Abstract
Purpose
Aim of this study is to compare late degenerative MRI changes in a subset of patients operated on with ACDF to a second subset of patients presenting indication to ACDF but never operated on.
Methods
Patients from both subgroups received surgical indication according to the same criteria. Both subgroups underwent a cervical spine MRI in 2004–2005 and 10 years later in 2015. These MRI scans were retrospectively evaluated with a cervical spine ageing scale.
Results
Comparing the two subset of patients both suffering from clinically relevant single-level disease returns no statistically significant difference in the degenerative condition of posterior ligaments, presence of degenerative spondylolisthesis, foraminal stenosis, diameter of the spinal canal, Modic alteration, and intervertebral discs degeneration at 10-year follow-up.
Conclusions
The adjacent segment degeneration represents, in the present cohort, a result of the natural history of cervical spondylosis rather than a consequence of fusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Background and rationale
Cervical spondylosis appears as an elusive entity since it is strictly related to physiological phenomena: the development of degenerative changes in the ageing spine [1, 2]. Many papers in literature compare radiological and clinical results of different treatments; however, many of these papers lack a rigorous methodology and thus conclusions suffer from major bias. In a previous work, we proposed a new evaluation score of the degenerative changes in the cervical spine (CS) linked with ageing [3], capable of standardizing populations for research in CS surgery. On the ground of this scale, we retrospectively investigated a rigorously standardized population of patients suffering from cervical spine degenerative disease (C-SDD), comparing a subset of patients who underwent fusion surgery (ACDF) versus a non-surgical subset of patients in search of remarks about one of the major concerns in CS surgery: the adjacent segment degeneration (ASD).
Objectives
Aim of this paper is to investigate the long-term effects of fusion on the elements composing the functional spinal unit (FSU) and its adjacent levels (one and two FSUs above and below), in order to describe what really occurs to the CS after fusion surgery over a span of 10 years.
Materials and methods
Study design and setting
The final cohort is composed of 71 patients suffering from single-level cervical degenerative disc disease (C-DDD), surgically treated or listed for surgical treatment between January 2004 and December 2005, at the Neurosurgical Divisions of Rome Army Hospital “Celio” and “Sant’Andrea” University Hospitals of “Sapienza”.
Participants and eligibility
All the patients included in the final cohort were diagnosed with a single-level C-DDD with a 1.5-T MRI scan with T1w and T2w axial and sagittal without gadolinium.
In relation to clinical history and neurological findings, all these C-DDD were referred to surgical treatment. Surgical indication criteria used at that time were:
-
1.
Signs of cervical myelopathy and/or radiculopathy,
-
2.
Symptoms of pain in the neck and radiating to the upper limbs, refractory to conservative treatment and severely disabling lasting more than 2 months.
All the patients included suffered from a single-level pathology. We selected the patients in order to minimize the influence of sagittal balance, age, sex, and level involved as follows:
-
1.
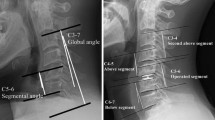
Sagittal cervical balance was evaluated with the aid of Risser–Ferguson method. In the final cohort were included only patients with a value of such angle between 10° and 15°. We preferred to consider sagittal alignment evaluated according to Risser–Ferguson method to the evaluation of the intervertebral angles because of its demonstrated effect on degeneration of the whole cervical spine (as widely expressed in discussion paragraph). Furthermore, intervertebral angles, considered as a “segmental alignment”, appear to be the expression of the degenerative conditions of a single intervertebral disc, rather than an anatomical “native” condition [3].
-
2.
All the patients included in the final cohort were between 45 and 60 years old. As previously demonstrated [3], chronological age is not necessary related to biological ageing of the CS.
-
3.
All the patients included in the final cohort suffered from a C5–C6 FSU disease.
-
4.
In order to minimize the influence of the individual differences in C5–C6 FSU degeneration, according to the previously mentioned score we included only patients with intervertebral discs scored at least 4 and with a cumulative C5–C6 FSU score between 9 and 15 (Table 1).
Table 1 Spine ageing evaluation scale in detail (CS score)
The final cohort is composed of two subgroups. In the first subgroup, patients underwent ACDF. Interbody fusion was realized with PEEK cages. No plating was performed. In first or second post-operative day, all the patients underwent a standard CS X-Ray film to verify the proper cage positioning. We still perform the first MRI scan at 30 days from surgery, exception done for cases in which a surgical or neurological complication forces us to anticipate the scan.
The second subgroup was composed of patients suffering from single-level C5–C6 C-SDD with surgical indication to ACDF according to the same aforementioned criteria. These patients due to several different reasons (Fig. 1b) never underwent ACDF.
Patients from both subgroups met the following exclusion criteria:
-
1.
Other surgical procedure in cervical region.
-
2.
Inflammatory, neoplastic, traumatic of the CS, and spinal cord.
-
3.
Unavailable or low-quality preoperative MRI, incomplete surgical or clinical records.
-
4.
Patients operated on (in ACDF subgroup) presenting surgical complication such as subsidence, misplacement of the cage, pseudoarthrosis, and neurological post-operative worsening.
-
5.
Did not accept re-evaluation.
Further details are shown in Fig. 1. The final cohort was never meant to be representative of the initial cohort, because it derives from an intentional process of ultra-selection of patients to enrol in the study. This strong selection was guided by the rationale that all the confounding variables, such as age, level of surgery, sagittal alignment, and surgical complications, had to be strictly controlled because of their potential confounding impact on the final radiological outcome of an ACDF surgery.
Variables and data sources
Patients from both subgroups were contacted by phone to undergo a standard CS 1.5-T MRI scan. To minimize the influence of follow-up length, all the patients included in the study received surgical indication and/or underwent ACDF procedure in the period between January 2004 and December 2005 and were re-evaluated between January and December 2015. MRI scans were subsequently evaluated with the CS score [3].
This work is similar to our previous paper [3], and it is of radiological evaluation of the degenerative changes in the CS after ACDF. The CS score interrater bias when analysing the MRI images has been assessed in the previous study [3]: it was as high as .891 and extremely significant from a statistical point of view. This is a radiological study, and therefore, clinical data are intentionally missing, exception done for what previously mentioned. In order to avoid any kind of bias, the authors, never consulted the clinical records of 10 years ago, and consequently, were completely blind towards the neurological outcomes of the patients. When we gathered the first database we believed that is an effect of fusion surgery, yet we found no difference between operated and non-operated patients. Therefore, the final database is unspoiled from any bias in data collection or prejudice.
Statistical methods, missing data, and potential sources of bias
The study size is given by selection of the inclusion criteria. As previously stated, we addressed no missing data since incomplete records were exclusion criteria. On the post hoc estimated power tests, the size of the sample was found to be excellent (1−β) = .912 (for α .05, effect size .8).
As previously mentioned, we included an analysis of the interobserver correlation as high as .891 in the previous study [3]. In order to assess the reliability of the CS score, we analysed the scores collected from all the different researchers and submitted to a Cronbach’s alpha reliability analysis that returned an interobserver correlation as high as .794, confirming once again, as in our previous study [3], that CS score is stable and no coarse interobserver bias is expected.
Statistical methods
The entire cohort was analysed with SPSS version 18. ANOVA analysis was used to compare means of the FSUs scores between the subgroups. Paired samples T-tests were used to compare means of scores between first and follow-up MRI scans, bivariate correlation was used for continuous variables (according to Pearson method), and univariate ANOVA and repeated measures ANOVA were used to compare means between the two subgroups and between first MRI scan and follow-up.
Ethical and legal issues
All the patients of the surgical subgroup expressed consent to the surgical procedure after appropriate information. All the patients gave informed explicit consent to undergo the follow-up MRI; before performing the MRI they were elucidated about the purpose of the study. The local ethic committees of our institutions had a favourable pronunciation about ethical issues of the study because of its retrospective nature, because no treatment-randomization was performed, and because MRI is of no harm towards the individuals included in the final cohort. Moreover, data reported have been completely anonymized. This study is perfectly consistent, in any of its aspect, with WMA Helsinki Declaration of Human Rights.
The platform of study protocol approval relied on the following considerations:
-
1.
MRI scans do not deviate from current normally accepted clinical practice about radiological investigation for cervical spondylosis.
-
2.
CS MRI scans were performed after written explicit informed consent of all the patients included in the final cohort.
-
3.
CS MRI scans were performed for free, patients did not undergo any additional fee, they were completely informed about their radiological and neurological conditions after any follow-up MRI scan, and they were not exposed to any kind of biological or psychological risks of harm.
-
4.
All the patients received a clear benefit from a free consultation from experienced spine surgeons after the MRI scan.
-
5.
The benefits for society and for future patients rely on the purpose of the study, about gaining important conclusions about the effectiveness of ACDF in treating degenerative conditions of the cervical spine.
Results
Participants
In the period between January 2004 and December 2005, a total of 70 ACDF surgeries were performed at Rome Army Hospital “Celio” and “Sant’Andrea” University Hospital. Among these patients, 33 were included in the final cohort. In the same period, in the outpatient services of the same hospitals were evaluated a total of 118 patients suffering from single-level C-SDD with indication to perform ACDF. Among these patients, 38 were included in the final cohort. All the details are shown in the flow chart (Fig. 1a, b).
None of the 33 patients included in the final cohort have been reoperated after first fusion at 10-year follow-up, and none of the 38 patients of the non-surgical subgroup have been operated during the 10-year follow-up between our first evaluation and indication and the end of the 10 years.
Descriptive data
The final cohort was thus composed a total of 71 patients divided into two subgroups of 33 and 38 patients, respectively (ACDF vs. no surgery), which were contacted by phone call, from 10 to 10.5 years later, during 2015, and accepted to be re-evaluated with a CS MRI scan. Forty-one patients were females and 30 males (average age 49.1 ± 3.64 years). 2004–2005’s and 2015’s CS MRI were evaluated according to the same score [3] (Table 2).
Statistical inference: outcome data and main results
ACDF patients compared to non-surgical subgroup showed no differences at follow-up for what intervertebral disc degeneration is concerned. The overall sum of C2–C7 disc scores, C4–C5, C6–C7, C3–C4, and C7–T1 intervertebral discs showed no statistical difference between the subgroups (ANOVA, respectively, p = .210, .836, .355, .058, .329; Fig. 2a, b).
ANOVA analysis showing no statistically significant difference between surgical versus non-surgical subgroup in degenerative changes at 10-year follow-up for what concerns the intervertebral discs of the level a C6–C7 and b C4–C5. Note that the scores reported on the left (“Y” axis) refer to the score of the C4–C5 and C5–C6 intervertebral discs only (see CS score in Table 1 for details about the evaluation of the intervertebral discs)
Ten years later, the surgical subgroup showed statistically significant lower scores in respect of non-surgical for what concerns: posterior ligament complex score at C5–C6 (ANOVA p = .004), foraminal stenosis at C5–C6 (ANOVA p < .001), and vertebral canal diameter at C5–C6 (ANOVA p < .001). These differences between the two subgroups were not statistically significant evaluating the scores of the first MRI scan, in 2005. These data outline the effectiveness of ACDF surgery in slowing the degenerative cascade of the CS.
Sex did not affect significantly CS degeneration.
Paired samples T test outlined statistically significant differences between the average scores of the following variables in 2004–2005 versus follow-up: intervertebral disc degeneration at C6–C7 (Mean—1.0 p < .001), at C4–C5 (Mean—1.15 p < .001), overall score of the same FSUs (respectively, Mean—3.15 p < .001 and—2.55 p < .001). C4–C5 and C6–C7 had averagely higher scores in respect of C3–C4 and C7–T1 at follow-up (respectively, Mean—2.20 p = .006 and—2.67 p = .009), thus indicating that not only the adjacent discs but the entire FSUs (upper and lower) tend to acquire an higher quantity of degenerative changes in respect of others.
C4–C5 and C6–C7 intervertebral discs degeneration scores were found to be different only in ACDF group (Paired samples T test p = .004), in our cohort the adjacent space degeneration (present in both subgroups) was most severe at the level above the non-functional FSU in respect of the one below, while in non-surgical subgroup this statistical significance was not present (p = .106).
In this cohort, no treatment-related differences have been outlined, at 10-year follow-up between C4–C5 and C6–C7 as far as concerns spinal canal diameter (p = .652), state of posterior ligaments (p = .074), Modic changes in the soma (p = .658), the presence of foraminal stenosis (p = .873).
Ligaments, foramina, Modic changes in the soma, spinal canal diameter, and as previously stated intervertebral discs showed an obvious statistically significant different degeneration between the first MRI and follow-up re-evaluation consistently with our previous work.
As predictable on the ground of our previous study, age is correlated both before and after 10 years with all the investigated features. Moreover, C3–C4 to C7–T1 intervertebral discs scores in 2004–2005 were correlated with their conditions in 2015 (bivariate correlation r = .473; p = .002) and same statistical interactions were revisable for what the others features are concerned.
Other analyses
Univariate ANOVA analysis was performed, highlighting no statistically significant difference among the two subgroups for what concerns C5–C6 versus C4–C5 and C6–C7 overall FSU score at follow-up (p = .484 and .732, respectively). Similar results derived from C4–C5 versus C6–C7, C4–C5 versus C3–C4, C6–C7 versus C7–T1, C3–C4 versus C7–T1 overall FSU average scores comparison (p = .379, .889, .094, .745, respectively). To sum up, in the present cohort no evidence was found of increased degeneration rate of ACDF patients if they are compared to non-operated patients.
Repeated measures ANOVA was performed to outline statistically relevant differences among the two subgroups at 10 years for C4–C5, C6–C7, overall FSU scores outlining no significant differences between the subgroups (p = .833, .927, .093). Nevertheless, the differences between the aforementioned FSU anatomical components were significant regardless if surgical treatment was performed (p = .002, < .001, < .001; Fig. 3a, b).
ANOVA repeated measures analysis showed statistically significant difference between the overall FSU scores of a C6–C7 and b C4–C5, respectively, in 2004–2005 and 2015. No significant treatment-related difference between the two subgroups was outlined; the difference between 2004–2005 and 2015 evaluation was significant. Note that the scores reported on the left (“Y” axis) refer to the score of the C4–C5 and C5–C6, not only the intervertebral discs but the algebraic sum of the scores assigned to 1. the degeneration of intervertebral discs, 2. the degeneration of yellow ligaments, 3. the degeneration of vertebral bodies, 4. the possible presence of spondylolisthesis, 5. the presence or absence of foraminal stenosis, and 6. the diameter of the spinal canal. (a single FSU score = 1 + 2 + 3 + 4 + 5 + 6 for that level; see CS score in Table 1 for details.) Estimated marginal means refer to the aforementioned variables used for this analysis
Discussion
Cervical spondylosis is a common spine disorder, regarded as the most common cause of myelopathy and radiculopathy in the adult [1, 4]; its impact on quality of life can be extremely variable [5], since many patients remain completely asymptomatic despite major radiological findings.
Over the past 60 years, ACDF has gained wide acceptance among spine surgeons as the gold standard for the treatment of CS degenerative disorders with a rate of relief of myeloradiculopathic signs as high as 90% [6, 7].
As early enthusiasm concerning the favourable effects of ACDF increased, so did the awareness of the main complication of fusion surgery: the adjacent space degeneration (ASD). Hilibrand et al. [8] classified degeneration of adjacent segments as “degeneration” and “disease”, outlining the difference between a radiological and a clinical aspects of this syndrome since radiological changes in CS do not necessarily correlate with a neurological impairment [1, 4, 9].
It is estimated that radiological ASD prevalence from 5 to 20 years after fusion surgery can be as high as 37.4% while ASD syndrome’s prevalence amounts to 2.5–4.0% [10]; the annual incidence is estimated 3–4% and to 25.6% of cumulative incidence at 10 years [11, 12]. Most variable factors have been advocated as causative of ASD, among which: raised intradiscal pressure caused by adjacent fusion [13], compensatory increase in range of motion of levels adjacent to a previously fused one [14], C5–C6 and C6–C7 levels [11], surgical effects on the anatomy of the CS [15], sagittal alignment [15, 16], or even acidosis [10].
Key results and interpretation
The rationale behind the study design of the present paper was to make “constant”, the vast majority of the previously identified risk factors for ASD development. We gathered a highly selected cohort of patients standardized according to a previously published evaluation scale; age range was reduced; for both surgical and non-surgical groups, follow-up duration was fixed at 10 years.
Our idea was that ASD is an effect of fusion surgery, yet we found no difference between operated and non-operated patients. Instead, a significant difference in global degenerative conditions of the CS was notable between 2004–2005 and 2015 MRI scans for both groups, while a higher degree of degenerative conditions in 2004–2005 predicted the worse degeneration in 2015 with no impact of surgery.
Matsumoto et al. [17] compared a cohort of patients who underwent ACDF to healthy controls. ACDF patients showed a higher rate of disc degeneration, but this conclusion suffers from two major bias. The first is that ACDF patients were averagely 6.2 years older than controls. The second is that control group was made of healthy individuals and therefore not comparable to patients suffering from C-SDD and operated on.
To date, it is not clear whether degenerative changes at the adjacent levels are effect of fusion, or the natural history of cervical spondylosis and no incontrovertible evidence supporting “fusion theory” versus “natural progression theory” is currently available [1, 11, 18].
The data we obtained brought us to support the idea that adjacent segments degenerate in response to other rules than just increased biomechanical stress after fusion.
It is common to find that an entire FSU located within an abnormal sagittal alignment (mostly C5–C6 and C6–C7) suffers from a load that causes accelerated degeneration [11, 19, 20]. In such conditions, intervertebral disc, over a reduced span of years progressively fails in expressing its “articular” function, becomes naturally fused and thus reaches the endpoint of the CS degeneration cascade [21].
In our samples, overall FSU score of C4–C5 and C6–C7 did not differ between subgroups, while in both subgroups the same FSUs had a statistically significant higher degree of degenerative changes in respect of C3–C4 and C7–T1.
According to these concepts, it is no longer a surprise that arthroplasty failed in many trials in demonstrating its superiority in respect of ACDF for both clinical and radiological ASD incidence [1, 10, 22, 23]. Consistently, Lunsford reported an annual incidence of 2.5% of new ASD after anterior cervical discectomy without differences between ACDF and simple discectomy patients [24]. Moreover, Goffin [19] reported a lower incidence of ASD in the subgroup of young patients undergoing ACDF due to CS trauma in respect of patients suffering from C-SDD.
Limitations and generalizability
The main limitation of this study lies in the exiguity of the sample. Though a great effort has been made towards a rigorous methodology in patients eligibility, conclusions may suffer from underrepresentation bias. However, the impact of many factors has been completely excluded. Moreover, with the aid of the evaluation score, CS degenerative conditions have been rigorously homogenized.
Conclusions
Comparing a subset of ACDF to a subset of non-operated but surgically indicated patients suffering from single-level C-DDD returns no statistically significant difference in the degenerative conditions of the CS degeneration at 10-year follow-up. According to our data, ASD is a part of the natural history of cervical spondylosis rather than a complication of ACDF.
Abbreviations
- ACDF:
-
Anterior cervical discectomy and fusion
- CS:
-
Cervical spine
- C-SDD:
-
Cervical spine degenerative disease
- C-DDD:
-
Cervical degenerative disc disease
- ASD:
-
Adjacent segment degeneration
- FSU:
-
Functional spinal unit
- MRI:
-
Magnetic resonance imaging
References
Yang B, Li H, Zhang T, He X, Xu S (2012) The incidence of adjacent segment degeneration after cervical disc arthroplasty (CDA): a meta analysis of randomized controlled trials. PLoS ONE 7(4):e35032. doi:10.1371/journal.pone.0035032
Okada E, Matsumoto M, Ichihara D, Chiba K, Toyama Y, Fujiwara H, Momoshima S, Nishiwaki Y, Hashimoto T, Ogawa J, Watanabe M, Takahata T (2009) Aging of the cervical spine in healthy volunteers: a 10-year longitudinal magnetic resonance imaging study. Spine 34(7):706–712 (Phila Pa 1976)
Wierzbicki V, Pesce A, Marrocco L, Piccione E, Colonnese C, Caruso R (2015) How old is your cervical spine? Cervical spine biological age: a new evaluation scale. Eur Spine J 24(12):2763–2770. doi:10.1007/s00586-014-3673-4 (Epub 2014 Nov 23)
Song KJ, Choi BW, Jeon TS, Lee KB, Chang H (2011) Adjacent segment degenerative disease: is it due to disease progression or a fusion-associated phenomenon? Comparison between segments adjacent to the fused and non-fused segments. Eur Spine J 20(11):1940–1945. doi:10.1007/s00586-011-1864-9 (Epub 2011 Jun 8)
Noriega DC, Kreuger A, Brotat M, Ardura F, Hernandez R, Muñoz MF, Barrios C (2013) Long-term outcome of the Cloward procedure for single-level cervical degenerative spondylosis. Clinical and radiological assessment after a 22-year mean follow-up. Acta Neurochir (Wien) 155(12):2339–2344. doi:10.1007/s00701-013-1902-y (Epub 2013 Oct 10)
Robinson RA, Smith GW (1955) Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp 96:223–224
Bohlman HH, Emery SE, Goodfellow DB, Jones PK (1993) Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 75:1298–1307
Hillibrand AS, Robbins M (2004) Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine 4:190S–194S (Phila Pa 1976)
Chung JY, Kim SK, Jung ST, Lee KB (2014) Clinical adjacent-segment pathology after anterior cervical discectomy and fusion: results after a minimum of 10-year follow-up. Spine J 14(10):2290–2298. doi:10.1016/j.spinee.2014.01.027
Xia XP, Chen HL, Cheng HB (2013) Prevalence of adjacent segment degeneration after spine surgery: a systematic review and meta-analysis. Spine 38(7):597–608. doi:10.1097/BRS.0b013e318273a2ea (Phila Pa 1976)
Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH (1999) Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am 81:519–528
Cho SK, Riew KD (2013) Adjacent segment disease following cervical spine surgery. J Am Acad Orthop Surg 21(1):3–11
Dmitriev AE, Cunningham BW, Hu N, Sell G, Vigna F, McAfee PC (2005) Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine 30(10):1165–1172 (Phila Pa 1976)
Elsawaf A, Mastronardi L, Roperto R, Bozzao A, Caroli M, Ferrante L (2009) Effect of cervical dynamics on adjacent segment degeneration after anterior cervical fusion with cages. Neurosurg Rev 32(2):215–224. doi:10.1007/s10143-008-0164-2 (Epub 2008 Oct 10, discussion 224)
Nassr A, Lee JY, Bashir RS, Rihn JA, Eck JC, Kang JD, Lim MR (2009) Does incorrect level needle localization during anterior cervical discectomy and fusion lead to accelerated disc degeneration? Spine 34(2):189–192 (Phila Pa 1976)
Lundine KM, Davis G, Rogers M, Staples M, Quan G (2014) Prevalence of adjacent segment disc degeneration in patients undergoing anterior cervical discectomy and fusion based on pre-operative MRI findings. J Clin Neurosci 21(1):82–85. doi:10.1016/j.jocn.2013.02.039 (Epub 2013 Sep 11)
Matsumoto M, Okada E, Ichihara D, Watanabe K, Chiba K, Toyama Y, Fujiwara H, Momoshima S, Nishiwaki Y, Iwanami A, Ikegami T, Takahata T, Hashimoto T (2010) Anterior cervical decompression and fusion accelerates adjacent segment degeneration: comparison with asymptomatic volunteers in a ten-year magnetic resonance imaging follow-up study. Spine 35(1):36–43 (Phila Pa 1976)
Baba H, Furusawa N, Imura S, Kawahara N, Tsuchiya H, Tomita K (1993) Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine 18:2167–2173 (Phila Pa 1976)
Goffin J, Geusens E, Vantomme N, Quintens E, Waerzeggers Y, Depreitere B, van Calenbergh F, van Loon J (2004) Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech 17(2):79–85
Levin DA, Hale JJ, Bendo JA (2007) Adjacent segment degeneration following spinal fusion for degenerative disc disease. Bull NYU Hosp Jt Dis 65(1):29–36
Prescher A (1998) Anatomy and pathology of the aging spine. Eur J Radiol 27(3):181–195
Nunley PD, Jawahar A, Kerr EJ 3rd, Gordon CJ, Cavanaugh DA, Birdsong EM, Stocks M, Danielson G (2012) Factors affecting the incidence of symptomatic adjacent-level disease in cervical spine after total disc arthroplasty: 2- to 4-year follow-up of 3 prospective randomized trials. Spine 37(6):445–451. doi:10.1097/BRS.0b013e31822174b3 (Phila Pa 1976)
Coric D, Kim PK, Clemente JD, Boltes MO, Nussbaum M, James S (2013) Prospective randomized study of cervical arthroplasty and anterior cervical discectomy and fusion with long-term follow-up: results in 74 patients from a single site. J Neurosurg Spine 18(1):36–42
Lunsford LD, Bissonette DJ, Jannetta PJ, Sheptak PE, Zorub DS (1980) Anterior surgery for cervical disc disease. Part 1: treatment of lateral cervical disc herniation in 253 cases. J Neurosurg 53(1):1–11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that they have no conflict of interest; they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript. The authors confirm their adherence to ethical standards and have no financial disclosures that would be a potential conflict of interest with this publication.
Research guidelines
Online Enhancing the Quality and Transparency Of health Research check was performed and returned STROBE. All the subheadings included in each paragraph are coherent with STROBE research protocol. This work reaches the STROBE research guidelines required standards.
Research involving human participants and/or animals and informed consent
This is a research project involving human subjects. It is perfectly consistent, in any of its aspect, with WMA Helsinki Declaration of Human Rights. All the patients of the surgical subgroup expressed consent to the surgical procedure after appropriate information. All the patients from both subgroups gave informed explicit consent to undergo the follow-up MRI; before performing the MRI they were elucidated about the purpose of the study.
Rights and permissions
About this article
Cite this article
Pesce, A., Wierzbicki, V., Piccione, E. et al. Adjacent segment pathology: natural history or effect of anterior cervical discectomy and fusion? A 10-year follow-up radiological multicenter study using an evaluation scale of the ageing spine. Eur J Orthop Surg Traumatol 27, 503–511 (2017). https://doi.org/10.1007/s00590-017-1936-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-017-1936-6