Abstract
Objectives
Hydroxyapatite (HA) is commonly used as bone substitute in clinical practices. However, only few studies have compared the relationship between the mixture ratio of bone graft in the actual clinical field and fusion rate according to bone graft volume. The study aimed to analyze the fusion rate according to the mixture ratio and the amount of bone graft in minimally invasive transforaminal lumbar interbody fusion (MI-TLIF).
Methods
A total number of 88 subjects who completed a 2-year follow-up after MI-TLIF participated in this study. Subjects were divided into three groups: Group 1 with local autograft, Group II with a mixture of HA and autobone of over 50 %, and Group III with a mixture of HA and autobone of less than 50 %. Subjects were also grouped into two groups: Group A with a graft volume of less than 12 ml and Group B with more than 12 ml. The correlation of mixture ratio and the graft volume with fusion rate was analyzed. For clinical analysis, visual analogue scale for pain and Oswestry Disability Index were used. Bone integration was evaluated based on the classification methods described in the Burkus study.
Results
Fusion rates are increased according to the ratio of autograft in all groups: 90.9 % in Group I, 87.8 % in Group II, and 85.7 % in Group III. However, there were no significant differences between groups (p = 0.22). The fusion rates significantly increased as the amount of bone graft increased to over 12 ml, showing 81.5 % in Group A and 92.0 % in Group B (p = 0.03).
Conclusions
A high rate of fusion was achieved in MI-TLIF in graft volume of more than 12 ml. We therefore recommend at least 12 ml of bone graft volume for successful fusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many studies have suggested minimally invasive neuronal decompression and fusion procedures to overcome complications such as soft tissue damage and muscular atrophy resulting from conventional posterior approach procedures. Among lumbar interbody fusions, anterior fusion is widely used as it allows anterior column support and high bone fusion rates by relieving compression of intervertebral space. In particular, minimally invasive transforaminal interbody fusion (MI-TLIF) is designed to minimize the damage to the soft tissue, reducing complications such as low back pain, muscular atrophy, and hemorrhage [1–5].
Bone fusion is critical to successful lumbar interbody fusion. However, it is difficult to obtain an adequate amount of local bone for graft in MI-TLIF. Consequently, additional bone graft is needed. And autogenous bone graft is considered ideal for bone formation, bone conduction, and guided bone. However, autograft accompanies various complications, including donor site bleeding, hematoma, pain, walking disorder, sensing abnormalities, the infection of incised area, a prolonged surgical time, and an increased blood loss. Studies aimed to overcome these issues are currently underway [6–11].
Hydroxyapatite (HA) is commonly used as bone substitute in clinical practices. Spivak et al. [12] achieved a satisfactory bone fusion using HA in lumbar interbody fusions. Chung et al. [13] also reported successful clinical results in posterior lumbar interbody fusion, compared to HA block-based and metal peek cage-based bone fusions. Although the combination of autobone and HA has been used in earlier studies, the association of bone fusion with mixture ratio and the amount of bone graft has rarely been investigated.
This study was aimed to investigate the effects of mixture ratio of autograft and allograft (HA) and the amount of bone graft on bone fusion success in MI-TLIF.
Materials and methods
Subjects
A total number of 88 subjects underwent a MI-TLIF from January 1 to March 2011, followed by a minimum of 2-year follow-up. The average follow-up period was 28.6 months (24–54 months). The subjects’ symptoms were characterized by low back pain and radicular pain caused by the instability of single lumbar segment or the lumbosacral region. Their pain was accompanied by spondylolisthesis, spinal canal stenosis, foraminal stenosis or lateral recess stenosis, calling for the removal of more than 50 % of facet joints. The criteria for segment instability included the average range of intervertebral motion of over 10° or over 4 mm displacement of the vertebral body in the standing lateral flexion and extension radiographs of the lumbosacral region. This study excluded the following groups of people/subjects: those with grade III/IV spondylolisthesis showing no motion in radiographs; those with a history of spinal surgery; those in need of multi-segment decompression or fusion surgeries; and finally, those with spinal infections and trauma.
In order to determine the effects of mixture ratio of graft on fusion rates, three groups were formed; Group I consisted of 11 subjects with local autograft while Group II was made up of 49 subjects with over 50 % mix of autograft with HA; finally, Group III composed of 28 subjects with less than 50 % mix of autograft. Only 11 subjects underwent autograft alone as autograft was prevalent in the early years covered in this study. And there were no other criteria for classification. The mean graft volume was 12.9 ml in Group I, 12.6 ml in Group II and 12.7 ml in Group III. But there were no significant differences between groups. To determine the effects of bone graft volume on fusion rates, subjects were also grouped into Group A with less than 12 ml of graft volume and Group B with more than 12 ml of graft volume. Based on the retrospective measurement of the amount of bone graft, the mean volume was 7.4 ml (5.0–10.0 ml) for the autograft group and 5.3 ml (3.0–10.0 ml) for the combination group. And the combined mean volume was 12.7 ml (8.0–18.5 ml), so 12 ml was set as the cutoff volume. The volume of the bone was measured with 20 cc syringe.
No statistical differences were found between groups in terms of sex, age, follow-up period, bone matrix density (BMD), and body mass index (BMI) (Table 1).
Surgical method
MI-TLIF involved making an incision of 2.5 cm length 2.5 cm off from the midline and dividing the muscles in order to separate multifidus and longissimus and to approach the laminar and the facet joint. Once lumbar facet joints are visualized, we set a delicate spinal traction and removed the inferior and the upper half of the superior articular processes along with yellow ligaments using a surgical microscope to visualize the nerve roots and the dura mater passing over the superior pedicle. And we performed lumbar discectomy. We then distracted the lumbar intervertebral distance gradually using a shaver and denuded the superior and inferior end plates in preparation for the fusion. We filled a spine cage (Capstone; Medtronic, Memphis, TN, USA) with a local autobone. It was obtained from articular process and lamina during decompression. We removed cartilage and soft tissue from obtained local autobone through rongeur and cut obtained local autobone about 3 mm sized.
A single cage was used for each patient. For Group I, a local bone was additionally harvested from the iliac crest for autograft. In case of Group II and III, the leftover autobone from cage filling was mixed with HA and used to fill the intervertebral space to allow bone fusion outside the cage (Fig. 1). After inserting the cage, we fixed the posterior cage with screws.
Clinical and radiologic analysis
Clinical analysis was performed based on the visual analogue scale (VAS) for radicular pain, Oswestry disability index (ODI) measured 2 weeks prior to and after the operation and at the final follow-up. Two orthopedic surgeons performed radiologic assessment by evaluating the bone fusion for each subject using the 2-year-old standing lateral flexion–extension radiographs and lumbar CT images. And their evaluations were compiled to calculate the mean value using the classification method described by Burkus et al. study (Table 2) [14]. Based on the radiographs, we defined the range of intervertebral motion at 3° or lower, vertebral body displacement of less than 3 mm, and continued formation of trabecular or new bone inside or outside the cage as bone fusion. The level of bone fusion was classified into four grades: Grade I (definitely solid), II (possibly solid), III (probably not solid), IV (definitely not solid). Only Grades I and II were regarded as bone fusion.
Statistical analysis of the differences between groups was performed using SPSS, version 19.0. The differences were tested by Kolmogorov–Smirnov test. P value of p < 0.05 was considered as statistical significance based on the results of ANOVA test, Student t test and Chi-square test.
Results
Assessment of clinical results
In total patient population, VAS for radicular pain was 6.5 before surgery, 1.9 after surgery and 1.0 at the final follow-up. VAS for low back pain was 6.2 before operation, 2.0 after surgery and 0.9 at the final follow-up. ODI was 25.8 before surgery, 12.8 after and 9.4 at the final follow-up, showing improvement in the outcomes. However, the differences between groups were not statistically significant (p ≥ 0.05) (Table 3).
Assessment of radiologic results
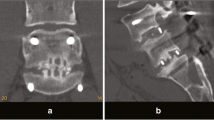
Of the 88 subjects, 77 had a bone fusion with a fusion rate of 87.5 %, and 11 did not have the bone fusion (Fig. 2, 3). The radiographs of 11 non-fusion subjects revealed no postoperative complications, including segment instability, dislocation of the implants trials and cage and screw-related complications. Revision surgery was therefore not performed.
The bone fusion rate was 90.9 % in Group I with 100 % autograft, 87.8 % in Group II with over 50 % autograft and 85.7 % in Group III with less than 50 % autograft; however, the differences between groups were not statistically significant (p = 0.22) (Table 4). However, bone fusion exhibited significant differences between graft volume-based groups showing 81.5 % in Group A with less than 12 ml of bone graft and 92.0 % in group B with over 12 ml (p = 0.03) (Table 5).
Discussion
Among lumbar interbody fusions, anterior fusion is widely used for degenerative spinal disorder as it allows anterior column support and high bone fusion rates by relieving compression of intervertebral space. In particular, MI-TLIF is designed to minimize the damage to the soft tissue, the surrounding muscles, and blood loss; hospital stay and antibiotics use are also reduced as a result [1–5, 14–16].
In lumbar interbody fusion operations, autograft is considered the most ideal for lumbar reconstruction since it is body-friendly, cytotoxicity-free and effective for bone formation, conduction, and guided bone. However, a limited availability of autogenous bone and various complications, including donor site bleeding, is keeping the use of autobone from a widespread use. Studies are currently underway to develop bone substitutes capable of reducing donor site complications while increasing bone fusion [6–11].
Among bone substitutes, HA is a bioactive ceramic containing main mineral deposition; its multiporous structure facilitates absorption and bone conduction, leading to a high integration with bone in the body. HA have advantages of easy shaping and absorption, a high biocompatibility without foreign body reactions, and producing no metabolites that are likely to interfere with bone formation [12, 13, 17–22].
Recent studies have discussed the role of HA in bone fusion. Korovessis et al. [23] found autograft to be better than the mixture of local autograft and HA in a posterior lumbar vertebrae fusion. Bucholz et al. [24] however, reported no differences in bone fusion between autograft and allograft (HA) based on the clinical and radiographic results of patients with proximal tibia fracture. They described HA as an effective/good alternative to autobone in their study. Boden et al. [25] also stated that fusion rate was similar between the autograft group and the HA combined group in which HA was added to the autograft as an extender at 1:1 ratio in a rabbit model. In this study, we found a positive correlation between the fusion rate and the ratio of autograft, although the differences between the autograft group and the combination groups were not significant.
Karabekir et al. [26] measured the intervertebral volume of 25 healthy people aged between 22 and 49 using MRI images and reported the mean volume between lumbar segment 3 and 4 to be 21.6 cm3 in men and 18.4 cm3 in women; the mean volume between lumbar segment 4 and 5 was 22.7 cm3 in men and 19.3 cm3 in women. However, Pfirrmann et al. [27] reported that the volume of intervertebral disk space significantly decreased as age increased with a mean volume of intervertebral disk space of 7.1 cm3 using MRI performed in 70 patients aged between 20 and 78 years. Decreased intervertebral disk space volume is associated in most patients with degenerated disks requiring surgical treatment. Therefore, the outcome indicated that an adequate space for bone graft is present when sufficient corpectomy and interbody distraction are performed intraoperatively.
Simple X-ray images revealed a fusion rate of more than 90 % in the anterior interbody fusion of Riouallon et al. [28] and in the transforaminal lumbar interbody fusion of Zairi et al. [29] over the follow-up period of more than 2 years. According to Park et al. [5], CT revealed a fusion rate of 77.3 % over the follow-up period of more than 2 years on MI-TLIF. Moreover, Thalgott et al. [17] reported that HA is an effective bone graft extender in patients with difficulty achieving bone fusion. The fusion rate was 92.5 % in posterolateral lumbar fusion by adding a 15 cc of HA in each segment to the autobone collected from the iliac bone. To acquire a higher fusion rate in this study, the authors tried to perform bone grafting in a large amount after sufficient corpectomy and interbody distraction intraoperatively. According to the CT results of fusion rate by bone graft volume, a statistically significant difference was shown in volumes of more than 12 cc. To achieve a satisfactory fusion rate of more than 90 %, at least more than 12 cc of bone graft volume is required.
According to Park et al. [5], the bone fusion rate was 77 % (41 subjects in 66 subjects who had a MI-TLIF and a 2-year follow-up. They did not perform any revision operation for 15 non-fusion subjects (23 %) despite the presence of a high level of low back pain because their ODI and low limb radicular pain did not show significant differences. In this study, low back pain was prominent in the non-fusion group, but there was no significant difference in the outcomes between the ODI and the low limb radicular pain. Neither implant complication was observed, and we did not perform a revision operation (Table 6).
This study, however, has some limitations: (1) it is a retrospective study, (2) the reliability of graft volume is low because the measurement was performed by us in the absence of any objective measurement methods, and (3) each group had a small number of parameters for statistical analysis. We therefore recommend further studies based on a reliable assessment tool, a large sample size, and a longer follow-up period. Nonetheless, this study provides useful information on the association of mixture ratio and the amount of bone graft on fusion success.
Conclusion
We found no statistically significant relationship between the mixture ratio of autograft and the fusion rate in MI-TLIF. However, the fusion rate significantly increased to 92.5 % in graft volume of more than 12 ml. We therefore suggest that enough amount of bone graft is crucial to MI-TLIF and that at least 12 ml graft is needed to achieve a satisfactory bone fusion.
References
Dhall SS, Wang MY, Mummaneni PV (2008) Clinical and radiographic comparison of mini-open transforaminal lumbar interbody fusion with open transforaminal lumbar interbody fusion in 42 patients with long-term follow-up. J Neurosurg Spine 9(6):560–565. doi:10.3171/SPI.2008.9.08142
Isaacs RE, Podichetty VK, Santiago P, Sandhu FA, Spears J, Kelly K, Rice L, Fessler RG (2005) Minimally invasive microendoscopy-assisted transforaminal lumbar interbody fusion with instrumentation. J Neurosurg Spine 3(2):98–105. doi:10.3171/spi.2005.3.2.0098
Mummaneni PV, Rodts GE, Jr (2005) The mini-open transforaminal lumbar interbody fusion. Neurosurgery 57(4):256–261 discussion 256–261
Park P, Foley KT (2008) Minimally invasive transforaminal lumbar interbody fusion with reduction of spondylolisthesis: technique and outcomes after a minimum of 2 years’ follow-up. Neurosurg Focus 25(2):E16. doi:10.3171/FOC/2008/25/8/E16
Park Y, Ha JW, Lee YT, Sung NY (2011) The effect of a radiographic solid fusion on clinical outcomes after minimally invasive transforaminal lumbar interbody fusion. Spine J 11(3):205–212
Aspenberg P, Albrektsson T, Thorngren KG (1989) Local application of growth-factor IGF-1 to healing bone. Experiments with a titanium chamber in rabbits. Acta Orthop Scand 60(5):607–610. doi:10.3109/17453678909150132
Cook SD, Dalton JE, Prewett AB, Whitecloud TS, 3rd (1995) In vivo evaluation of demineralized bone matrix as a bone graft substitute for posterior spinal fusion. Spine (Phila Pa 1976) 20 (8):877–886. doi:10.1097/00007632-199504150-00002
Cook SD, Dalton JE, Tan EH, Whitecloud TS, 3rd, Rueger DC (1994) In vivo evaluation of recombinant human osteogenic protein (rhOP-1) implants as a bone graft substitute for spinal fusions. Spine (Phila Pa 1976) 19 (15):1655–1663. doi:10.1097/00007632-199408000-00002
Louis-Ugbo J, Murakami H, Kim HS, Minamide A, Boden SD (2004) Evidence of osteoinduction by Grafton demineralized bone matrix in nonhuman primate spinal fusion. Spine (Phila Pa 1976) 29(4):360–366 discussion Z361
Martin GJ, Jr, Boden SD, Titus L, Scarborough NL (1999) New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine (Phila Pa 1976) 24(7):637–645
Choi DJAD, Lee SL et al (2008) The effect of demineralized bone matrix as a graft enhancer in posterior lumbar interbody fusion using cage and local bone chips. J Korean Soc Spine Surg 15(3):97. doi:10.4184/jkss.2008.15.3.157
Spivak JM, Hasharoni A (2001) Use of hydroxyapatite in spine surgery. Eur Spine J 10(Suppl 2):S197–S204. doi:10.1007/s005860100286
Chung JMCB, Lee CK, Lee JH et al (2009) Posterior lumbar interbody fusion using hydroxyapatite block—comparison with metal and PEEK cages. J Korean Soc Spine Surg 16(4):7. doi:10.4184/jkss.2009.16.4.243
Burkus JK, Foley K, Haid RW, LeHuec JC (2001) Surgical Interbody Research Group—radiographic assessment of interbody fusion devices: fusion criteria for anterior lumbar interbody surgery. Neurosurg Focus 10(4):E11
Foley KT, Holly LT, Schwender JD (2003) Minimally invasive lumbar fusion. Spine (Phila Pa 1976) 28 (15 Suppl):S26–35. doi:10.1097/01.BRS.0000076895.52418.5E
Schwender JD, Holly LT, Rouben DP, Foley KT (2005) Minimally invasive transforaminal lumbar interbody fusion (TLIF): technical feasibility and initial results. J Spinal Disord Tech 18(Suppl):S1–S6
Thalgott JS, Giuffre JM, Fritts K, Timlin M, Klezl Z (2001) Instrumented posterolateral lumbar fusion using coralline hydroxyapatite with or without demineralized bone matrix, as an adjunct to autologous bone. Spine J 1(2):131–137
Hwang CJBJ, Koo KH, Lee JH, Yeom JS, Chang BS, Lee CK (2007) A comparative experimental study of allograft and porous hydroxyapatite as bone substitutes. J Korean Orthop Assoc 42:7. doi:10.4055/jkoa.2007.42.4.545
Finkemeier CG (2002) Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 84-A(3):454–464. doi:10.3923/javaa.2010.1055.1067
Sartoris DJ, Gershuni DH, Akeson WH, Holmes RE, Resnick D (1986) Coralline hydroxyapatite bone graft substitutes: preliminary report of radiographic evaluation. Radiology 159(1):133–137. doi:10.1016/S1067-2516(96)80058-5
Cheng USKD, Cho JL et al (2008) Comparison of the effect of hydroxyapatite and allogeneous bone as an adjunct to autogenous iliac bone grafting in posterolateral spinal fusion. J Korean Orthop Assoc 43:5
Kang HJKT, Kweon SH et al (2011) The usefulness of hydroxyapatite bone transposition in treatment of metaphyseal fracture with bone defect. J Korean Musculoskelet Transpl Soc 11(1):5
Korovessis P, Koureas G, Zacharatos S, Papazisis Z, Lambiris E (2005) Correlative radiological, self-assessment and clinical analysis of evolution in instrumented dorsal and lateral fusion for degenerative lumbar spine disease. Autograft versus coralline hydroxyapatite. Eur Spine J 14(7):630–638. doi:10.1007/s00586-004-0855-5
Bucholz RW (2002) Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res 395:44–52
Boden SD, Martin GJ, Jr., Morone M, Ugbo JL, Titus L, Hutton WC (1999) The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar spine fusion. Spine (Phila Pa 1976) 24(4):320–327
Karabekir HS, Gocmen-Mas N, Edizer M, Ertekin T, Yazici C, Atamturk D (2011) Lumbar vertebra morphometry and stereological assesment of intervertebral space volumetry: a methodological study. Ann Anat 193(3):231–236. doi:10.1016/j.aanat.2011.01.011
Pfirrmann CW, Metzdorf A, Elfering A, Hodler J, Boos N (2006) Effect of aging and degeneration on disc volume and shape: a quantitative study in asymptomatic volunteers. J Orthop Res 24(5):1086–1094. doi:10.1002/jor.20113
Riouallon G, Lachaniette CH, Poignard A, Allain J (2013) Outcomes of anterior lumbar interbody fusion in low-grade isthmic spondylolisthesis in adults: a continuous series of 65 cases with an average follow-up of 6.6 years. Orthop Traumatol Surg Res 99(2):155–161. doi:10.1016/j.otsr.2012.12.009
Zairi F, Arikat A, Allaoui M, Assaker R (2013) Transforaminal lumbar interbody fusion: comparison between open and mini-open approaches with two years follow-up. J Neurol Surg A Cent Eur Neurosurg 74(3):131–135. doi:10.1055/s-0032-1330956
Conflict of interest
No conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoo, JS., Min, SH. & Yoon, SH. Fusion rate according to mixture ratio and volumes of bone graft in minimally invasive transforaminal lumbar interbody fusion: minimum 2-year follow-up. Eur J Orthop Surg Traumatol 25 (Suppl 1), 183–189 (2015). https://doi.org/10.1007/s00590-014-1529-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-014-1529-6