Abstract
Background
The treatment of periprosthetic femoral fractures is a great challenge for the orthopedic surgeon and requires a knowledge of bone fracture fixation as well as skills and experience in revision surgery. The aim of this retrospective study was to evaluate the functional and radiological outcomes of periprosthetic femoral fractures surgically treated in our department from 2010 to 2016.
Materials and methods
This study involved 73 patients with a periprosthetic femoral fracture after total hip arthroplasty or hemiarthroplasty. Periprosthetic femoral fractures were classified using the Vancouver system. Functional outcomes were assessed using Harris hip score, Palmer Parker score, SF-36 score and ambulatory status. Radiological findings were classified using Beals and Tower’s criteria.
Results
The mean age of patients was 79.6 years old. Local risks factors were identified in 67% of the patients, principally osteoporosis (63.0%), followed by osteolysis (26.0%) and loosening of the stem (8.2%). According to the Vancouver classification, there were 10 type A, 49 type B and 14 type C fractures. Of the type B fractures, 26 were B1, 17 were B2 and 6 were B3. Applying Beals and Tower’s criteria, radiological results were excellent in 24 patients (32.9%), good in 35 (47.9%) and poor in 14 (19.2%). The mean Harris hip score post-operatively was 72.5.
Conclusions
These kinds of fractures should be assessed individually and the optimal treatment plan should be made in accordance with the bone stock quality, stem stability, location of the fracture and patient expectations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, periprosthetic femoral fractures (PFFs) are a serious increasing complication after hip replacements as the number of patients undergoing primary and revision total hip arthroplasty (THA) continues to rise [1, 2].
Data from the literature estimate an incidence of 1% in the first implants up to 6% in revision surgery. Different reasons have determined the increase in PFF; first of all, the high number of hip prosthesis that are implanted every year in correlation with the continuous increase in the number of cases of coxarthrosis due to the aging of the general population. In particular, the number of primary and revision arthroplasty in the USA is expected to increase by 174 and 137%, respectively, within 2030 [3]. Other factor to underline is the enlargement of indications since nowadays: thanks to innovated prosthetic materials and improvement in operating technique, surgery is realized more and more in old patients with poor quality bone and loss of fixation of the components associated with it.
Literature studies agree to recognize in the early detection of risk factors a crucial moment to the management of these patients. Among the risk factors for PFFs are to be included, general factors, as osteoporosis with loss of bone stock, and local factors as loosening of the femoral stem, periprosthetic osteolysis, or environmental factors that could cause traumatic events of modest entity, but enough to cause a PFF.
According to the Swedish National Hip Arthroplasty Register of 2010, PFFs are the third most frequent complication after THA (9.4%), only preceded by aseptic loosening of the stem (60.1%) and recurrent prosthesis mobilization (13.1%) [2, 4]. They also lead to an increase in mortality, and their management is extremely expensive.
Materials and methods
A total of 73 patients with PFFs surgically treated in our Department from February 2010 to November 2016 were enrolled in this retrospective study; 51 of them (69.9%) were women and 22 (30.1%) were men. Nineteen patients (26.0%) were lost to clinical follow-up because died before being interviewed.
PPFs were classified according to the Vancouver system, proposed by Duncan and Masri [5], which incorporates the site of the fracture, the stability of the implant and the quality of the surrounding bone [6].
X-rays and reports were studied to identify the location of the fracture rhymes, the causes and type of procedure used for first implant (THA, revision of arthroplasty, hemiarthroplasty (HA)), the type of fixation used (cemented or cementless), the year in which it was performed and the presence of risk factors (osteolysis, cortical defects, stress riser and stem loosening).
Functional outcomes were assessed with the use of Harris hip score (HHS), Palmer Parker score (PPS) [7], SF-36 score and ambulatory status. The mean follow-up time was 41 months (range 4–69).
Therefore, pain was evaluated using the Visual Analogue Scale (VAS) and comorbidities were examined using the Charlson Comorbidity Index (CCI) [8] and the American Society of Anesthesiologists (ASA) score.
Radiological findings were classified using Beals and Tower’s criteria [9]. Following this classification, outcomes were assessed as excellent (stable prosthesis with minimal deformity), good (stable prosthesis with moderate deformity) or poor (loosening, non-union, severe deformity, sepsis or new fracture).
A prosthesis was graded as stable if there were not radiolucent lines around the stem. The femoral components were considered loose if there were progressive radiolucencies of ≥ 2 mm wide involving (1) > 50% of the bone–cement interface, (2) cement column fractures surrounding the prosthesis or (3) femoral component migration.
We also subdivided patients unrolled in this study into four groups according to the prosthetic first implant: the first group includes cemented THA, the second group cementless THA, the third group revision THA and the forth group hemiarthroplasty. So, we searched significative differences between the first two groups.
Initially, an exploratory analysis of sample data was performed, the results of this study were reported as means, with the standard derivation (SD) preceded by ±; frequencies and percentages were calculated for qualitative data. Statistical significance was researched with the use of Anova test, Chi-squared test and Kruskal–Wallis test using the program “MedCalc 12.14.0” (Medcalc Software, Mariakerke, Belgium).
Results
The mean age of the patients was 79.6 ± 9.8 years (range 53–96) with 80.3 ± 9.9 years for women (range 54–96) and 78 ± 9.6 years for men (range 53–91). The mean BMI of all patients at the time of surgery was 26.3 ± 7.4.
The most frequent fractures, 56.2% (n. 41), occurred on the right side, while 43.8% (n. 32) on the left side.
Reasons for first prosthetic implant were coxarthrosis in 48 patients (65.8%), fractures in 21 (28.8%), avascular necrosis of the femoral head in 3 (4.1%) and developmental dysplasia of the hip (DDH) in 1 (1.4%).
The PFF involved a primary THA in 58 cases (79.5%), a revision THA in 9 (12.3%) and a HA in 6 (8.2%).
The type of fixation was cemented in 30 cases (41.1%), while cementless in 43 (58.9%). The mean time from primary procedure to fracture was 8.6 years. No intraoperative fractures occurred during first prosthetic implants.
As for the comorbidities, 14 patients (19.2%) had a Deyo-Charlson index > 3, 12 (16.4%) a score of 3, 12 (16.4%) a score of 2, 12 (16.4%) a score of 1 and 23 (31.5%) had no comorbidities.
At the time of surgery, ASA score was 4 in 24 patients (32.8%), 3 in 41 (56.2%) and 2 in 8 (11.0%).
The mean operative time was 111 ± 43.4 min. The mean delay between the hospital admission and surgery was 5.7 days (range 0–22 days), and the mean hospital stay was 21 days (range 5–108 days).
We found a high incidence of blood transfusions in patients with surgical treatment (all cases except four). The mean number of units transfused was 3.9 (range 0–13), with the most number in patients with type B3 fractures (5.3 ± 2.8).
Radiographic and clinical assessment at 6 months revealed bone union in 25 patients (34.2%).
In our study, by statistical calculation, we found that gender, BMI, side and type of fracture, ASA, presence of contralateral prosthesis and type of surgery were not statistically significant on post-operative functional outcomes.
The mean HHS post-operatively was 72.5 ± 16.8 (fair result). The lowest HHS scores were obtained in the group of patients where PFFs had occurred after cementless arthroplasty. In particular, examinations conducted at 12 months after surgical treatment showed poor results (HHS < 70) in 24 patients (44.4%); 15 of them (27.8%) after cementless THA, 5 (9.3%) after primary revision surgery, 3 (5.6%) after hemiarthroplasty and 2 (3.7%) after cemented THA.
Moreover, according to the Vancouver classification, we found HHS > 90 (excellent result) in 33.3% type A, in 5.0% type B1, in 23.1% type B2, in 20.0% type B3 and in 22.2% type C fractures.
Post-operative weight-bearing was allowed in 21 patients (28.8%), including 17 (23.3%) with partial weight-bearing and 4 (5.5%) with full weight-bearing; the remaining 52 (71.2%) were not allowed it. In particular, following the Vancouver classification, we found allowed weight-bearing in 40% patients with type A, in 38.5% with type B1, in 23.5% with type B2, in 33.3% with type B3 and in 7.1% with type C fractures.
According to the Beals and Tower’s criteria, we obtained excellent or good radiological findings in 59 patients (80.8%). Particularly, excellent results were found in 6 patients of 10 (60%) with type A, in 9 of 26 (34.6%) with type B1, in 6 of 17 (35.3%) with type B2, in 1 of 6 (16.7%) with type B3 and in 2 of 14 (14.3%) with type C fractures.
Moreover, poor results were found in 2 patients of 10 (20%) with type A, 2 of 26 (7.7%) with type B1, 4 of 17 (23.5%) with type B2, 1 of 6 (16.7%) with type B3 and 5 of 14 (35.7%) with type C fractures. So we can suggest type A fractures had a better radiological result, while type C had a worse radiological result at 12 months from the surgery.
Evaluating the walking ability of the patients after surgery, we subdivided the PPS into three groups: PPS < 4 (poor results), PPS 4–6 (good results), PPS > 6 (excellent results). Considering the type of fracture according to the Vancouver classification, we found excellent results in 55.6% type A, 26.3% type B1, 33.3% type B2 and C and 0% type C fractures; poor results in 11.1% type A, 26.3% type B1, 16.7% type B2, 20.0% type B3 and 22.2% type C fractures. From these data, we can say the best results were in type A fractures, while the worst results in type B1.
Moreover, the mean VAS score was 3.4 ± 2.4. Dividing the VAS score into three groups (0–2 mild pain, 3–5 moderate pain, > 5 high pain), we found in the first group (mild pain) 12 patients of 22 (54.6%) had been operated with ORIF (plate and screws), 5 (22.7%) with metal loops and 5 (22.7%) with revision of the stem. Furthermore, following the Vancouver classification, we found mild pain (VAS < 3) in 66.7% type A, 42.1% type B1, 25.0% type B2, 40.0% type B3 and 33.3% type C fractures. So we can suggest type A fractures were related with less pain (Fig. 1).
We also found 20 of 46 patients (43.5%) with osteoporosis were in anti-osteoporotic therapy before fracture; the mean post-operative HHS in this group was 76.6 (fair result), while the mean PPS was 6.5. In the other group of 26 patients (56.5%) without anti-osteoporotic therapy, the mean HHS was 70.8 (fair result), while the mean PPS was 5.2.
After that, comparing the cemented THAs (group 1; 21 cases) with cementless THAs (group 2; 37 cases) as cause of first implant, we found in the first group the mean age of patients with PFF was 83 ± 9.3 years, the mean BMI was 27.8 ± 8.4, the mean ASA was 3.5 ± 0.6 and the mean CCI was 2.9 ± 1.5, while in the second group, respectively, 76 ± 8.6 years, 25.7 ± 3.6, 3.0 ± 0.6 and 3.3 ± 2.0. So, there were no significative differences about CCI in these two groups, while there was a slight difference about ASA, age and BMI (all higher in cemented THA).
Moreover, in the first group, the mean time of surgery was 127.8 ± 45.2 min, the mean days of hospitalization was 23.3 ± 23.2, the mean time from first implant to fracture was 12.4 ± 8.3 years, and the mean units of blood transfused was 4.2 ± 2.8, while in the second group, respectively, 105.1 ± 44.8 min, 18.7 ± 12.6, 5.9 ± 7.1 years and 3.3 ± 2.0. As to be expected, the surgical time was longer in cemented implants. Even the days of hospitalization, the mean time from first implant to fracture and the mean units of blood transfused were higher in patients with cemented THA.
As for the Vancouver classification, we found in the first group 0 type A, 7 (33.3%) type B1, 7 (33.3%) type C, 4 (19.1%) type B2 and 3 (14.3%) type B3 fractures, while in the second group, 12 (32.4%) type B1, 12 (32.4%) type B2, 9 (24.3%) type A, 3 (8.1%) type B3 and 1 (2.7%) type C fractures. From these results, we can note there were no fractures around the trochanteric region in cemented implants, while fractures below the tip of the stem (the most frequent in cemented implants) were found the least common after cementless implants.
According to the Beals and Tower’s criteria, in cementless implants, we found poor radiographic results in six patients (16.2%), good results in 14 (37.8%) and excellent results in 17 (45.9%), while in cemented implants, poor results in 3 patients (14.3%), good results in 14 (66.7%) and excellent results in 4 (19.0%).
As for the functional outcomes, in the first group, we found the mean HHS was 80.9 ± 11.1, the mean PPS was 6.1 ± 2.2, the mean ISF (index of physical health) according to the SF-36 was 40.1 ± 10.2 (physical activity 57.1 ± 30.1; physical role restrictions 60.7 ± 33.6; physical pain 68.8 ± 19.7), while in the second group, respectively, 72.6 ± 18.2, 5.8 ± 2.0, 36.9 ± 11.5 (physical activity 53.7 ± 26.6; physical role restrictions 48.4 ± 36.5; physical pain 57.8 ± 20.2). All these data show better functional results in cemented THA compared to cementless THA (Fig. 2).
Discussion
According to the literature, conservative treatment in patient with PFFs results in a high rate of complications, which include non-union, pulmonary embolism and atelectasis [10]. For that, surgical treatment is recommended for elderly patients [11]. We chose surgical management for all patients in this study because of its advantages of early joint exercise and ambulation to avoid the problem of conservative treatment.
Surgical treatment of PFFs also demonstrated mortality in the literature, generally showing a prevalence of approximately 10% [12]. In our study, we found 8 patients (10.9%) had died from complications unrelated to the fracture, 4 (5.5%) for cardiogenic shock, 3 (4.1%) for respiratory failure, 2 (2.7%) for kidney failure and 2 (2.7%) for bed rest syndrome.
The time interval between primary THA and fracture varies among different studies, ranging from 7.4 years (Swedish Registry) to 8.1 years (Mayo Clinic Total Joint Registry). Furthermore, the Swedish Registry reported the time interval between revision surgery and fracture to average 3.9 years [13]. As in the literature, we found the mean time to fracture from the primary THA was 8.3 ± 8.2 years, while in spite of the Swedish Registry data, in our study, the mean time to fracture from last revision surgery was 12 ± 7.2 years. In particular, we found the shortest time between the primary implant and PFFs was after cemented hemiarthroplasty (5.8 ± 10.9 years).
Following the Beals and Tower’s criteria, we obtained excellent or good radiological findings in 59 patients (80.8%). On the contrary, Beals and Tower showed a higher proportion of poor results (52%), in spite of the fact that the patients they studied were younger than our sample (mean of 67 vs. 79.6 years old) [14]. These better results are probably attributable to the use of new fixation devices with locking screws, the evolution of modular stems and the less invasive techniques, but further studies should be conducted to confirm this.
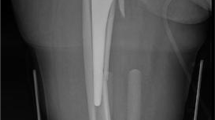
The Vancouver classification has proved to be reliable and valid, showing good correlation between radiographic evaluation and intraoperative findings, even when used by unexperienced people [15]. According to this classification, in our cases, the most common PFFs were type B1 (26 patients, 35.6%), followed by type B2 (17 patients, 23.3%), type C (14 patients, 19.2%), type A (10 patients, 13.7%) and type B3 (6 patients, 8.2%). Type A fractures were treated using metal mesh with loops or plates (Fig. 3a, b). Type B1 fractures were stabilized with Intrauma O’Nil plates, in some cases supported with metal loops (Fig. 4a, b). Type B2 fractures were treated by replacing the prosthetic stem with a new one with and without additional strengthening as a plate or metal loops (Fig. 5a, b). Revision surgery was performed using long cementless stems to achieve fixation at the femoral isthmus. Type C fractures were stabilized with Intrauma O’Nil or Synthes plates (Fig. 6a, b).
The results presented by other authors are similar to ours and show that type B fractures occur most often and constitute about 80% of all fractures, especially in patients after primary hip replacement surgery [16].
In agreement with our results, many studies have shown that in an elderly population similar to ours the mean HHS is between 59 and 73 [17, 18]. Like us, Montaldi et al. [19] found poor HHS values but good radiological results in according with the Beals and Tower classification.
In the present study, we did not find statistical differences in the final results depending on the type of fracture, the type of surgery, or the previous comorbidities. On the other hand, we found that the presence of previous local risk factors is associated with a poorer outpatient state after the fracture, although we did not identify an independent risk factor.
In the literature, it has been suggested that the type of fixation has an influence on the functional outcomes. Specifically, Foster et al. [20] found a significantly higher risk of PFFs in uncemented Austin-Moore hemiarthroplasties (7%) than Thompson cemented hemiarthroplasties (0%). These and other authors recommended cemented fixation in elderly patients, as bone cement may act to reinforce the osteoporotic proximal femur, improving load distribution. In our study, uncemented fixation was used in 97.3% of the patients, but this factor did not have an effect on the final outcome. Nevertheless, further research should be conducted in our institution to assess this factor as a risk factor of PFFs.
Then, the use of cement is considered by many reports as a protective factor for PFFs [21]. In accordance with them, in our study, we found that in cemented implants, PFFs occurred on average 6.5 years later than in cementless implants.
Many studies in the literature reported that osteoporosis is a significant risk factor for PFFs. In our study, we reported that the most risk factors were osteoporosis in 46 cases (63.0%), following by osteolysis in 19 (26.0%), loosening of the stem in 6 (8.2%) and weakening of the cortical bone due to stress shielding in 3 (4.1%).
Furthermore, we found 30 patients of 73 (41.1%) had a local or systemic post-operative complications. Although our overall complication rate is high, it is consistent with that reported in the literature (26–43%) [22]. We reported 21 cases of local complications (28.8%), including 7 wound infections, 7 urinary tract infections, 6 deep venous thrombosis and 1 case of hemorrhagic wound. The systemic complications were 20 (27.4%), including 7 respiratory failures, 4 kidney failures, 4 cardiogenic shocks, 2 ischemic stokes, 2 hypokinetic syndromes and 1 septic shock.
Then, 8 patients of 73 (10.9%) had post-operative complications requiring re-intervention: 3 aseptic loosening (4.1%), 2 recurrent dislocations (2.7%), 2 periprosthetic infections (2.7%) and 1 fracture (1.4%). Our incidence of re-intervention results lesser than other data reported in the literature from 23% [22] to 32% [18].
Conclusions
Many studies underline that PFFs are a very important problem because they will be destined to increase and there is not an only type of treatment, but the correct therapeutic choice depends on the level of fracture, quality of bone, prosthesis stability and general patient conditions. Thanks to the literature and our own results, we suggest that local risk factors contribute to a poorer final result in elderly patients in terms of functional outcomes with relatively low Harris hip scores and an elevated risk of mortality. In particular, in our study, we found patients with type A fractures and with cemented THA as first implant had the best radiological and functional outcomes.
Moreover, this kind of surgery is expensive, difficult and elaborated to realize; in some cases, despite a preceding planning, the surgeon is forced to change the typology of surgical procedure. Therefore, prevention is the only valid weapon in the hand of the orthopedic before a definitely demanding surgery; according to our case studies, the surgery performed by an equipe with experience in prosthetic implants and traumatology brings the best guarantee of treatment. So, careful pre-operative planning and appropriate intraoperative management in the hand of experienced surgeons may increase the chances of successful treatment [23]. Seeing that periprosthetic femoral fractures are an increasing complication and their treatment is a complex clinical challenge, it is believed that further studies are necessary to identify other related risk factors.
References
Marsland D, Mears SC (2012) A review of periprosthetic femoral fractures associated with total hip arthroplasty. Geriatr Orthop Surg Rehabil 3:107–120
Lewallen DG, Berry DJ (1998) Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect 47:243–249
Kurtz S, Ong K, Lau E, Mowat F, Halpern M (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89(4):780–785
Kärrholm J (2010) The swedish hip arthroplasty register. Acta Orthop 81(1):3–4
Duncan CP, Masri BA (1995) Fractures of the femur after hip replacement. Instr Course Lect 44:293–304
Brand S, Ettinger M, Omar M, Hawi N, Krettek C, Petri M (2015) Concepts and potential future developments for treatment of periprosthetic proximal femoral fractures. Open Orhop J 9:405–411
Parker MJ, Palmer CR (1993) A new mobility score for predicting mortality after hip fracture. J Bone Joint Surg Br 75:797–798
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Beals RK, Tower SS (1996) Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res 327:238–246
Bethea JS 3rd, DeAndrade JR, Fleming LL, Lindenbaum SD, Welch RB (1982) Proximal femoral fractures following total hip arthroplasty. Clin Orthop Relat Res 170:95–106
McElfresh EC, Coventry MB (1974) Femoral and pelvic fractures after total hip arthroplasty. J Bone Joint Surg Am 56:483–492
Griffiths EJ, Cash DJ, Kalra S, Hopgood PJ (2013) Time to surgery and 30-day morbidity and mortality of periprosthetic hip fractures. Injury 44:1949–1952
Lindahl H, Malchau H, Odén A, Garellick G (2006) Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br 88:26–30
Singh JA, Jensen MR, Harmsen SW, Lewallen DG (2013) Are gender, comorbidity, and obesity risk factors for postoperative periprosthetic fractures after primary total hip arthroplasty? J Arthroplasty 28(1):126–131
Brady OH, Garbuz DS, Masri BA, Duncan CP (2000) The reliability and validity of the Vancouver classification of femoral fractures after hip replacement. J Arthroplasty 15(1):59–62
Holley K (2007) Periprosthetic fractures of the femur after hip arthroplasty: an analysis of 99 patients. HSS J 3(2):190–197
Scholz R, Pretzsch M, Matzen P, von Salis-Soglio GF (2003) Treatment of periprosthetic femoral fractures associated with total hip arthroplasty. Z Orthop Ihre Grenzgeb 141(3):296–302
Lindahl H, Garellick G, Regnér H, Herberts P, Malchau H (2006) Three hundred and twenty-one periprosthetic femoral fractures. J Bone Joint Surg Am 88(6):1215–1222
Montalti M, Pilla F, Guerra G, Traina F (2013) Periprosthetic femoral fractures: treatments and outcomes. An analysis of 47 cases. HIP Int 23(4):380–385
Foster AP, Thompson NW, Wong J, Charlwood AP (2005) Periprosthetic femoral fractures: a comparison between cemented and uncemented hemiarthroplasties. Injury 36(3):424–429
McGraw IWW, Spence SC, Baird EJ, Eckhardt SM, Ayana GE (2013) Incidence of periprosthetic fractures after hip hemiarthroplasty: are uncemented prostheses unsafe? Injury 44(12):1945–1948
Zuurmond RG, van Wijhe W, van Raay JJAM, Bulstra SK (2010) High incidence of complications and poor clinical outcome in the operative treatment of periprosthetic femoral fractures: an analysis of 71 cases. Injury 41(6):629–633
Kim Y, Tanaka C, Tada H (2015) Treatment of periprosthetic femoral fractures after femoral revision using a long stem. BMC Musculoskelet Disord 16:113–119
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the local University Hospital Human Subject Research Ethics Committee, and data collection and analysis were performed in compliance with the Declaration of Helsinki.
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Caruso, G., Milani, L., Marko, T. et al. Surgical treatment of periprosthetic femoral fractures: a retrospective study with functional and radiological outcomes from 2010 to 2016. Eur J Orthop Surg Traumatol 28, 931–938 (2018). https://doi.org/10.1007/s00590-017-2082-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-017-2082-x