Abstract
Osteoporotic distal radius fractures are a public health problem that medical osteoporosis treatment is unable to check. We have described a bone substitute method to improve mechanical properties in bone. Our current study aims at improving the technique by maximising the filling of the distal radius. Cavities were created by curettage in the distal radius using a styloid process approach in ten randomly selected wrists from the sample population of ten cadavers. All radii were injected percutaneously with calcium phosphate cement. Cement quantities injected were calculated and corrected for observed cement leakage. Our results show that distal radius curettage before cementoplasty can double the amount of calcium phosphate cement injected. Prevention of osteoporotic distal radius fractures by cementoplasty seems a promising technique. Slowly absorbed micro-porous biomaterial use is preferable in long-term preventive treatment. Nevertheless, in the future, this technique should apply in specific cases, such as medical osteoporosis treatment failure with contra-lateral wrist fracture.
Résumé
Les fractures du poignet ostéoporotique sont un problème majeur de santé publique. En effet, le traitement médical de l’ostéoporose ne parvient pas à enrayer la progression de cette épidémie. Dans ce contexte, nous avons mis au point une technique de cimentoplastie préventive du radius distal lors d’un précédent travail expérimental. Le but de la présente étude est d’améliorer cette technique d’injection percutanée perosseuse d’un ciment phosphocalcique pour obtenir un remplissage maximum de la cavité médullaire du radius distal. Les vingt poignets de dix sujets anatomiques ont été préparés à l’Ecole de Chirurgie de l’Assistance Publique-Hôpitaux de Paris. Dans dix poignets tirés au sort, une cavité a été réalisée par curetage dans le radius distal par voie styloïdienne punctiforme. Puis les vingt radius ont été injectés en percutané perosseux sous fluoroscope par un ciment phosphocalcique. Les moyennes de remplissage de ciment en mm3 des vingt poignets ont été calculées, avant et après correction en fonction des fuites de ciment constatées. Nos résultats démontrent que la préparation initiale du radius distal par curetage avant cimentoplastie double la quantité de ciment phosphocalcique injecté. La prévention des fractures du radius distal ostéoporotique par cimentoplastie percutanée perosseuse au ciment phosphocalcique injectable nous paraît être une voie intéressante à explorer. L’utilisation d’un biomatériau microporeux à résorption lente est indispensable dans un traitement préventif fait pour durer. Cette technique devra néanmoins s’appliquer à des indications ciblées telles contrindication ou échec de traitement médical de l’ostéoporose, fracture du poignet controlatéral.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporotic wrist fractures represent an ever-increasing proportion of health care administered in developed countries, due notably to increased life expectancy. These fractures are often indicators of osteoporotic disease, and their appearance should systematically lead to a diagnostic assessment [6]. However, even when osteoporosis is diagnosed, treatment is limited to, at best, improving bone mineralisation and, at worst, preventing its reduction [12] without acting directly on bone resistance that is not proportional to the degree of mineralisation [24]. The treatment of osteoporosis relies on the use of various therapeutic agents where the ratio of effectiveness to side effects does not allow reliable reproducible results. Although bisphosphonates have an overall effectiveness of 50% [3], the principle of hormone replacement therapy in menopausal women has been called into question due to the risk of cancer [2].
In this context, during an anatomical trial [13], we suggested the basis for a preventive surgical treatment of osteoporotic fractures of the distal radius. The objective was to increase bone resistance and, therefore, raise the threshold of resistance to fracture by percutaneous injection of calcium phosphate cement. In this study, we found that the centro-medullary filling of the distal radius with the calcium phosphate cement was uneven. This depended notably on the trajectory imposed on the injection stylet, the degree of mineralisation, the microarchitecture and the initial bone strength. The lower the initial mechanical bone strength, the greater the degree of filling with calcium phosphate cement. After cementoplasty, the mechanical strength measured with peripheral quantitative computed tomography was proportional to the degree of filling seen on the fluoroscope. The space occupied by the injected cement spread most often to the edge of the cone scooped out by the removal of the stylet, without reaching either the shaft or the sub-chondral area of the distal radius [15].
The objective of the current study is to improve this calcium phosphate cement injection technique by maximising the filling of the distal radius medullary cavity, from the subchondral bone to the metaphyseal–diaphyseal area. As the technique was designed for eventual clinical application in humans, it appears essential to develop a reliable, reproducible and minimally invasive technique as soon as possible. The principle of single percutaneous cement injection through the bone satisfies the last criterion. In order to deal with the other criteria in the specification, we sought to standardise our technique by preparation of the distal radius by curettage in order to obtain a homogeneous cavity ready for filling.
The calcium phosphate cement Cémentek LV (TEKNIMED) was chosen from among the injectable bone substitutes available because of its interesting physicochemical properties [11]. This particular synthetic bone substitute has a calcium–phosphate ratio of 1.63, approaching that of natural bone. The crystallisation process that characterises it leads to a chemically non-stoechiometric stable hydroxyapatite, meaning that ionic exchanges between the crystal surfaces and the extra-cellular environment are high [17]. Its micro-porosity explains its relatively slow biodegradation rate [14] whereas the absence of macro-porosity provides a resistance to compression rupture of the order of 20 Mpa, placing it between that for trabecular and cortical bone.
Methods
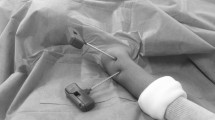
A preliminary study was undertaken on two separate anatomical subjects in order to determine the ideal entry point for the injection stylet (Fig. 1). To artificially form the best possible intrabone cavity in the distal radius, from the subchondral bone to the metaphyseal–diaphyseal junction, we made a special tool using a hand-shaped, double-curved number 18 Kirschner wire manipulated with a removable handle. The curve and counter-curve were adapted to the shape of the distal radius.
In one case (Fig. 1a), the entry point was proximal metaphyseal–diaphyseal and in the other, distal, the trocar point therefore being penetrated by the tip of the radial stylus (Fig. 1b). In both cases, the rectilinear, trocar-tipped stylet was pushed from the lateral to the medial edge of the radius, descending when the point of entry was proximal and ascending when the point of entry was distal. It was then replaced by the double-curved wire manipulated simultaneously in three dimensions—in rotation, backwards and forwards—to collapse the remaining bone honeycombs and obtain the largest possible injectable cavity.
It became obvious from reading the fluoroscope images (Fig. 1) that preparation at the proximal entry point did not allow us to collapse all the space occupied by the trabecular bone in the distal radius. It proved impossible to reach the styloid area and difficult to reach the medial area with regard to the distal radio-ulnar (Fig. 1a). On the other hand, preparation at the distal entry point allowed easy access to the centro-medullary distal radius area (Fig. 1b). The probable reason is that the trocar-tipped stylet and the double-curved wire in the upward direction could be moved to trace a cone at the styloid tip. On the other hand, when the point of entry was proximal, the only direction possible for the stylet and the double-curved pin was rectilinear, oblique at the bottom and inward. As a result, we decided to use only the styloid entry for the study.
Subjects
Ten Caucasian anatomical subjects, five men and five women, were prepared by the Ecole de Chirurgie de l’Assistance Publique-Hôpitaux de Paris (Table 1). The average age at death was 86.7 years (73–95). No wrist from any of the subjects, 20 wrists in total, presented any previous consolidated fracture lesions of the distal radius.
Technique
The author carried out the anatomical procedure on the 20 wrists. A percutaneous orifice was created across the tip of the radial styloid using a 10-cm-long number 11 trocar-tipped stylet pushed along its bisectrix until it reached the medial cortical of the radius without perforating it. In two cases where the resistance of the bone was particularly weak, an un-wanted perforation of the cortical was noted while pushing the stylet.
Two groups were subsequently defined. In group 1, calcium phosphate cement injection was carried out after preparation of the cavity, as described above. In group 2, calcium phosphate cement injection was carried out without any special preparation. It was decided that the two wrists from each subject should be allocated to a different group in order to obtain a matched series. To avoid bias due to manual dominance, the side chosen for preparation was selected at random.
For both groups, a 20-mg dose of Cementek LV was hand mixed to obtain a malleable homogenous paste and introduced into a BoC system (Grifols) injection syringe to which the stylet had been adapted. The injection was subsequently carried out using a BVgold (Philipps) fluoroscope to check the progress of the cementoplasty. The cementoplasty of the distal radius was stopped either when cement leakage was seen or when the operator judged the filling to be satisfactory. Leaks appeared either because of the medial cortical perforation caused by the thrust of the trocar point or at the entry hole for the injection stylet. The torsion-torque resistance of the syringe was 1,000 psi, the syringe automatically breaking when this value was exceeded.
Measurement method
We used a BoC system syringe graduated in millimetres cubed to measure the quantity of cement injected. To avoid measurement errors, the syringe was flushed before each injection. Extra-osteal leakage obviously increased the measured volume of cement injected into the distal radius. The measured injected volume in millimetres cubed was corrected for leaks by subtracting the leak volume estimated using the fluoroscope.
The average cement fill volumes in millimetres cubed were calculated both before and after observed leakage correction for both groups.
Data analysis
The average volumes and the standard deviations were calculated for three variables: injected volume, leakage volume and corrected volume for both groups. The Shapiro-Wilk test for normality was applied to the distribution for the difference in results between the two groups. If normality was refused, the Wilcoxon matched-pair test was applied to the difference or, failing that, Student’s pair test was used. A value of p<0.05 was considered significant.
Results
Age and gender characteristics for the ten subjects in groups 1 and 2 are shown in Table 1 along with the results for the volume of cement injected, the cement leakage volumes and the corrected volumes of cement after subtraction of the leaks following cementoplasty of the distal radius. Fluoroscopy images examples in groups 1 and 2 are shown in Fig. 2. Statistical analysis results are shown in Table 2.
Discussion
Our results clearly show that pre-preparation of the distal radius by curettage before calcium phosphate cement injection doubles the quantity of cement injected whether leaks are taken into account or not (Table 2). Although the cement leakage volumes were estimated rather than measured, extrapolation was allowable, as the total leakage volume differences between the two groups were not statistically significant. In qualitative terms, the distribution of the cement injected followed the path of the stylet in group 2 whereas in group 1, it tended to be evenly distributed from the sub-chondral bone to the metaphyseal–diaphyseal area. Nevertheless, our method is not dedicated to modify the evolution of the osteoporosis disease itself, even at the metaphyseal–diaphyseal area.
Certain authors consider that displaced radial fractures in elderly or dependent persons, particularly when they present with osteoporosis, should undergo conservative treatment [4, 23]. The argument put forward is that any attempt to reduce these fractures, regardless of the treatment applied, leads, in most cases, to a fresh displacement [16]. It is because of the unfortunate risk of displacement recurrence that these authors have abandoned surgical treatment of such fractures rather than a question of poor tolerance.
In this context, the prevention of such fractures by percutaneous transosseous cementoplasty with injectable calcium phosphate cement seems to us to be an interesting route worth exploring. The extrapolation of this technique to human clinical practice should, nevertheless, depend on specific indicators. In the first place, it appears logical to offer it to osteoporotic menopausal women presenting certain pre-existing conditions from among the following: cancer contra-indicating the use of hormone replacement therapy, failure of a properly run course of medical treatment (vitamin and calcium supplementation, bisphosphonates, raloxifene) or contra-lateral wrist fracture.
The use of biomaterials to strengthen the distal radius is not new, and methylmethacrylate has already been used in elderly patients with acceptable results [9]. However, the long-term fate of acrylic cement is detachment due to the total absence of bioactivity at the surface without any bone rehabilitation. On the other hand, bone substitutes are bioactive and usually allow effective osteointegration. Their rapid re-absorption, varying from a few weeks to a few months, depending on the case, is a disadvantage for preventive treatment designed to last. But the calcium phosphate cement used in this study is strictly micro-porous, which allows re-absorption over several years [14] theoretically only requiring a top-up injection every 10 years.
This type of curettage followed by the filling of the space contained within the cortical bone circumscribing the distal radius, could be suspected of inducing complications, particularly vascular and/or mechanical complications. However, the preparation of the distal radius for the implantation of complete wrist prostheses, a clinical model equivalent to our curettage filling, did not lead to any bone necrosis or metaphyseal–diaphyseal fractures after 4 years in 72 cases of porotic bone in rheumatoid polyarthritis [5]. The major problem with these prostheses is detachment at the interface between the acrylic cement and the prosthesis whereas the interface between the calcium phosphate cement and the bone tissue showed successful osteointegration in living subjects [1]. Another feature is that the calcium phosphate cement used here crystallises following a succession of non-exothermic chemical reactions [25], as opposed to acrylic cements that harden by exothermic polymerisation and which could be accused of causing burn lesions through heating of the osteogenic cells [19]. Finally, the vascularisation of the distal radius, coming essentially from the periosteal arteries that penetrate the cortical bone [7, 18], is not affected by a centro-medullary curettage.
The styloid piecing route first used is considerably less abrading than the distal radius preparation required for the implantation of a complete wrist prosthesis. Even in this case, fat embolisms, described in cases of long-bone reaming with pressure-injected acrylic cement, appear in a totally different context and have not been found in the literature concerning the distal radius. As far as cement embolisms are concerned, they have not been described with calcium phosphate cement, as the injection pressure is less than 1,000 psi, a pressure above which the injection syringe is designed to break. The curettage may even be beneficial, as the collapsing of the remaining trabecular honeycombs, even in porotic bone, may liberate osteogenic factors likely to reinforce cortical bone solidity, the product of the curettage being effectively automatically forced against the deep cortical face during the manoeuvres associated with the curettage and the calcium phosphate injection.
The question of the stylet penetration point was discussed in the “Methods” section. We consider that we have demonstrated that the radial styloid choice was better than the metaphyseal–diaphyseal area. We have not studied a third possible entry point that has, nevertheless, been regularly used in the literature [8, 10]—the middle metaphyseal area, the usual source of the horizontal line in distal radius fractures. We have already stressed the disadvantages of this (pre) fracturing route [15]. It is a classically fragile area where the posterior cortical of the radius risks being made even more fragile by the reciprocating and rotary movements of the stylet necessary to obtain the best-possible filling.
If, on the face of it, the curettage is not harmful to the mechanical properties of the distal radius, the geometric distribution of the calcium phosphate cement injected into the distal radius is important. In this study, we tried to fill the distal radius as far as possible, from the sub-chondral bone to the epiphyseal–metaphyseal area, as a completely filled volume. We have shown in a previous study that, although the measured mechanical strength of the distal radius is greater after percutaneous injection of a bone substitute, this increase is enhanced when the distribution of the cement is concentrated at the periphery [15]. Moreover, for an identical bone mass for a given volume, a normal bone of small section with thick cortical layers is stronger than a porotic bone of much greater section but with thinner cortical layers [22]. The porotic bone behaves as though its cortical layers have become thinner relative to the trabecular bone [21, 26]. Thus, hormone replacement therapy acts on the mineral distribution between cortical and trabecular bone by moving the interface between trabecular and cortical bone towards the cortical bone rather than acting to increase bone mineral density [20]. It would, therefore, be advisable during a future study to further improve the technique by leaving a central void within the distal radius, concentrating the distribution of the calcium phosphate cement around the periphery in contact with the cortical layer and then measuring the impact of this modification in terms of the mechanical strength of the bone.
In conclusion, we think there is a place for a prolonged action, preventive, therapeutic treatment for porotic bone fractures using a minimally invasive surgical technique aimed at areas of the skeleton at risk, the target of which is improved mechanical bone strength. From this point of view, the objective of our work was to develop a reliable, reproducible and minimally invasive technique for curettage filling of the distal radius by percutaneous transosseous calcium phosphate cement injection.
References
Apelt D, Theiss F, El-Warrak AO, Zlinsky K, Bettschart-Wolfisberger R, Bohner M, Matter S, Auer JA, Von Rechenberg B (2004) In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 25: 1439–1451
Beral V, Million Women Study Collaborators (2003) Breast cancer and hormone-replacement therapy in the Million Women Study. The Lancet 362:419–427
Chapurlat R, Meunier PJ (1998) Biphosphonates et remodelage osseux: efficacité dans la maladie de Paget, la dysplasie fibreuse et l’ostéoporose. Revue de chirurgie orthopédique 84:743–751
Delle Santa D., Sennwald G (2001) Y a t-il une place pour le traitement conservateur de la fracture du radius distal chez l’adulte? Chirurgie de la main 20: 426–435
Fourastier J, LeBreton L, Alnot JY, Langlais F, Condamine JL, Pidhorz L (1996) La prothèse totale radio-carpienne Guépar dans la chirurgie du poignet rhumatoïde. À propos de 72 cas revus. Revue de chirurgie orthopédique 82:108–115
Freedman K, Kaplan F, Bilker W, Strom B, Lowe R. T(2000) Treatment of osteoporosis: are physicians missing an opportunity? J Bone Joint Surg Am 82(8):1063–1070
Haerle M, Schaller E, Mathoulin C (2003) Vascular anatomy of the palmar surfaces of the distal radius and ulna: its relevance to pedicled bone grafts at the distal palmar forearm. J Hand Surg Br 28(2):131–136
Jeyam M, Andrew JG, Muir LTSW, McGovern A (2002) Controlled trial of distal radial fractures treated with a resorbable bone mineral substitute. J Hand Surg Br 27(2):146–149
Kofoed H (1983) Comminuted displaced Colles’ fractures. Treatment with intramedullary methylmethacrylate stabilisation. Acta Orthop Scand 54:307–311
Kopylov P, Jonsson K, Thorngren KG, Aspenberg P (1996) injectable calcium phosphate in the treatment of distal radial fractures. J Hand Surg Br 21:(6)768–771
Lacout JL, Mejdoubi E, Hamad M (1996) Crystallisation mechanism of calcium phosphate cement for biological uses. Mater Sci Mater Med 7:371–374
Liberman UA, Weiss SR, Bröll J, et al (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The alendronate phase III osteoporosis treatment study group. N Engl J Med 333:(22)1437–1443
Liverneaux P (2003) Cement-plasty of distal radius using a calcium phosphate bone cement: anatomical experimental study in 20 wrists. Arch Physiol Biochem 111(S):65
Liverneaux P (2003) Utilisation d’un ciment phosphocalcique dans le traitement de la dysplasie fibreuse des os: à propos d’un cas. Revue de chirurgie orthopédique 89:532–536
Liverneaux P (2004) Augmentation expérimentale de la résistance du radius distal ostéoporotique par un ciment phosphocalcique. Chirurgie de la main 23(1):37–44
Mc Queen MM, Court-Brown CM (2003) Increasing age and fractures of the distal radius. Curr Orthop 17:360–368
Mejdoubi E, Lacout JL, Heughebaert C, Michaud P (1994) Optimization of hydraulic calcium phosphate cement. Advanced Materials Research 1:163–172
Menck J, Schreiber HW, Hertz T, Bürgel (1994 ) Angioarchitektur von ulna und radius und ihre praktische relevanz. Langenbecks Arch Chir 379:70–75
Mjöberg B, Pettersson H, Rosenqvist R, Rydholm A (1984) Bone cement, thermal injury and the radioluct zone. Acta Orthop Scand 55:597–600
Muller ME, Webber CE, Adachi JD (2003) Hormone replacement therapy improves distal radius bone structure by endocortical mineral deposition. Can J Physiol Pharmacol 81:952–958
Nijs J, Westhovens R, Joly J, Cheng XG, Borghs H, Dequeker J (1998) Diagnostic sensitivity of peripheral quantitative computed tomography measurements at ultradistal and proximal radius in postmenopausal women. Bone 22:(6)659–664
Russo CR, Lauretani F., Bandinelli S, Bartalli B, Di Toria A, Volpato S, Guralnik JM, Harris T, Ferrucci L (2003) Aging bone in men and women: beyond changes in bone mineral density. Osteoporos Int 14:531–538
Saffar P, Mazodier F, Werther JR (2000) Les fractures du sujet âgé – Faut-il opérer les sujets de plus de 75 ans? In: Dumontier C et Herzberg G, éd. Les fractures fraîches du radius distal chez l’adulte. Revue de Chirurgie Orthopédique 87 (Suppl 5):130–135
Schiessl H, Ferreti JL, Tysarczik-Niemeyer G, Willnecker J (1996) Noninvasive bone strength index as analysed by peripheral quantitative computed tomography (pQCT). In Schönau E (ed) Paediatric osteology: new developments in diagnostics and therapy. Elsevier Science 141–146
Takagi S, Chow LC, Ishikawa K (1998) Formation of hydroxyapatite in new calcium phosphate cements. Biomaterials 19:1593–1599
Van der Linden JC, Day JS, Verhaar JAN, Weinans H (2004) Altered tissue properties induce changes in cancellous bone architecture in aging and diseases. J Biomech 37:367–374
Acknowledgements
Our grateful thanks are due to the following people and organisations for technical assistance during the study: Statistical analysis by Nicolas Molinari, Laboratoire de Biostatistiques, IURC, 641 avenue Gaston Giraud, 34093 Montpellier, France; anatomical subjects provided by the Laboratoire d’Anatomie de l’Ecole de Chirurgie du Fer à Moulin, Paris, France; calcium phosphate cement supplied by Société Teknimed, Vic-en-Bigorre, France. This study was supported financially by GRETO, Groupe de recherche et d’Etudes en Traumatologie de l’Ostéoporose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liverneaux, P.A. Osteoporotic distal radius curettage–filling with an injectable calcium phosphate cement. A cadaveric study. Eur J Orthop Surg Traumatol 15, 1–6 (2005). https://doi.org/10.1007/s00590-004-0182-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-004-0182-x