Abstract
Purpose
While many studies have explored the association between paravertebral muscle (PVM) change and low back pain/lumbar spinal stenosis, little is known about PVM change in adult degenerative scoliosis (ADS). The present study explored the PVM change in ADS and investigated its association with bony structural parameters.
Methods
We evaluated 78 patients in ADS without radiculopathy (ADS group) and 65 healthy persons without degenerative lumbar diseases (control group). Percentage of fat infiltration area (%FIA) of multifidus muscle at L1 to S1 disk level was measured by MRI, using ImageJ software. Lumbar scoliosis Cobb’s angle, lumbar lordotic angle, lateral vertebral translation, and apical vertebral rotation were recorded in ADS group, and relationship between PVM change and these factors was analyzed.
Results
In the control group, the mean %FIA of multifidus muscle was not significantly different between the bilateral sides at all levels (P > 0.05). In the ADS group, the mean %FIA was significantly higher on the concave side than the convex side at all levels (P < 0.05). Asymmetric degree of multifidus muscle change was 8.55% ± 4.91%, which was positively correlated with lumbar scoliosis Cobb’s angle, lateral vertebral translation, and apical vertebral rotation (0 < r < 1, P < 0.05), but negatively weak-correlated with lumbar lordotic angle (− 1 < r < 0, P < 0.05).
Conclusions
Asymmetric PVM change in ADS is more often seen on the concave side, which is positive to evaluate the progression of scoliosis. Its asymmetric degree increases with progression of lumbar scoliosis Cobb’s angle and decreased lumbar lordotic angle. Apical vertebral rotation and lateral vertebral translation can aggravate the asymmetric degree.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adult degenerative scoliosis (ADS) is common among the aging population and is a spinal deformity in skeletally mature individuals, with a curve measuring ≧ 10° according to the Cobb method [1]. The deformity often leads to low back pain (LBP) and radiating pain to the legs due to foraminal stenosis and seriously affects the quality of life [2, 3]. Although the prevalence of ADS is difficult to evaluate precisely, it has been reported to reach 64–68% among the aging population [4, 5]. Generally, ADS is believed to derive from disks degeneration first, followed by the posterior column [5], but its pathogenesis has not been completely elucidated.
In the lumbar spine level, the paravertebral muscle (PVM) comprises three parts—multifidus, longissimus, and iliocostalis lumborum muscles (from medial to lateral direction)—which can be considered to have two functions: to produce movements of the trunk and to provide stability to the spinal column [6]. There have been numerous studies about the association between spinal disorders and morphological change of PVM. Hyun et al. [7] found that there were significantly higher signal intensities and degree of fatty change of the multifidus in the patients with degenerative lumbar kyphosis (DLK) than healthy volunteers. Jiang et al. [8] demonstrated a significant imbalance between muscle volume and fatty infiltration in deep PVM of adolescent idiopathic scoliosis (AIS) patients. Lee et al. [9] found that there were significantly higher signal intensity and a larger area of fat infiltration of back muscle in the patients with degenerative lumbar flat back than in normal people. These studies support that spinal deformity may be correlated with PVM change. Nevertheless, few published reports have performed quantitative evaluation of PVM in ADS, and the relationship between PVM and bony structural parameters in ADS is unclear.
The objective of this study was to investigate the PVM change in ADS and to ascertain whether the PVM on the convex/concave side was abnormal and correlated with bony structural parameters. It was important to understand the mechanism of the onset and progression in ADS, which was conducive to explore its pathogenesis and to treat the disease.
Materials and methods
Subjects
This was a prospective cross-sectional study. The ADS group consisted of 78 consecutive ADS patients who were diagnosed at the Third Hospital of Hebei Medical University during the period from June 2005 to December 2012. Inclusion criteria were: aged over 50 years at the initial presentation of ADS (Cobb angle > 10°) with no radiculopathy, and patients who did not receive physical therapy, acupuncture, or brace treatment were included. The control group consisted of 65 healthy persons who had a complete checkup at the Third Hospital of Hebei Medical University during the period from January 2006 to June 2013. Inclusion criteria were age over 50 years, without degenerative lumbar diseases, and people who did not receive physical therapy, acupuncture, or brace treatment were included. There were no significant differences between the two groups in sex, age, and BMI (Table 1).
Imaging procedures
All the patients underwent radiography and MRI examinations. The radiography system used in this study was a 500 mA Siemens DR system (Siemens Corporation, Germany) with automatic exposure control system. Usage parameters were: Electric Current was 500 mA and voltage was 75 kV on anteroposterior position, 85 kV on lateral position. The MRI system was a 1.5 Tesla Imaging System (Siemens Magnetom Symphony, Germany). T1-weighted images (T1WI) and T2-weighted images (T2WI) of sagittal views of lumbar intervertebral were obtained using a spin echo sequence system for T1WI and a fast spin echo sequence system for T2WI. A surface coil was used. Slice width was 4 mm and inter-slice gap was 1 mm. The acquisition matrix was 512 × 256. The sequence parameters were repetition time (TR) 482 ms (ms)/echo time (TE) 10 ms (ms) for TIWI, and TR 2300 ms/TE 99 ms for T2WI.
Imaging evaluation
For each patient in the ADS group, the apical vertebral level, curve direction, apical vertebral rotation, lateral vertebral translation, and lumbar scoliosis Cobb’s angle were recorded and measured in the standard anteroposterior radiography, according to previously reported research methods [10, 11]. The lumbar lordotic angle between the L1 upper endplate and the L5 lower endplate was measured in the standard lateral radiography. Two doctors who have more than 10 years work experience measure the indicators. All these data were analyzed using ImageJ software (Version 1.43u, Wayne Rasband, NIH, USA).
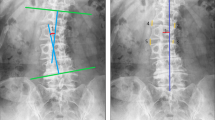
Lateral vertebral translation was classified into two conditions: “yes” was the presence of a lateral vertebral translation of > 3 mm; otherwise, it was “no.” Apical vertebral rotation was classified into five grades according to Nash–Moe method [11] (if the apex was disk, the apical vertebra was under the apex): Grade 0: convex and concave no asymmetry; Grade 1: convex migrates within first segment and concave may start disappearing; Grade 2: convex migrates to second segment and concave gradually disappears; Grade 3: convex migrates to middle segment and concave is not visible; and Grade 4: convex migrates past midline to concave side of vertebral body and concave was not visible. Grade 4 was less common, so we merged Grade 4 and Grade 3 into Grade 3 (Fig. 1).
Male, 75Y, degenerative lumbar scoliosis: a showing that apical vertebrae was L3 vertebrae and its rotation belonged to Grade 2, no lateral translation; lumbar scoliosis Cobb’s angle was 21.2°; Main curve was located on lumbar segment with left scoliosis, and compensatory curve whose orientation was contrary to main curve was located on lumbosacral segment; b showing that lordotic angle was 41.1°
Three T2WI-images were taken at each L1-S1 disk level, and the center slice of each was considered as the research object. The %FIA of PVM was measured in the following steps. The first step was to set the scale pixel/cm, converting pixels into cm measurement units through the ruler present in the photographic image, and then each image was converted into grayscale 8-bit image. The second was to measure the cross-sectional area (CSA) of targeted PVM at each level by outlining its region freehand using ImageJ software [12, 13]. The third was to measure the FIA using a threshold technique [9, 13]. In the technique, the value of the threshold was selected automatically by using “default” and “dark background” method, and the detailed technical process was as follows: image/adjust/threshold/dark background and default/auto. Fat tissue in the 8-bit image was colored in red using the threshold tool of the program, and the red area was measured as FIA. The last was to calculate the %FIA, which was FIA divided by the CSA. We used the paired t test to evaluate the change of PVM, and if there was a significant difference between the convex side and the concave side, its different value was considered as the asymmetric degree of PVM change (Figs. 2 and 3).
Male, 75Y, degenerative lumbar scoliosis: a–e showing the bright pixels of fat tissue were colored in white in MR images, and the %FIA of multifidus muscle on the concave side was 37.21%, 34.41%, 28.19%, 30.72%, 32.36% and on the convex side was 18.63%, 18.79%, 18.89%, 25.22%, 29.43% at L1–2, L2–3, L3–4, L4–5, and L5–S1 levels; the asymmetric degree was 10.39%
Statistical analysis
Statistical analysis was conducted using SPSS 13.0 (IBM Corp, Armonk, NY). The nonparametric tests were used to compare the age and BMI between the two groups and to analyze the correlation between asymmetric degree of PVM change and lateral vertebral translation in the ADS group. The paired t test was used to compare the bilateral PVM difference (left/right side and convex/concave side) in the two groups. The Chi-square test was used to compare the sex distribution data between the two groups. The intra- and inter-observer reliability of %FIA in PVM was calculated by the intraclass correlation coefficient(ICC) using a two-way random model and absolute agreement with a confidence interval of 95%, and the ICC value was considered highly reliable, moderate, and less reliable when the value was ≧ 0.80, between 0.60 and 0.79, and < 0.60, respectively. Linear regression analysis was used to analyze the correlation between the asymmetric degree and lumbar scoliosis Cobb’s angle or lumbar lordotic angle. The nonparametric tests and independent-samples t test were performed to assess the correlation between the asymmetric degree and apical vertebral rotation.
Results
In the ADS group, each patient had two curves in the standard anteroposterior radiography, comprising a main curve and a compensatory curve. There were 17 (21.79%) patients whose main curve was located on the thoracolumbar segment, including five (6.41%) with right scoliosis and 12 (15.38%) with left scoliosis, and 61 (78.21%) patients whose main curve was located on the lumbar segment, including 14 (17.95%) with right scoliosis and 47 (60.26%) with left scoliosis. The compensatory curve was located on the lumbosacral segment, and its direction was contrary to the main curve. Twenty-eight (35.90%) patients had three vertebras in the main curve, 38 (48.72%) had four vertebras, and 12 (15.38%) had five vertebras. Apical vertebra in the main curve was located on L1 in three (3.85%) cases, L1–2 in seven (8.97%) cases, L2 in 12 (15.38%) cases, L2–3 in 42 (53.85%) cases, L3 in 11 (14.10%) cases, and L3–4 in three (3.85%) cases, while apical vertebra in the compensatory curve was located on L4 in three (3.85%) cases, L4–5 in nine (11.54%) cases, L5 in 25 (32.05%) cases, and L5–S1 in 41 (52.56%) cases. Lumbar scoliosis Cobb’s angle ranged from 11.1°to 38.5° (average 20.8° ± 6.4°), and lumbar lordotic angle ranged from 7.5°to 43.3° (average 24. 5° ± 8.7°).
Evaluation of multifidus muscles in the ADS and control group
In the ADS group, the average %FIA of multifidus muscle on the convex side was 10.17% ± 5.22%, 12.06% ± 7.49%, 16.73% ± 10.44%, 24.72% ± 11.67%, 35.09% ± 13.70% and on the concave side was 15.60% ± 7.22%, 21.09% ± 10.90%, 25.81% ± 11.25%, 36.88% ± 14.85%, 44.22% ± 17.36% at L1–2, L2–3, L3–4, L4–5, and L5–S1 levels, which showed significant differences between the both sides (t = − 7.718, − 8.256, − 9.062, − 9.973, − 8.457; P < 0.05). The asymmetric degree of multifidus muscle change between the both sides was 8.55% ± 4.91% (Tables 2, 3).
In the control group, the average %FIA of multifidus muscle on the left side was 8.64% ± 7.03%, 11.02% ± 6.73%, 15.18% ± 11.35%, 22.92% ± 9.17%, 27.80% ± 10.53% and on the right side was 8.51% ± 7.14%, 10.82% ± 6.83%, 15.61% ± 11.86%, 23.25% ± 9.12%, 28.47% ± 11.74% at L1–2, L2–3, L3–4, L4–5, and L5–S1 levels, which showed no significant differences between the both sides (t = − 0.532, 0.778, − 1.123, 1.129, − 1.265; P > 0.05) (Tables 4, 5).
Analysis of associated factors with multifidus muscle change
According to correlation analysis, the asymmetric degree of multifidus muscle change was positively correlated with lumbar scoliosis Cobb’s angle (0 < r < 1, P < 0.05) (Fig. 4), but negatively correlated with lumbar lordotic angle (− 1 < r < 0, P < 0.05) (Fig. 5). Linear regression existed between the asymmetric degree and lumbar scoliosis Cobb’s angle, as well as lumbar lordotic angle (R2 = 0.610 and 0.339, respectively) (Table 6). There was a correlation between the asymmetric degree and apical vertebral rotation, as well as lateral vertebral translation (P < 0.05), which showed that the asymmetric degree was positively correlated with apical vertebral rotation degree and lateral vertebral translation (P < 0.05) (Tables 7 and 8, Fig. 6).
Discussion
PVM plays a vital role in the stability and functional movement of the lumbar vertebral column [6]. Multifidus muscle, the largest and most medial of the lumbar PVM, is sensitive to pathologic change [14], and it provides stability to the spine biomechanically [6, 15, 16]. In our previous trial, we found that there existed a significant relationship between PVM degeneration and lumbar stability, and multifidus muscle degeneration was more obvious than erector spinae [17]. Fatty infiltration in the lumbar multifidus muscle was common in adults and strongly associated with LBP [18]. Besides, in the rehabilitation of lumbar PVM dysfunction in LBP, multifidus was often considered as a core muscle to be researched [19,20,21,22]. For these reasons, we chose the multifidus muscle as our study subject.
There are several ways to explore the PVM change, such as ultrasound, needle electromyogram(EMG), computerized tomography(CT) and MRI [6, 9, 12, 13, 17, 20, 21, 23,24,25,26]. Using ultrasound, Kliziene et al. [20] explored the effects of core stability exercises on multifidus muscles in healthy women and women with chronic LBP. Measuring intramuscular EMG activity of the multifidus, Kiesel et al. [26] investigated whether experimentally induced LBP affected multifidus activity during functionally oriented tasks, weight shifting and upper extremity lifting. Murakami et al. [25] measured the CSA of PVM by using CT scan to predict scoliosis progression. Comparing the intra- and inter-reliability of PVM using CT and MRI, authors advised MRI other than CT for PVM measurements of CSA and FIA [24]. MRI can clearly show muscle mass and intramuscular fat and simply identify the character in different groups of muscles and the difference in signal intensity between fat and muscles. Therefore, MRI appears to be an ideal imaging modality for assessing the intramuscular morphology of lumbar PVM. The method described in the present study provides a valuable tool for evaluating the change of PVM in ADS.
CSA and FIA are major indicators to evaluate PVM change. Several studies have confirmed that PVM change is characterized by an increase in the amount of fat deposits or a decrease in the size of the muscle [6,7,8,9, 17, 27]. Investigating PVM change in patients with chronic unilateral lumbar radiculopathy and back pain, Chon et al. and Barker et al. found that the CSA was smaller than these in controls [14, 28]. Kang et al. [6] regarded the %FIA as a major indicator to compare the PVM change in patients with DLK and chronic LBP, and they concluded that patients with DLK had a higher %FIA in the multifidus and erector spinae muscle. In our study, %FIA was considered as the major indicator to assess PVM change in ADS, consistent with previous reports.
There are several factors affecting PVM change, such as age, sex, BMI, denervation, and so on. Kalichman [27] found that PVM density decreased with age and increased with BMI. A 15-yr longitudinal MRI study supported the idea that an increase in fat content was caused by age and BMI [29]. The CSA and density of PVM in men were higher than in women [27, 30, 31]. Stokes et al. [32] researched that CSA of multifidus muscle was larger in males, but age had no effect. Chon et al. [28] concluded that there existed asymmetric atrophy of PVM in patients with chronic unilateral lumbar radiculopathy. Denervation of multifidus muscle could lead to muscle atrophy and replacement of fatty tissues after nerve root injury [13, 28, 33, 34]. In our study, there were no significant differences in the age, sex, and BMI between the two groups, and patients with radiculopathy were excluded from the research. Regardless of these factors, we could better focus on the relationship between bony structural parameters and PVM change in ADS.
In the ADS group, we found that the degree of multifidus muscle change on the concave side was significantly higher than on the convex side, and its asymmetric degree between the both sides was 8.55% ± 4.91%. In the control group, there was no significant difference in the multifidus muscle between the bilateral sides. Generally, there is equivalent stress load on the bilateral PVM in normal people. Scoliosis leads to different stress on the both sides, and tension load on the convex side is larger than on the concave side. On the concave side, fat content in PVM was supposed to be a result of long-lasting inactivity [35], while increase of EMG activity was an effect of PVM stretching on the convex side [36]. This biomechanical change results in the asymmetric change of PVM. The result was consistent with earlier researches about scoliosis. Multifidus muscle on the convex side was shorter than on the concave side at the apex in idiopathic scoliosis (IS) patients, and this was considered as a theory of primary muscular imbalance causing the spinal deformity [37]. In the patients with AIS, larger back volume was slightly more frequent on the concave than on the convex side, and skin-fold thickness was always greater on the concave side at the apex region [38]. Chan et al. [39] agreed with the idea that multifidus muscle on the concave side was morphologically abnormal at the apex of scoliotic curve and there was a significant association between abnormal signal change and scoliosis. Similar findings also demonstrated that there was significant imbalance of muscle volume and FIA in deep PVM in the AIS patients [8]. All the researches about AIS were identical with our study, and we considered that ADS and AIS may have the same mechanical principle.
Our study found that the asymmetric degree of multifidus muscle change was positively correlated with lumbar scoliosis Cobb’s angle, lumbar vertebrae translation, and apical vertebral rotation: first, the larger scoliosis Cobb’s angle and apical vertebral rotation degree, the more asymmetric mechanical load between the concave side and the convex side. Second, lateral vertebral translation influenced the stability of spine. We considered that these two points were the main reasons for the aggravation of asymmetric degree. A biomechanical study confirmed that PVM on the convex side would bear more than 1.5 kg/cm2 load, when apical vertebrae deviated from the midline each 1 cm [40]. Cobb angle of ≧ 30°, apical vertebral rotation of ≧ grade 2, and lateral vertebral translation of ≧ 6 mm were important risk factors, predicting scoliosis progression [41]. Cobb angle was positive with lateral vertebral translation [42] and lateral vertebral translation associated with vertebral rotation [43, 44]. Asher et al. [45] found that the vertebra translated and rotated always toward the apex. The differences between bilateral back muscle volume were more frequent at the apex of the curve [38]. To research the relationship between EMG of PVM and progression of the scoliotic curve, Cheung et al. [46] found that the asymmetry in paraspinal EMG activity at the lower end vertebra of the curve was associated with progressiveness of the scoliosis. Greater EMG activity in the convex side was due to severity of scoliotic curvatures [47], and curvatures smaller than 25° Cobb angle had a myoelectric activity not different from that of normal people [40]. In the mild AIS, multifidus muscle thickness on the concave side was greater than the corresponding convex side [48]. All of them supported our view directly or indirectly.
Besides, we also found that the asymmetric degree of multifidus muscle change was negatively correlated with lumbar lordotic angle, although the correlation is weak. First, lumbar lordotic angle was negatively correlated with lumbar scoliosis Cobb’s angle, and loss of lumbar lordotic angle caused the PVM asymmetric change by increase of scoliosis Cobb’s angle. Murata et al. [49] found that lumbar scoliosis Cobb’s angle was reduced by at least 5° as the lordotic angle increased 10°. Second, a recent meta-analysis concluded that lumbar lordotic angle was associated positively with disk degeneration, and patients with LBP had smaller lumbar lordotic angle than healthy controls [50]. Disk degeneration was one of the most important factors to cause the ADS [5], and chronic LBP caused atrophy of the paraspinal isolated multifidus muscle [14, 27, 33, 34]. In a word, we considered that lumbar lordotic angle was correlated with the asymmetric degree of multifidus muscle change by the above factors, including lumbar scoliosis Cobb’s angle, disk degeneration, and LBP.
There are some limitations in this study that need to be further discussed and investigated. First, the sample size in the study is small, although comparable with other similar studies. Second, there is a lack of studies on EMG, histochemistry, cytology, and pathology to support our results. Further studies with adequate sample sizes through multicenter recruitment and using these methods are needed.
Conclusions
There exists asymmetric PVM change in ADS, more often seen on the concave side, which is regarded as indicating the progression of scoliosis. Its asymmetric degree increases with progression of lumbar scoliosis Cobb’s angle and decrease of lumbar lordotic angle. Apical vertebral rotation and lateral vertebral translation can aggravate the asymmetric degree.
Abbreviations
- PVM:
-
Paravertebral muscle
- MRI:
-
Magnetic resonance image
- CT:
-
Computerized tomography
- EMG:
-
Needle electromyogram
- T1WI:
-
T1-weighted images
- T2WI:
-
T2-weighted images
- TR:
-
Repetition time
- TE:
-
Echo time
- FIA:
-
Fat infiltration area
- CSA:
-
Cross-sectional area
- ADS:
-
Adult degenerative scoliosis
- DLK:
-
Degenerative lumbar kyphosis
- AIS:
-
Adolescent idiopathic scoliosis
- IS:
-
Idiopathic scoliosis
- LBP:
-
Low back pain
References
Aebi M (2005) The adult scoliosis. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. Eur Spine J 14(10):925–948. https://doi.org/10.1007/s00586-005-1053-9
Fu KM, Rhagavan P, Shaffrey CI, Chernavvsky DR, Smith JS (2011) Prevalence, severity, and impact of foraminal and canal stenosis among adults with degenerative scoliosis. Neurosurgery 69(6):1181–1187. https://doi.org/10.1227/NEU.0b013e31822a9aeb
Bess S, Boachie-Adjei O, Burton D, Cunningham M, Shaffrey C, Shelokov A, Hostin R, Schwab F, Wood K, Akbarnia B (2009) Pain and disability determine treatment modality for older patients with adult scoliosis, while deformity guides treatment for younger patients. Spine 34(20):2186–2190. https://doi.org/10.1097/BRS.0b013e3181b05146
Jimbo S, Kobayashi T, Aono K, Atsuta Y, Matsuno T (2012) Epidemiology of degenerative lumbar scoliosis: a community-based cohort study. Spine 37(20):1763–1770. https://doi.org/10.1097/BRS.0b013e3182575eaa
Silva FE, Lenke LG (2010) Adult degenerative scoliosis: evaluation and management. Neurosurg Focus 28(3):E1. https://doi.org/10.3171/2010.1.focus09271
Kang CH, Shin MJ, Kim SM, Lee SH, Lee CS (2007) MRI of paraspinal muscles in lumbar degenerative kyphosis patients and control patients with chronic low back pain. Clin Radiol 62(5):479–486. https://doi.org/10.1016/j.crad.2006.12.002
Hyun SJ, Bae CW, Lee SH, Rhim SC (2016) Fatty degeneration of the paraspinal muscle in patients with degenerative lumbar kyphosis: a new evaluation method of quantitative digital analysis using MRI and CT scan. Clin Spine Surg 29(10):441–447. https://doi.org/10.1097/BSD.0b013e3182aa28b0
Jiang J, Meng Y, Jin X, Zhang C, Zhao J, Wang C, Gao R, Zhou X (2017) Volumetric and fatty infiltration imbalance of deep paravertebral muscles in adolescent idiopathic scoliosis. Med Sci Monitor Int Med J Exp Clin Res 23:2089–2095
Lee JC, Cha JG, Kim Y, Kim YI, Shin BJ (2008) Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: comparison with the normal controls. Spine 33(3):318–325. https://doi.org/10.1097/BRS.0b013e318162458f
Wu HL, Ding WY, Shen Y, Zhang YZ, Guo JK, Sun YP, Cao LZ (2012) Prevalence of vertebral endplate modic changes in degenerative lumbar scoliosis and its associated factors analysis. Spine 37(23):1958–1964. https://doi.org/10.1097/BRS.0b013e31825bfb85
Kohno S, Ikeuchi M, Taniguchi S, Takemasa R, Yamamoto H, Tani T (2011) Factors predicting progression in early degenerative lumbar scoliosis. J Orthop Surg (Hong Kong) 19(2):141–144. https://doi.org/10.1177/230949901101900202
Ranson CA, Burnett AF, Kerslake R, Batt ME, O’Sullivan PB (2006) An investigation into the use of MR imaging to determine the functional cross sectional area of lumbar paraspinal muscles. Eur Spine J Off Pub Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 15(6):764–773. https://doi.org/10.1007/s00586-005-0909-3
Shafaq N, Suzuki A, Matsumura A, Terai H, Toyoda H, Yasuda H, Ibrahim M, Nakamura H (2012) Asymmetric degeneration of paravertebral muscles in patients with degenerative lumbar scoliosis. Spine 37(16):1398–1406. https://doi.org/10.1097/BRS.0b013e31824c767e
Barker KL, Shamley DR, Jackson D (2004) Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain: the relationship to pain and disability. Spine 29(22):E515–E519
McGill SM, Grenier S, Kavcic N, Cholewicki J (2003) Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol Off J Int Soc Electrophysiol Kinesiol 13(4):353–359
Hansen L, de Zee M, Rasmussen J, Andersen TB, Wong C, Simonsen EB (2006) Anatomy and biomechanics of the back muscles in the lumbar spine with reference to biomechanical modeling. Spine 31(17):1888–1899. https://doi.org/10.1097/01.brs.0000229232.66090.58
Guo X, Zhang X, Ding W, Yang D, Ma L, Xie D, Wang H, Wang H, Lu K, Yang S (2014) Imaging study of paravertebral muscle degeneration in degenerative lumbar instability. Zhonghua wai ke za zhi [Chin J Surg] 52(8):571–575
Kalichman L, Carmeli E, Been E (2017) The Association between imaging parameters of the paraspinal muscles. Spinal Degener Low Back Pain 2017:2562957. https://doi.org/10.1155/2017/2562957
Danneels LA, Vanderstraeten GG, Cambier DC, Witvrouw EE, Bourgois J, Dankaerts W, De Cuyper HJ (2001) Effects of three different training modalities on the cross sectional area of the lumbar multifidus muscle in patients with chronic low back pain. Br J Sports Med 35(3):186–191
Kliziene I, Sipaviciene S, Klizas S, Imbrasiene D (2015) Effects of core stability exercises on multifidus muscles in healthy women and women with chronic low-back pain. J Back Musculoskel Rehabil 28(4):841–847. https://doi.org/10.3233/bmr-150596
Deckers K, De Smedt K, Mitchell B, Vivian D, Russo M, Georgius P, Green M, Vieceli J, Eldabe S, Gulve A, van Buyten JP, Smet I, Mehta V, Ramaswamy S, Baranidharan G, Sullivan R, Gassin R, Rathmell J, Gilligan C (2017) New therapy for refractory chronic mechanical low back pain-restorative neurostimulation to activate the lumbar multifidus: one year results of a prospective multicenter clinical trial. Neuromodul J Int Neuromod Soc. https://doi.org/10.1111/ner.12741
Russo M, Deckers K, Eldabe S, Kiesel K, Gilligan C, Vieceli J, Crosby P (2017) Muscle control and non-specific chronic low back pain. Neuromod J Int Neuromod Soc. https://doi.org/10.1111/ner.12738
Tunnell J (2009) Needle EMG response of lumbar multifidus to manipulation in the presence of clinical instability. J Manual Manip Ther 17(1):E19–E24. https://doi.org/10.1179/jmt.2009.17.1.19E
Hu ZJ, He J, Zhao FD, Fang XQ, Zhou LN, Fan SW (2011) An assessment of the intra- and inter-reliability of the lumbar paraspinal muscle parameters using CT scan and magnetic resonance imaging. Spine 36(13):E868–E874. https://doi.org/10.1097/BRS.0b013e3181ef6b51
Murakami N, Obata K, Abe Y, Oto Y, Kido Y, Itabashi H, Tsuchiya T, Tanaka Y, Yoshino A, Nagai T (2012) Scoliosis in Prader–Willi syndrome: effect of growth hormone therapy and value of paravertebral muscle volume by CT in predicting scoliosis progression. Am J Med Gen Part A 158(7):1628–1632. https://doi.org/10.1002/ajmg.a.35429
Kiesel KB, Butler RJ, Duckworth A, Halaby T, Lannan K, Phifer C, DeLeal C, Underwood FB (2012) Experimentally induced pain alters the EMG activity of the lumbar multifidus in asymptomatic subjects. Manual Therapy 17(3):236–240. https://doi.org/10.1016/j.math.2012.01.008
Kalichman L, Hodges P, Li L, Guermazi A, Hunter DJ (2010) Changes in paraspinal muscles and their association with low back pain and spinal degeneration: cT study. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 19(7):1136–1144. https://doi.org/10.1007/s00586-009-1257-5
Chon J, Kim HS (2017) Asymmetric atrophy of paraspinal muscles in patients with chronic unilateral lumbar radiculopathy. Ann Rehab Med 41(5):801–807. https://doi.org/10.5535/arm.2017.41.5.801
Fortin M, Videman T, Gibbons LE, Battie MC (2014) Paraspinal muscle morphology and composition: a 15-yr longitudinal magnetic resonance imaging study. Med Sci Sports Exerc 46(5):893–901. https://doi.org/10.1249/mss.0000000000000179
Bulcke JA, Termote JL, Palmers Y, Crolla D (1979) Computed tomography of the human skeletal muscular system. Neuroradiology 17(3):127–136
Kalichman L, Klindukhov A, Li L, Linov L (2016) Indices of paraspinal muscles degeneration: reliability and association with facet joint osteoarthritis: feasibility study. Clin Spine Surg 29(9):465–470. https://doi.org/10.1097/BSD.0b013e31828be943
Stokes M, Rankin G, Newham DJ (2005) Ultrasound imaging of lumbar multifidus muscle: normal reference ranges for measurements and practical guidance on the technique. Manual Therapy 10(2):116–126. https://doi.org/10.1016/j.math.2004.08.013
Hyun JK, Lee JY, Lee SJ, Jeon JY (2007) Asymmetric atrophy of multifidus muscle in patients with unilateral lumbosacral radiculopathy. Spine 32(21):E598–E602. https://doi.org/10.1097/BRS.0b013e318155837b
Battie MC, Niemelainen R, Gibbons LE, Dhillon S (2012) Is level- and side-specific multifidus asymmetry a marker for lumbar disc pathology? Spine J Off J North Am Spine Soc 12(10):932–939. https://doi.org/10.1016/j.spinee.2012.08.020
Goubert D, Oosterwijck JV, Meeus M, Danneels L (2016) Structural changes of lumbar muscles in non-specific low back pain: a systematic review. Pain Physician 19(7):E985–E999
Butterworth TR Jr, James C (1969) Electromyographic studies in idiopathic scoliosis. South Med J 62(8):1008–1010
Fidler MW, Jowett RL (1976) Muscle imbalance in the aetiology of scoliosis. J Bone Joint Surg Br 58(2):200–201
Zoabli G, Mathieu PA, Aubin CE (2007) Back muscles biometry in adolescent idiopathic scoliosis. Spine J Off J North Am Spine Soc 7(3):338–344. https://doi.org/10.1016/j.spinee.2006.04.001
Chan YL, Cheng JC, Guo X, King AD, Griffith JF, Metreweli C (1999) MRI evaluation of multifidus muscles in adolescent idiopathic scoliosis. Pediatr Radiol 29(5):360–363. https://doi.org/10.1007/s002470050607
Reuber M, Schultz A, McNeill T, Spencer D (1983) Trunk muscle myoelectric activities in idiopathic scoliosis. Spine 8(5):447–456
Sapkas G, Efstathiou P, Badekas AT, Antoniadis A, Kyratzoulis J, Meleteas E (1996) Radiological parameters associated with the evolution of degenerative scoliosis. Bull Hosp Joint Dis NY 55(1):40–45
Easwar TR, Hong JY, Yang JH, Suh SW, Modi HN (2011) Does lateral vertebral translation correspond to Cobb angle and relate in the same way to axial vertebral rotation and rib hump index? A radiographic analysis on idiopathic scoliosis. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 20(7):1095–1105. https://doi.org/10.1007/s00586-011-1702-0
Kotwicki T, Napiontek M, Nowakowski A (2006) Transverse plane of apical vertebra of structural thoracic curve: vertebra displacement versus vertebral deformation. Stud Health Technol Inform 123:164–168
Kotwicki T, Napiontek M (2008) Intravertebral deformation in idiopathic scoliosis: a transverse plane computer tomographic study. J Pediatr Orthop 28(2):225–229. https://doi.org/10.1097/BPO.0b013e3181647c4a
Asher MA, Cook LT (1995) The transverse plane evolution of the most common adolescent idiopathic scoliosis deformities. A cross-sectional study of 181 patients. Spine 20(12):1386–1391
Cheung J, Halbertsma JP, Veldhuizen AG, Sluiter WJ, Maurits NM, Cool JC, van Horn JR (2005) A preliminary study on electromyographic analysis of the paraspinal musculature in idiopathic scoliosis. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 14(2):130–137. https://doi.org/10.1007/s00586-004-0780-7
Zetterberg C, Bjork R, Ortengren R, Andersson GB (1984) Electromyography of the paravertebral muscles in idiopathic scoliosis. Measurements of amplitude and spectral changes under load. Acta orthopaedica Scandinavica 55(3):304–309
Zapata KA, Wang-Price SS, Sucato DJ, Dempsey-Robertson M (2015) Ultrasonographic measurements of paraspinal muscle thickness in adolescent idiopathic scoliosis: a comparison and reliability study. Pediatr Phys Ther Off Publ Sect Pediatr Am Phys Therapy Assoc 27(2):119–125. https://doi.org/10.1097/pep.0000000000000131
Murata Y, Takahashi K, Hanaoka E, Utsumi T, Yamagata M, Moriya H (2002) Changes in scoliotic curvature and lordotic angle during the early phase of degenerative lumbar scoliosis. Spine 27(20):2268–2273. https://doi.org/10.1097/01.brs.0000029261.05130.1a
Chun SW, Lim CY, Kim K, Hwang J, Chung SG (2017) The relationships between low back pain and lumbar lordosis: a systematic review and meta-analysis. Spine J Off J North Am Spine Soc 17(8):1180–1191. https://doi.org/10.1016/j.spinee.2017.04.034
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xie, D., Zhang, J., Ding, W. et al. Abnormal change of paravertebral muscle in adult degenerative scoliosis and its association with bony structural parameters. Eur Spine J 28, 1626–1637 (2019). https://doi.org/10.1007/s00586-019-05958-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-019-05958-7