Abstract
The body exhaust suit (BES) of Charnley creates ‘negative pressure’ inside the gown using intake/outtake tubing. Modern ‘space suit’ (SS) systems incorporate helmet-based intake fans, which use the hood material as a filter and create ‘positive pressure’ inside the gown. While early studies of BES demonstrate a clear reduction in infection rates following arthroplasty, recent clinical data on SS use has paradoxically reported a marked increase. We hypothesized that the positive pressure inside the gown could carry air and particles via the unsealed area around the surgeon’s cuff into the operative field. We performed 12 simulated operations with the surgeons hands covered in fluorescent 0.5 micron powder that approximates the size of shedded skin squames. Photographs under UV light and air particle counts were used to compare potential contamination rates between SS and conventional gowns using a standardised scoring system. The highest powder migration was seen in the SS group with a score of 15.3 out of 28. No powder migration was seen in the standard gown group (p = 0.028). This study provides a plausible explanation for the increase in infection rates seen with SS use. We recommend SS be considered for personal protection only and supplemented with sealant tape around the inner glove.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and background
Deep infection following total joint replacement is a devastating complication. Early arthroplasty series reported infection rates as high as 9.5 % [1] leading to a considerable efforts to reduce this rate. Charnley introduced the body exhaust suit [2] (BES), aiming to reduce wound contamination caused by bacterial shedding from the surgical team. In the large MRC trial reported by Lidwell [3, 4], BES resulted in a further 90 % reduction in infection rates (0.7 vs. 0.06 %) in patients given both prophylactic antibiotics and operated on in ultraclean theatres. These results led to the widespread introduction of BES.
BES were designed with both air intake and outlet tubing to create ‘negative pressure’ inside the gown, ensuring any shed particles are extracted away from the surgical field. However, such tubing is cumbersome, which led to the development of more portable ‘space suit’ (SS) systems such as the T4 Steri-Shield (Stryker Instruments, Kalamazoo, MI, USA), the Provision Surgical Helmet (DePuy, Warsaw, Indiana), and Stackhouse FreedomAire (Stackhouse Incorporated, Palm Springs, CA, USA). Space suit systems have an intake valve on the helmet itself, which draws air in from outside using the hood material as a filter. The air is then blown down across the surgeon’s face and neck, creating a ‘positive pressure’ situation inside the surgeon’s gown.
This positive pressure is of concern, as theatre staff are thought to be the primary source of microbial contamination in up to 98 % of cases [5, 6]. Air under positive pressure will escape though any gaps that are not sealed, such as that between the glove and the surgeon’s cuffs. In addition, turbulent flow around the surgeon’s hood may disrupt normal theatre airflow.
A recent large registry study [7] reported markedly increased infection rates when SS are used in arthroplasty compared to conventional gowns. We hypothesized that increased egress of air and bacteria around the surgeon’s cuffs with SS could provide a mechanism with which to explain higher infection rates. Fluorescent powder has previously been used to track aerosol spread in sterile environments [8, 9], and the aim of this study was to use this technique to investigate potential routes for contamination of the SS. We also sought to measure particle levels around the hood to assess whether these were altered by the presence of the intake fan on the helmet.
Methods
A pilot study was carried out in a cadaver lab where total knee replacements were performed with the surgeons gowned using Sterishield Togas and T4 helmets (Stryker Instruments, Kalamazoo, MI, USA) and conventional gowns (Microcool, Kimberly-Clark, Ohio). After surgical scrub and prior to gowning, the surgeon covered his hands in fluorescent powder (Neon Red AX, DAYGLO Corp, Ohio). This powder has a mean particle size of 0.5 micron, similar to the size of shedded skin squames that are thought to carry bacteria.
On the basis of this pilot study, a 30 min simulated knee replacement was developed which was carried out on sawbones (Synbone, Malans, Switzerland) in a theatre environment using conventional ventilation operating at 22 air changes per hour. Each operation commenced with the surgeon performing a surgical scrub in a separate room, after which the hands of the surgeon were rubbed in fluorescent powder to the level of the distal wrist crease. The surgeon then moved to a second room and gowned with assistance in one of 4 ways: with a SS (Stryker T4 helmet system and Stryker toga), SS but with inner gloves taped down to the cuffs using drape tape, SS with the fan off and plastic facemask cut away to allow breathing, and finally a standard gown (Microcool, Kimberly-Clark, Ohio). Double gloving was performed in all scenarios (Biogel Eclipse, Mölnlycke, Gothenburg, Sweden). Gowning was standardised such that the cuff of the gown finished at the metacarpophalangeal joint of the thumb, and gloves were drawn proximally over the wrist.
After gowning, the surgeon stepped into a clean anaesthetic room and ultraviolet light was used to check for any fluorescent particles on the exterior of the gown, gloves, or hood. If any particles were found the surgeon returned to rescrub and regown. The surgeon then moved into theatre and performed a simulated TKR.
A resting particle count was taken using a laser particle counter (PMS Handilaz Mini) for comparison. During the simulated operation, a total of three particle counts were taken 10 cm from the right ear of the surgeon and the results averaged. This site was chosen to better evaluate the effect of the fan on air particle counts.
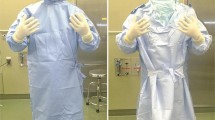
At the conclusion of the procedure, standardised photos were taken of the extensor and flexor surfaces of the left and right forearms, the right aspect of the hood, the fan intake of the hood, and the anterior aspect of the chest. Migration of powder was then graded by the number of particles in the image according to the following system: 0 particles = score of 0, 1–5 a score of 1, 5–10 a score of 2, 10–100 a score of 3, and >100 a score of 4. The average result of two observers gave the recorded grade.
Statistical evaluation
Data was evaluated using Mann–Whitney U tests, and p values were considered to be statistically significant when <0.05. We performed a power calculation based on a pilot study in which joint replacements were performed on cadavers; out of six suits used, all three that were used with the fan in operation had powder migration, whilst all with the fan off had none. Using a continuity correction, we calculated a need for four patients with the fan on and four with the fan off to achieve 80 % power.
Results
All of the full suit procedures showed migration onto the flexor surface of both arms; the only group to have no visible powder on any part of the gown was the standard gown group (Fig. 1). The highest mean powder migration was within the spacesuit group with a score of 15.3/28, and the lowest mean score was in the standard gown group of 0/20 (p = 0.028). The region with the highest powder migration was the flexor surface of the surgeon’s dominant right hand in the spacesuit group, with a mean score of 3.75 (Table 1). The addition of surgical tape around the cuff of the spacesuit appeared to prevent forearm powder migration with a mean score of 0. In the two groups that had a fan in use, powder migration around the fan was seen with a mean score of 2 in the spacesuit group and a score of 3 in the spacesuit with taped cuffs group.
The highest particle counts were seen when using a spacesuit with the average total particle count of 17,583 in the spacesuit group and 21,666 in the spacesuit with taped cuffs. The lowest total particle counts were present with the spacesuit with the fan off and the standard gown with an average total particle count of 10,533 and 12,855, respectively. When the data were analysed for particle size, the spacesuit group had a higher 5 μm particle count of 958 versus 254 with the fan off (p = 0.045) (Table 2).
Discussion
Reports of lower infection rates with BES systems led to their widespread introduction; however, the more modern SS design differs significantly from Charnley’s original concept. Using the New Zealand joint registry, Hooper et al. [7] reported the first clinical data on modern SS and infection rates. Among surgeons who had completed at least 50 TKRs both with and without a SS, there was a tenfold increase in the rate of early revision due to deep infection when they had used a SS compared to conventional gowns (0.251 vs. 0.028 %, p = 0.016). The revision rate increased further when space suits were combined with laminar flow.
Our study provides two potential explanations. Egress of skin squames or other particles around the surgeon’s cuff, maximal on the flexor surface of his dominant right arm, provides a convenient entry point for bacterial contamination into the wound (Fig. 1). Contamination from direct contact with the surgeons cuffs may explain the lack of correlation between wound contamination and bacterial air counts seen in other studies [10]. Cuff contamination would be exacerbated by vertical laminar flow, as it is interposed between the air pathway and the wound [11].
Secondly, the visual powder migration and higher particle counts seen around the hood when a fan is in operation probably reflect disruption of laminar theatre ventilation as a result of the suction effect of the fan. The presence of fluorescent particles on the outside of the hood was an unexpected finding of this study, as presumably these particles exited from inside the suit either around the cuff or from below the surgeon’s gown. Recent studies have found the outer aspect of the hood to show bacterial contamination in 49–80 % of cases [12, 13] suggesting hoods should not be considered sterile.
There are a number of limitations to our study. Firstly, the relationship between the study outcomes (visual powder migration and particle counts) and the clinical outcome of interest (deep infection rates) is unknown. This is common among studies of the theatre environment, since prophylactic antibiotics have lowered infection rates to a level where clinical studies are difficult to power adequately. ‘Surrogate’ markers for infection are therefore used, such as air particle counts or wound washings [10, 14–17], assuming a relationship between these markers and clinical infection rates. Our visual powder migration score is arbitrary, and particle counts have been criticised for their inconsistent relationship to microbiological wound contamination [18]. Secondly, we conducted a sham operation to allow the study to be conducted in a theatre environment, but this does not correspond to clinical reality. Finally, in our study, the surgeon’s hands were covered in 5 micron size particles after scrubbing; a situation very different to the normally ‘clean’ hands post scrubbing. Thus, the actual amount of skin or other particles that egress through this route is unknown.
However, the dramatic difference in visual cuff powder migration between the clothing types suggests this is a feasible mechanism. Positive pressure inside the gown is an obvious explanation, but particle egress occurred even when the fan was off suggesting that this may not the only factor. The toga used in this study is made of a thicker waterproof material than the conventional gown, which may lead to an imperfect seal under the glove.
Our findings are consistent with previous comparative studies (Table 3); all of which failed to show an advantage of SS over conventional gowns in reducing contamination of the sterile field. Der Tavitian et al. [10] used perhaps the most plausible surrogate for infection, measuring wound bacterial counts with tetrazolium-stained membrane imprinting in 50 TKR patients. Overall, contamination was seen in 64 % of SS versus 60 % of conventional gown wounds; however, there was a trend for SS wounds to become more contaminated as the procedure went on (1.5 colonies per membrane versus 0.5 at last sample point).
Our study provides a plausible mechanism to explain the failure of modern SS designs to match the reduction in infection rates seen with Charnley’s original BES. Modern SS are known to provide a clear advantage over conventional clothing in terms of surgeon safety from fluid splash [19]; therefore, we recommend that if used they be considered for personal protection only and supplemented with tape around the inner glove.
References
Charnley J (1964) A clean‐air operating enclosure. Br J Surg 51(3):195–202
Charnley J (1979) Low friction arthroplasty of the hip. Springer, Berlin
Lidwell O, Lowbury E, Whyte W (1982) Effect of ultraclean air in operating rooms on deep sepsis in the joint after total hip or knee replacement: a randomised study. Br Med J 285:10–14
Lidwell OM (1988) Air, antibiotics and sepsis in replacement joints. J hosp infect 11 Suppl C:18–40
Owers KL, James E, Bannister GC (2004) Source of bacterial shedding in laminar flow theatres. J hosp infect 58(3):230–232. doi:10.1016/j.jhin.2004.06.028
Whyte W, Hodgson R, Tinkler J (1982) The importance of airborne bacterial contamination of wounds. J Hosp Infect 3(2):123–135
Hooper GJ, Rothwell AG, Frampton C, Wyatt MC (2011) Does the use of laminar flow and space suits reduce early deep infection after total hip and knee replacement?: the ten-year results of the New Zealand Joint Registry. J Bone Joint Surg Br 93(1):85–90. doi:10.1302/0301-620X.93B1.24862
Oberyszyn AS, Robertson FM (2001) Novel rapid method for visualization of extent and location of aerosol contamination during high-speed sorting of potentially biohazardous samples. Cytometry 43(3):217–222
Boekel P, Blackshaw R, Van Bavel D, Riazi A, Hau§ R (2012) Sterile stockinette in orthopaedic surgery: a possible pathway for infection. ANZ J Surg 82:838–843
Der Tavitian J, Ong SM, Taub NA, Taylor GJS (2003) Body-exhaust suit versus occlusive clothing. A randomised, prospective trial using air and wound bacterial counts. J Bone Joint Surg Br 85(4):490–494
Taylor GJ, Bannister GC (1993) Infection and interposition between ultraclean air source and wound. J Bone Joint Surg Br 75(3):503–504
Kearns K, Witmer D, Makda J, Parvizi J et al SpringerLink—Clinical Orthopaedics and Related Research®. Online First™. Clinical Orthopaedics and …
Singh VK, Hussain S, Javed S, Singh I, Mulla R, Kalairajah Y (2011) Sterile surgical helmet system in elective total hip and knee arthroplasty. J Orthop Surg (Hong Kong) 19(2):234–237
Blomgren G, Hambraeus A, Malmborg AS (1983) The influence of the total body exhaust suit on air and wound contamination in elective hip-operations. J Hosp Infect 4(3):257–268
Bohn WW, McKinsey DS, Dykstra M, Koppe S (1996) The effect of a portable HEPA-filtered body exhaust system on airborne microbial contamination in a conventional operating room. Infect Control Hosp Epidemiol 17(7):419–422
Shaw JA, Bordner MA, Hamory BH (1996) Efficacy of the steri-shield filtered exhaust helmet in limiting bacterial counts in the operating room during total joint arthroplasty. J Arthroplasty 11(4):469–473
Pasquarella C, Pitzurra O, Herren T, Poletti L, Savino A (2003) Lack of influence of body exhaust gowns on aerobic bacterial surface counts in a mixed-ventilation operating theatre. A study of 62 hip arthroplasties. J Hosp Infect 54(1):2–9. doi:10.1016/S0195-6701(03)00077-X
Friberg B, Friberg S, Ostensson R, Burman LG (2001) Surgical area contamination—comparable bacterial counts using disposable head and mask and helmet aspirator system, but dramatic increase upon omission of head-gear: an experimental study in horizontal laminar air-flow. J Hosp Infect 47(2):110–115. doi:10.1053/jhin.2000.0909
Singh VK, Kalairajah Y (2009) Splash in elective primary knee and hip replacement: are we adequately protected? J Bone Joint Surg Br 91(8):1074–1077. doi:10.1302/0301-620X.91B8.22079
Acknowledgments
We thank associate Professor Chris Frampton, for his statistical analysis.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Young, S.W., Chisholm, C. & Zhu, M. Intraoperative contamination and space suits: a potential mechanism. Eur J Orthop Surg Traumatol 24, 409–413 (2014). https://doi.org/10.1007/s00590-013-1178-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-013-1178-1