dexamethasone. Am J Case Rep 11:343–353
Centeno CJ, Busse D, Kisiday J et al (2008) Regeneration of meniscus cartilage in a knee treated with percutaneously implanted autologous mesenchymal stem cells. Med Hypotheses 71:900–908. https://doi.org/10.1016/j.mehy.2008.06.042
Emadedin M, Aghdami N, Taghiyar L et al (2012) Intra-articular injection of autologous mesenchymal stem cells in six patients with knee Osteoarthritis. Arch Iran Med 15:422–428
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502
Murphy JM, Fink DJ, Hunziker EB, Barry FP (2003) Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48:3464–3474. https://doi.org/10.1002/art.11365
Pawitan JA, Liem IK, Budiyanti E et al (2014) Umbilical cord derived stem cell culture: multiple-harvest explant method. Int J PharmTech Res 6:1202–1208
Dilogo IH, Primaputra MRA, Pawitan JA, Liem IK (2017) Modified Masquelet technique using allogeneic umbilical cord-derived mesenchymal stem cells for infected non-union femoral shaft fracture with a 12 cm bone defect: a case report. Int J Surg Case Rep 34:11–16. https://doi.org/10.1016/j.ijscr.2017.03.002
Litwic A, Edwards MH, Dennison EM, Cooper C (2013) Epidemiology and burden of osteoarthritis. Br Med Bull 105:185–199. https://doi.org/10.1093/bmb/lds038
Kong L, Zheng LZ, Qin L, Ho KKW (2017) Role of mesenchymal stem cells in osteoarthritis treatment. J Orthop Transl 9:89–103. https://doi.org/10.1016/j.jot.2017.03.006
Brittberg M, Lindahl A, Nilsson A et al (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895. https://doi.org/10.1056/NEJM199410063311401
Martel-Pelletier J, Wildi LM, Pelletier JP (2012) Future therapeutics for osteoarthritis. Bone 51:297–311. https://doi.org/10.1016/j.bone.2011.10.008
Alhadlaq A, Mao JJ (2004) Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev 13:436–448. https://doi.org/10.1089/scd.2004.13.436
Bruder SP, Fink DJ, Caplan AI (1994) Mesenchymal stem cells in bone development, bone repair, and skeletal regenaration therapy. J Cell Biochem 56:283–294. https://doi.org/10.1002/jcb.240560303
Cha J, Falanga V (2007) Stem cells in cutaneous wound healing. Clin Dermatol 25:73–78. https://doi.org/10.1016/j.clindermatol.2006.10.002
Gangji V, Toungouz M, Hauzeur J-P (2005) Stem cell therapy for osteonecrosis of the femoral head. Expert Opin Biol Ther. https://doi.org/10.1517/14712598.5.4.437
Centeno CJ (2014) Clinical challenges and opportunities of mesenchymal stem cells in musculoskeletal medicine. PM&R 6:70–77. https://doi.org/10.1016/j.pmrj.2013.08.612
Kim S-W, Han H, Chae G-T et al (2006) Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger’s disease and ischemic limb disease animal model. Stem Cells 24:1620–1626. https://doi.org/10.1634/stemcells.2005-0365
Baker CD, Abman SH (2013) Umbilical cord stem cell therapy for bronchopulmonary dysplasia: ready for prime time? Thorax 68:402–404. https://doi.org/10.1136/thoraxjnl-2012-202661
Gu Z, Akiyama K, Ma X et al (2010) Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus 19:1502–1514. https://doi.org/10.1177/0961203310373782
Li X, Duan L, Liang Y et al (2016) Human umbilical cord blood-derived mesenchymal stem cells contribute to chondrogenesis in coculture with chondrocytes. Biomed Res Int 2016:3827057. https://doi.org/10.1155/2016/3827057
Deng H, Liao L, Wu J et al (2017) Clinical efficacy of intravesical electrical stimulation on detrusor underactivity: 8 years of experience from a single center. Med (United States) 96:e8020. https://doi.org/10.1097/MD.0000000000008020
Zheng P, Ju L, Jiang B et al (2013) Chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells by co-culture with rabbit chondrocytes. Mol Med Rep 8:1169–1182. https://doi.org/10.3892/mmr.2013.1637
Mennan C, Wright K, Bhattacharjee A et al (2013) Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int 2013:916136. https://doi.org/10.1155/2013/916136
Maumus M, Jorgensen C, Noël D (2013) Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie 95:2229–2234. https://doi.org/10.1016/j.biochi.2013.04.017
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8:315–317. https://doi.org/10.1080/14653240600855905
Ding DC, Shyu WC, Chiang MF et al (2007) Enhancement of neuroplasticity through upregulation of β1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis 27:339–353. https://doi.org/10.1016/j.nbd.2007.06.010
Wang D, Chen K, Du WT et al (2010) CD14 + monocytes promote the immunosuppressive effect of human umbilical cord matrix stem cells. Exp Cell Res 316:2414–2423. https://doi.org/10.1016/j.yexcr.2010.04.018
Xu S, Liu H, Xie Y et al (2015) Effect of mesenchymal stromal cells for articular cartilage degeneration treatment: a meta-analysis. Cytotherapy 17:1342–1352. https://doi.org/10.1016/j.jcyt.2015.05.005
Vega A, Martín-Ferrero MA, Del Canto F et al (2015) Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation 17:1342–1352. https://doi.org/10.1097/TP.0000000000000678
Gupta PK, Das AK, Chullikana A, Majumdar AS (2012) Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther 3:25. https://doi.org/10.1186/scrt116
van Lent PLEM, van den Berg WB (2013) Mesenchymal stem cell therapy in osteoarthritis: advanced tissue repair or intervention with smouldering synovial activation? Arthritis Res Ther 15:112. https://doi.org/10.1186/ar4190
Zhang B, Wang M, Gong A et al (2015) HucMSc-exosome mediated-Wnt4 signaling is required for cutaneous wound healing. Stem Cells 33:2158–2168. https://doi.org/10.1002/stem.1771
Feng C, Luo X, He N et al (2018) Efficacy and persistence of allogeneic adipose-derived mesenchymal stem cells combined with hyaluronic acid in osteoarthritis after intra-articular injection in a sheep model. Tissue Eng Part A 24:219–233. https://doi.org/10.1089/ten.tea.2017.0039
Matas J, Orrego M, Amenabar D et al (2019) Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med 8:215–224. https://doi.org/10.1002/sctm.18-0053
O’Hanlon CE, Newberry SJ, Booth M et al (2016) Hyaluronic acid injection therapy for osteoarthritis of the knee: concordant efficacy and conflicting serious adverse events in two systematic reviews. Syst Rev 5:186. https://doi.org/10.1186/s13643-016-0363-9
Acknowledgements
We would like to thank Boenyamin Setiawan, PhD and National Innovation System Research Incentive Program (INSINAS) for funding our research, as well as Tri Kurniawati, BSc for her assistance throughout the grant administration process.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ismail Hadisoebroto Dilogo, Anissa Feby Canintika, Alberto Lastiko Hanitya, Jeanne Adiwinata Pawitan, Isabella Kurnia Liem and Jacub Pandelaki declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dilogo, I.H., Canintika, A.F., Hanitya, A.L. et al. Umbilical cord-derived mesenchymal stem cells for treating osteoarthritis of the knee: a single-arm, open-label study. Eur J Orthop Surg Traumatol 30, 799–807 (2020). https://doi.org/10.1007/s00590-020-02630-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-020-02630-5