Abstract
Background
Long bone posttraumatic osteomyelitis (PTOM) is a relatively common complication following surgical fixation of open fractures. There is a lacking consensus on ideal strategies for diagnostic evaluation of long bone PTOM. While open bone biopsy and culture is considered the ‘gold diagnostic standard,’ its cost and invasiveness are often prohibitive and have prompted the search for alternate diagnostic methods.
Objective
To evaluate the sensitivity and specificity of various diagnostic modalities relative to open bone biopsy and culture for the detection of long bone PTOM.
Design
Retrospective cohort study; Level of Evidence, III.
Setting
Urban Level I trauma center and safety-net institution.
Patients/participants
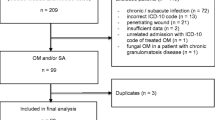
A consecutive cohort of 159 adult patients presenting with long bone PTOM at our Level I trauma center between January 1, 2004, and December 31, 2013, were retrospectively identified. All included patients fulfilled diagnostic criteria for PTOM (as defined by the Center for Disease Control and Prevention) that involved a long bone (femur, fibula, tibia, humerus, radius, and ulna). Patients with diabetic foot infection, septic arthritis, osteomyelitis of the spine/pelvis/hand, or insufficient medical records were excluded.
Main outcome measurements
Sensitivity and specificity of deep wound culture, soft tissue histopathologic examination, and elevated levels of acute phase reactants [C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and leukocyte count (WBC)] were determined using findings of open bone biopsy and culture as a reference standard.
Results
The most common pathogen isolated on open bone culture was staphylococci, contributing to 89 (57%) of 159 cases of long bone PTOM (p < 0.001). Relative to open bone biopsy and culture as the gold diagnostic standard, soft tissue histopathology demonstrated a sensitivity of 69.8% [95% confidence interval (CI) 53.7–82.3%] and specificity of 38.9% (95% CI 18.3–63.9%) for the detection of long bone PTOM. Deep wound culture exhibited a lower sensitivity of 66.0% (95% CI 56.1–74.8%) and specificity of 28.1% (95% CI 12.9–49.5%), a difference that was statistically significant (p = 0.021). Among inflammatory markers, elevated levels of CRP and ESR were equally sensitive for the detection of PTOM compared to open bone biopsy and culture, while WBC was significantly less sensitive (sensitivity 33.2%; 95% CI 25.3–43.7; p < 0.001).
Conclusion
Soft tissue histopathologic examination and deep wound culture are relatively poor substitutes for the diagnosis of long bone PTOM compared to open bone biopsy and culture. The accurate identification of causative pathogens underlying long bone PTOM is critical for diagnosis and choice of antibiotic treatment. Future studies investigating the use of higher-resolution diagnostic methods are merited.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long bone posttraumatic osteomyelitis (PTOM) is a relatively common complication following surgical fixation of long bone fractures, occurring in up to 10% of open fractures and 1% of closed fractures [1, 2]. Several factors may contribute to the pathogenesis of PTOM including direct inoculation of bacteria at the time of the fracture, macro- or microvascular damage, surgical contamination, or postoperative wound contamination [3,4,5]. Without early diagnosis and adequate treatment, long bone PTOM may result in fracture nonunion, sepsis, and need for limb amputation [4].
The gold standard for diagnosis of long bone PTOM is open bone biopsy and culture [6, 7]. Other diagnostic methods have been previously described, including percutaneous bone culture, deep wound culture, soft tissue histopathologic examination, and measurement of acute phase reactants such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and serum leukocyte count (WBC) [3, 5, 8, 9]; however, the validation of such methods, and comparison to open biopsy and culture, remains to be established in adequately powered, large-scale studies [5, 6, 8].
The purpose of the presented study was to evaluate the sensitivity and specificity of various diagnostic modalities relative to open bone biopsy and culture for the detection of long bone PTOM in patients presenting to a single Level I trauma center. Our initial hypothesis was that deep wound culture and soft tissue histopathologic examination would demonstrate statistically equivalent sensitivities for detection of long bone PTOM as compared to open bone biopsy and culture.
Patients and methods
Following institutional review board (IRB) approval, we conducted a retrospective analysis on a consecutive cohort of adult patients presenting with long bone PTOM to a single Level I trauma center between January 1, 2003, and December 31, 2013. The diagnosis of osteomyelitis was made in accordance with the most up-to-date criteria of the Center for Disease Control and Prevention (CDC), given by the presence of: (1) pathogenic growth on direct bone cultures, (2) evidence of osteomyelitis on direct examination of the bone during invasive procedures or histopathologic examination, or (3) at least two signs of infection (temperature >38 °C, localized edema, erythema, tenderness, or draining sinus tract) in addition to positive blood cultures, laboratory tests, or imaging findings suggestive of infection. Additional inclusion criteria were age of at least 18 years, infection localized to a long bone (humerus, radius, ulna, femur, tibia, or fibula), and diagnosis confirmed by open bone biopsy and culture. Patients were excluded given the presence of diabetic foot infection, septic arthritis, or osteomyelitis of the hand/spine/pelvis.
In addition to outcome variables, demographic data including age, gender, body mass index (BMI), medical comorbidities, history of alcohol and substance abuse, type of fracture, and location of fracture were retrieved for all patients. Manual chart review of electronic medical records was performed by four data abstractors.
Specimen collection
Levels of acute phase reactants including WBC, ESR, and CRP were measured approximately 1–2 days prior to direct bone sampling. Intraoperatively, the superficial wound site was carefully cleaned with 0.05% chlorhexidine aqueous solution to avoid contamination from overlying skin flora. Specimens for deep wound culture were prepared using a moistened swab to probe the deepest aspect of the wound, taking care to avoid contact with overlying soft tissue. For histopathologic examination, devitalized soft tissue immediately surrounding the site of infection was carefully resected to be sent for frozen section and Gram stain. Direct bone samples were obtained under using an 11-gauge biopsy needle. All specimens were transported to the clinical microbiology laboratory in sterile containers. Specimens were processed per routine laboratory protocol and set up for both aerobic and anaerobic bacterial cultures. Aerobic media plates were incubated at 35 °C and checked daily for growth for 5 days. Anaerobic media plates were incubated at 35 °C in an anaerobic environment and checked daily for growth beginning on the second day for a total of 5 days. Isolates were identified using standardized methods of biochemical testing.
Statistical analysis
All variables were evaluated for distribution of normality using a combination of histograms, Q–Q plots, and the Shapiro–Wilk tests. Descriptive statistics were summarized as means and standard deviations for quantitative variables and as counts and frequencies for categorical variables. Levels of acute phase reactants (ESR, CRP, and WBC) were dichotomized as ‘normal’ or ‘elevated’ for the purpose of comparative analysis. The sensitivity of each modality relative to open bone biopsy and culture as the ‘gold diagnostic standard’ was determined using McNemar’s test. Statistical significance for all comparisons was set at p < 0.05 (two-tailed). All analyses were conducted using IBM SPSS Statistics (version 23.0, IBM, Inc.).
Results
Participants and descriptive data
The study cohort was comprised of 159 patients (102 males, 57 females) with a mean age of 46.0 years [standard deviation (SD) 13.2 years], mean height of 172.3 cm (SD 10.6 cm), mean weight of 84.1 kg (SD 22.8 kg), and mean BMI of 28.3 kg/m2 (SD 7.1 kg/m2). Twenty (14.4%) patients had a prior diagnosis of chronic hepatitis (B or C), 15 (9.4%) of diabetes mellitus, and 2 (1.3%) of human immunodeficiency virus (HIV). Ninety-seven (61.0%) patients expressed a chronic history of smoking, 86 (54.1%) of drinking alcohol, and 47 (29.6%) of illicit substance abuse. Fifteen patients (9.4%) were homeless at the time of receiving medical care. Open fractures constituted the initial injury in 58 (36.5%) patients. The most common site of infection was the tibia, implicated in 52.2% of all cases of long bone PTOM (p < 0.001). Additional demographic and clinical characteristics of the study cohort are summarized in Tables 1 and 2.
Frequency of causative pathogens identified on open bone culture
The most common pathogen isolated from direct bone samples in patients with long bone PTOM was staphylococci, occurring in 57% of all cases (p < 0.001). Polymicrobial (mixed flora) and streptococcal species demonstrated statistically equivalent prevalence rates and together comprised 37% of all cases of long bone PTOM. All other pathogens isolated on open bone culture had significantly lower prevalence rates (p < 0.001; Table 3).
Sensitivity of diagnostic modalities for evaluation of long bone PTOM
Relative to open bone biopsy and culture as the gold diagnostic standard, soft tissue histopathology demonstrated a sensitivity of 69.8% (95% confidence interval [CI], 53.7–82.3%) and specificity of 38.9% (95% CI 18.3–63.9%) for the detection of long bone PTOM. By comparison, deep wound culture demonstrated a lower sensitivity of 66.0% (95% CI 56.1–74.8%) and specificity of 28.1% (95% CI 12.9–49.5%), a difference that was statistically significant (p = 0.021). Among inflammatory markers, elevated levels of CRP and ESR were equally sensitive for the detection of PTOM compared to open bone biopsy and culture, while WBC was significantly less sensitive (sensitivity 33.2%; 95% CI 25.3–43.7; p < 0.001); however, specificity of the former two modalities was limited to approximately 20% (Table 4). Sensitivities and specificities of each diagnostic modality relative to open bone biopsy and culture are presented in Table 4.
Discussion
The results of our study demonstrate that soft tissue histopathology and deep wound culture serve as relatively poor diagnostic substitutes compared to open bone biopsy and culture for the detection of long bone PTOM. While soft tissue histopathologic examination was slightly more sensitive than deep wound culture in detecting long bone PTOM, both methods suffered a relatively high rate of false positives, rendering poor specificity. Similarly, measurement of an elevated CRP level was more sensitive for detection of osteomyelitis infection as compared to other acute phase reactants; however, the specificity of this measure was limited to 18.1%.
There are several potential reasons that may explain the poor diagnostic capacity of these modalities. Swab samples are generally considered more susceptible to contamination than tissue or fluid specimens and may additionally inhibit the growth of certain pathogens, leading to false negatives [10]. Soft tissue histopathologic examination involves exposure of causative pathogens to fixative agents that may alter their classical staining characteristics; for example, staphylococci may not form clusters and streptococci may not form chains [10]. The presence of soft tissue ulcers, cellulitis, or peripheral vascular disease may further yield false positives on soft tissue histopathologic examination. With regard to levels of acute phase reactants, several studies have advocated for the establishment of new diagnostic threshold values in patients with osteomyelitis. Maharajan et al. [11] showed that a cutoff threshold of 0.4 ng/ml for serum procalcitonin was 85.2% sensitive and 87.3% specific for detection of acute osteomyelitis and septic arthritis infections, compared to a sensitivity of 66.7% and specificity of 91% using the conventional cutoff of 0.5 ng/ml. Michali et al. [12] reported sensitivities and specificities for detection of diabetic foot osteomyelitis to be 85 and 83% for CRP levels >14 mg/L, 84 and 75% for ESR > 67 mm/h, and 75 and 79% for WBC > 14 × 109/L. Although our study did not examine patients with foot osteomyelitis, we observed drastically reduced sensitivities and specificities of acute phase reactants for detection of PTOM using traditional diagnostic threshold values. In particular, an elevated WBC (>10 × 109/L) was present in only 33.9% of all cases of long bone PTOM diagnosed by open bone biopsy and culture. Consistent with our findings, Unkila-Kallio et al. [13] observed that only 35% of children with acute hematogenous osteomyelitis had leukocytosis at the time of admission. A systematic review of 36 papers by Harris et al. [14] also concluded that WBC is poorly correlated with the presence of osteomyelitis infection.
Another potential reason for the discrepancies between open bone biopsy and other diagnostic modalities may relate to the differing growth requirements of various pathogens implicated in osteomyelitis infection. Indeed, Khatri et al. identified 31 of 53 (58.4%) pathogenic isolates on open biopsy that failed to grow on wound cultures in patients with PTOM. Eight of these isolates were diphtheroids [15]. Similarly, Lewis et al. [16] observed that 90% of anaerobic infections were uniquely confined to bone. In our study, at least 30% of all patients with long bone PTOM had positive findings on open bone biopsy and culture but negative findings on soft tissue histopathologic examination or deep wound culture. The accurate identification of causative pathogens underlying long bone PTOM is critical for establishing a timely diagnosis and choice of antibiotic treatment. To this end, future studies investigating the use of higher-resolution diagnostic methods such as polymerase chain reaction (PCR) or fluorescence in situ hybridization (FISH) may be promising [8].
Strengths of the present study include its relatively large sample size and surveillance of multiple diagnostic modalities. However, we acknowledge some important limitations. Firstly, the retrospective design of our study inherently limits scientific objectivity of our reported findings. Additionally, our Level I trauma center functions as a ‘safety-net’ institution to a largely medically indigent population. Therefore, patients in the presented study may bear an increased incidence of medical comorbidities and risk factors for PTOM compared to patients encountered at other hospital centers. Finally, our study only included a few patients with infection at multiple foci. In such patients, accurate diagnosis may require the use of multiple modalities in tandem. Nonetheless, we believe that the results of our study are clinically meaningful and prompt further investigation of alternate diagnostic methods for the reliable detection of long bone PTOM.
Conclusion
Delayed diagnosis of long bone PTOM can be limb threatening. Soft tissue histopathology and deep wound culture demonstrate poor sensitivity for the detection of long bone PTOM compared to open bone biopsy and culture as a reference standard. Elevated levels of CRP are sensitive for detection of long bone PTOM but not specific. Further studies investigating the use of higher-resolution diagnostic methods are warranted.
References
Patzakis MJ, Zalavras CG (2005) Chronic posttraumatic osteomyelitis and infected nonunion of the tibia: current management concepts. J Am Acad Orthop Surg 13:417–427
Zalavras CG, Christensen T, Rigopoulos N et al (2009) Infection following operative treatment of ankle fractures. Clin Orthop Relat Res 467:1715–1720
Calhoun JH, Manring MM, Shirtliff M (2009) Osteomyelitis of the long bones. Semin Plast Surg 23:59–72
Ikpeme IA, Ngim NE, Ikpeme AA (2010) Diagnosis and treatment of pyogenic bone infections. Afr Health Sci 10:82–88
Sanders J, Mauffrey C (2013) Long bone osteomyelitis in adults: fundamental concepts and current techniques. Orthopedics 36:368–375
Sanders JO, Bozic KJ, Glassman SD et al (2014) Clinical practice guidelines: their use, misuse, and future directions. J Am Acad Orthop Surg 22:135–144
Palmer MP, Altman DT, Altman GT et al (2014) Can we trust open culture results in nonunions? J Orthop Trauma 28:384–390
Hake ME, Oh JK, Kim JW et al (2015) Difficulties and challenges to diagnose and treat post-traumatic long bone osteomyelitis. Eur J Orthop Surg Traumatol 25:1–3
Fritz JM, McDonald JR (2008) Osteomyelitis: approach to diagnosis and treatment. Phys Sportsmed 36:nihpa116823
Wilson ML, Winn W (2008) Laboratory diagnosis of bone, joint, soft-tissue, and skin infections. Clin Infect Dis 46:453–457
Maharajan K, Patro DK, Menon J et al (2013) Serum Procalcitonin is a sensitive and specific marker in the diagnosis of septic arthritis and acute osteomyelitis. J Orthop Surg Res 8:19
Michail M, Jude E, Liaskos C et al (2013) The performance of serum inflammatory markers for the diagnosis and follow-up of patients with osteomyelitis. Int J Lower extrem Wounds 12:94–99
Unkila-Kallio L, Kallio MJ, Peltola H (1994) The usefulness of C-reactive protein levels in the identification of concurrent septic arthritis in children who have acute hematogenous osteomyelitis. A comparison with the usefulness of the erythrocyte sedimentation rate and the white blood-cell count. J Bone Joint Surg Am 76:848–853
Harris JC, Caesar DH, Davison C et al (2011) How useful are laboratory investigations in the emergency department evaluation of possible osteomyelitis? Emerg Med Australas 23:317–330
Khatri G, Wagner DK, Sohnle PG (2001) Effect of bone biopsy in guiding antimicrobial therapy for osteomyelitis complicating open wounds. Am J Med Sci 321:367–371
Lewis RP, Sutter VL, Finegold SM (1978) Bone infections involving anaerobic bacteria. Medicine 57:279–305
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest in relation to the presented body of research.
Rights and permissions
About this article
Cite this article
Chadayammuri, V., Herbert, B., Hao, J. et al. Diagnostic accuracy of various modalities relative to open bone biopsy for detection of long bone posttraumatic osteomyelitis. Eur J Orthop Surg Traumatol 27, 871–875 (2017). https://doi.org/10.1007/s00590-017-1976-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-017-1976-y