Abstract
Purpose
‘After-hours’ non-elective spinal surgeries are frequently necessary, and often performed under sub-optimal conditions. This study aimed (1) to compare the characteristics of patients undergoing non-elective spine surgery ‘After-hours’ as compared to ‘In-hours’; and (2) to compare the perioperative adverse events (AEs) between those undergoing non-elective spine surgery ‘after-hours’ as compared to ‘in-hours’.
Methods
In this retrospective study of a prospective non-elective spine surgery cohort performed in a quaternary spine center, surgery was defined as ‘in-hours’ if performed between 0700 and 1600 h from Monday to Friday or ‘after-hours’ if more than 50% of the operative time occurred between 1601 and 0659 h, or if performed over the weekend. The association of ‘after-hours’ surgery with AEs, surgical duration, intraoperative estimated blood loss (IOBL), length of stay and in-hospital mortality was analyzed using stepwise multivariate logistic regression.
Results
A total of 1440 patients who underwent non-elective spinal surgery between 2009 and 2013 were included in this study. A total of 664 (46%) procedures were performed ‘after-hours’. Surgical duration and IOBL were similar. About 70% of the patients operated ‘after-hours’ experienced at least one AE compared to 64% for the ‘in-hours’ group (p = 0.016). ‘After-hours’ surgery remained an independent predictor of AEs on multivariate analysis [adjusted OR 1.30, 95% confidence interval (CI) 1.02–1.66, p = 0.034]. In-hospital mortality increased twofold in patients operated ‘after-hours’ (4.4% vs. 2.1%, p = 0.013). This association lost significance on multivariate analysis (adjusted OR 1.99, 95% CI 0.98–4.06, p = 0.056).
Conclusion
Non-elective spine surgery performed ‘after-hours’ is independently associated with increased risk of perioperative adverse events, length of stay and possibly, mortality. Research is needed to determine the specific factors contributing to poorer outcomes with ‘after-hours’ surgery and strategies to minimize this risk.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the Health Care Quality Initiative published by the Institute of Medicine, surgical complications are the second most common cause of preventable mortality and morbidity after medication-related complications [1]. Surgical team fatigue, prolonged working hours and sleep deprivation have been shown to impair surgical performance and increase technical errors in the operating room [2]. In addition, in many centers, ‘after-hours’ surgery is often performed with a perioperative team less familiar with the specific surgical techniques. Procedures requiring complex spinal instrumentation may be hindered by a lack of specialized biomedical, radiographic and implant personnel during the ‘after-hours’ period.
The association between adverse events and surgery performed ‘after-hours’ been reported in anesthesiology and a number of surgical specialties including orthopedic surgery, general surgery, cardiac surgery [3,4,5,6]. Dedicated daytime emergency orthopedic trauma rooms implemented to reduce after-hours surgery have improved operating suite flow and decreased complications [7, 8]. However, the relationship between surgical time of day and postoperative outcomes has been variable in different surgical populations, with other studies demonstrating no relationship [4, 9, 10].
At our institution, ‘after-hours’ surgery is frequently required due to a combination of system and patient factors. When assessed prospectively, spine surgery is associated with a high rate of perioperative adverse events, and we have previously reported an intraoperative and postoperative AE rate for spinal surgery of 10.5 and 73.5%, respectively [11]. However, the relationship between ‘after-hours’ spine surgery and perioperative AEs has never been studied. Given the vulnerability of the spine population and given the complexity of these interventions from an surgical and anesthetic point of view, the relationship between ‘after-hours’ surgery and perioperative AEs and mortality is important information. The timing of spine surgery is a potentially modifiable system factor, with the potential to improve patient outcomes. The objectives of this study were to (1) compare the characteristics of patients undergoing non-elective spine surgery ‘After-hours’ as compared to ‘In-hours’; and (2) to compare the perioperative AEs, surgical duration, IOBL, length of stay (LOS) and in-hospital mortality between those undergoing non-elective spine surgery ‘after-hours’ as compared to ‘in-hours’. We hypothesized that ‘after-hours’ performance of non-elective spine surgery is associated with increased perioperative adverse events, surgical duration, IOBL, LOS and mortality compared to similar cases performed during regular working hours.
Methods
We performed this retrospective study of a prospective cohort with ethics approval from our Institutional Research Ethics Board (H14-03364) with a waiver for informed consent.
Patients and procedures
All consecutive patients who underwent non-elective spine surgery at our institution between January 1st, 2009, to December 31st, 2013, were included in the study. Ours is a Level 1 Trauma Center and quaternary academic teaching center in a major metropolitan center in Canada, with a catchment population of over 4 million people. These patients came from the emergency room or were direct transfer from other peripheral hospitals. Cases were booked by the attending surgeon on a priority basis. At our institution during the period of the study, cases were triaged based on urgency, discussion with the surgeon(s) and time of booking by the in-charge anesthesiologist and nursing team, taking into consideration the urgency of the case as well as operative room access. The date range chosen reflected a period of relatively consistent surgical scheduling practices. Seven fellowship-trained spine surgeons (three neurosurgeons and four orthopedic surgeons), fellows and residents provided surgical care. The attending spine surgeon and anesthesiologist were present at the operation. Between 1600 and 0700 h the nursing, radiology and anesthesiology teams are not specifically subspecialized.
‘After-hours’ versus ‘in-hours’ classification
Patients were identified in two groups: the ‘in-hours’ group defined as surgery occurring between 0700 and 1600 h from Monday to Friday and the ‘after-hours’ group defined as surgery where greater than 50% of the case was performed between 16h01 and 06h59 from Monday to Friday or when surgery occurred at any time during the weekend (Saturday and Sunday). For example, a surgery starting at 14h00 on a Monday and finishing at 20h00 would be classified in the ‘after-hours’ group. This left 9 h in the ‘in-hours’ categery and 15 h in the ‘after-hours’ category. The definition of ‘after-hours’ is somewhat arbitrary and reflect our institution’s practice. At our hospital, from Monday to Friday, 07h00 until 16h00, spine surgery cases are performed with the support of a neuro-anesthesiologist and dedicated nursing and support staff, who are trained in, and experienced in spine surgery. Furthermore, implant/instrument industry representative is usually on site. The percentage of time spent ‘after-hour’ was calculated by dividing the minutes spent ‘after-hours’ (between 16h01 and 6h59 from Monday to Friday) by the total surgical duration.
Predictor variables
Based on diagnosis, surgical cases were grouped as follows:
-
Acute trauma an acute spinal fracture with or without spinal cord injury;
-
Emergent oncology pathologic fracture, epidural cord compression secondary to a neoplasm or acute neurologic deterioration of an intra-dural tumor;
-
Infection acute postoperative surgical site infection or primary spondylodiscitis/osteomyelitis/epidural abscess;
-
Degenerative typically acute disk herniation with nerve root deficit/cauda equina, rapidly progressive cervical myelopathy;
-
Other e.g., baclofen pump malfunction, postoperative epidural hematoma, active and symptomatic cerebrospinal fluid leak.
Age, gender, American Spinal Injury Association (ASIA) Impairment Scale (AIS) grade, neurologic level and specifics regarding surgery (type of approach, level(s) decompressed, instrumented or fused, type of reconstruction, bone graft utilization, operation date, surgical duration) were collected in a prospective standardized fashion. Comorbidity data were generated retrospectively by review of the Electronic Medical Record. The surgical complexity was calculated using the Spine Surgical Invasiveness Index system (SSII). All data elements for the SSII were prospectively collected in a comprehensive ‘operative details’ database [12]. The SSII is an instrument that accounts for the number of vertebral levels decompressed, fused and instrumented, as well as the surgical approach. The score ranges from 0 to 48 points, with a higher score indicating greater surgical invasiveness.
Outcome measures
Our primary outcome was any adverse event (AE). All postoperative outcome data were recorded prospectively using the Spine Adverse Event Severity system, version 2 [SAVES V2]. This validated and reliable tool has been used at our institution since 2008 (‘Appendix 1’ section) [13]. Length of hospital stay, in-hospital mortality, surgery start and end time and estimated blood loss were also collected prospectively.
Statistical analyses
Data were described using mean [standard deviation (SD)], median [interquartile range (IQR)] or percentage, as appropriate. We first compared the characteristics of patients who underwent ‘after-hours’ surgery with ‘in-hours’ surgery, using a Chi-squared test, independent t-test or analysis of variance as appropriate.
We examined the relationship between ‘after-hours’ surgery and our primary outcome of any adverse event. To analyze the relationship between ‘after-hours’ surgery and adverse outcomes, we first compared the patient and procedural characteristics of the populations with and without an adverse event using a Chi-squared test, independent t-test or analysis of variance, as appropriate. Unadjusted odds ratios were generated using logistic regression. We used a stepwise multivariate logistic regression model with backward elimination (with a significance level of p < 0.05 for inclusion in the model), with the variable ‘after-hours’ was forced into the model. Model performance was determined using assessments of discrimination (c-statistic) and calibration (Hosmer–Lemeshow goodness-of-fit test). When designing this study, it was anticipated that long surgeries (with potentially more AEs) would extend into the ‘after-hours’ period and be included in the ‘after-hours’ group. To further explore and validate the relationship between surgical timing and our primary composite outcomes, we performed a secondary analysis using the ‘percentage of time spent after-hours’, instead of the dichotomous variable ‘after-hours’ using a similar logistic regression model.
Other secondary analyses included examining the relationship between ‘after-hours’ surgery with hospital length of stay, and in-hospital mortality. These relationships were analyzed using Poisson regression, and logistic regression for mortality and length of stay, respectively. Finally, we compared intraoperative blood loss between the ‘in-hours’ and ‘after-hours’ groups using a Wilcoxon sign rank test. All data analysis was performed using SAS Version 9.4 (SAS Institute, Cary, North Carolina) and STATA 12.1 (StataCorp, Texas, USA). All tests were two-tailed and a p value < 0.05 was considered statistically significant.
Although we did not perform a formal sample size calculation, we used a prior study from our institution to estimate that the date range chosen would yield a sufficient sample size of approximately 2000 non-elective spine surgery patients with an 87% rate of adverse event [11].
Results
Study population
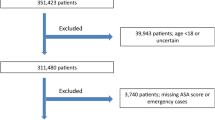
A total of 1440 patients underwent non-elective spine surgery and were included in the final analysis. The study population characteristics are summarized in Table 1. A total of 772 (53.8%) procedures were performed during ‘In hours’ and 664 (46.1%) procedures were performed ‘after-hours.’ Of these, 344 (52%), 180 (27%) and 140 (21%) occurred during weekday evenings or nights, weekend days and weekend evenings or nights, respectively. About 48% of the total operative time occurred in the ‘After-hours’ period. Of those grouped as ‘after-hours’, 96% of the operative time occurred in the after-hours periods. Conversely, only 7% of the operative time in the ‘in-hours’ group occurred in the after-hours period.
Comparison between ‘after-hours’ and ‘in-hours’ patient population
The group of patients who underwent ‘after-hours’ surgery was more likely to be younger, smokers, have concurrent polytrauma or malignancy, and have AIS A and B severities of neurologic impairment. SSII, male sex and other comorbidities were similar between the groups. In our multivariate logistic regression below, we controlled for these baseline differences.
Primary outcomes: any adverse event
A total of 962 AE occurred (overall AE rate of 67%). Urinary tract infection (25%), pneumonia (20%) and electrolyte imbalance (20%) were the most commons postoperative AEs. About 70% of the patients operated ‘after-hours’ experienced at least one AE compared to 64% for the ‘in-hours’ group (p = 0.029). With the exception of pneumonia, dysphagia and neuropathic postoperative pain (Table 2), the incidence of postoperative adverse events was comparable between the two groups.
One hundred ninety-one intraoperative AEs occurred in 168 patients, giving an overall intraoperative AE rate of 12% (Table 3). The mean number of total AEs per patient was greater in the ‘after hours’ group compared to the ‘in-hours’ group [3.0 (SD 5.9) vs. 2.4 (SD 4.4), respectively, p = 0.027]. There was no difference between the ‘after-hours’ and ‘in-hours’ groups with respect to hardware malposition, positioning-related AEs and dural tear.
The unadjusted OR of experiencing any adverse event if surgery occurred ‘after-hours’ was 1.30 (95% CI 1.04–1.60, p = 0.0196) (‘Appendix 2’ section). After stepwise multivariate logistic regression modeling, ‘after-hours’ status was associated with an adjusted OR 1.30 (95% CI 1.02–1.66, p = 0.034) of adverse events, with inclusion of age, SSII, diagnosis and ASIA impairment scale as independent predictors of any adverse event (Table 4). Our final model predicted AEs with good discrimination (c-statistic 0.77) and acceptable calibration (Hosmer–Lemeshow χ2 8.09, p = 0.4251).
When the analysis was repeated using percentage of time spent ‘after-hours’, the results were similar with an OR of 1.03 per 10% increase in time spent ‘After-hours’, (95% CI 1.006–1.06, p = 0.016), after adjusting for age, diagnosis, ASIA impairment scale and SSII. Weekend or weekday designation was not a predictor of AEs (p = 0.666).
Secondary outcomes
Mean length of the surgery was 189 (SD 113) minutes for the ‘after-hours’ and 190 (SD 118) minutes for the ‘in-hours’ group (p = 0.9922). Estimated blood loss was similar, 534 ml (SD 667) and 571 ml (SD 1101) in the ‘after-hours’ and ‘in-hours’ groups, respectively (p = 0.4087).
Hospital length of stay was significantly longer in the ‘after-hours’ group [14 (IQR 7–28) days] compared to the ‘in-hours’ group [13 (IQR 7–24) days]. The category ‘after-hours’ was associated with an increased length of stay (unadjusted coefficient 0.16, 95% CI 0.14–0.18, p < 0.0001). After adjusting multiple confounders, ‘after-hours’ remained a significant predictor of LOS (adjusted coefficient 0.04, 95% CI 0.017–0.063, p = 0.0.001) the model details are provided in ‘Appendix 3’ section.
The overall mortality rate in our cohort was 3.1%. Patients who had their surgery performed ‘after-hours’ were twice as likely to die in hospital as those operated on ‘in-hours’ with a 4.4% mortality rate versus 2.1% (p = 0.013). The unadjusted OR for death was 2.16 (95% CI 1.16–4.02, p = 0.015). On multivariate logistic regression analysis, the association between ‘after-hours’ status and in-hospital mortality was no longer significant (adjusted OR 2.00, 95% CI 0.98–4.05 p = 0.056). Age, ASIA impairment scale and a history of active neoplasm were independent predictors of any adverse event (model details are provided in ‘Appendix 4’ section). Prediction for in-hospital mortality had good discrimination (c-statistic 0.89) and acceptable calibration (Hosmer–Lemeshow χ2 10.39, p = 0.238).
Discussion
This is the first study to investigate the impact of performing non-elective spinal surgery cases during the ‘out of hours’ period on mortality and perioperative complications. Our results demonstrate a significant association between non-elective surgery done ‘after-hours’ and an increase in perioperative adverse events and hospital length of stay. Our findings were robust to different analyses. Our multivariate analysis demonstrated that ‘after-hours’, age, ASIA, SSII and the diagnosis category were predictors of AEs. This means even when controlling for ‘case surgical complexity, neurologic injury, age and diagnosis, ‘after-hours’ surgery was associated with more adverse events than the same complexity of surgery done ‘in hours’. In-hospital mortality was doubled in cases done ‘after-hours’, but this association only achieved borderline significance on multivariate regression analysis. These results are sufficiently compelling to warrant further investigation and raise important questions about the potential effects of surgical timing on patient outcomes in patients requiring non-elective spine surgery.
Although our study is novel in identifying this relationship between AE and surgical timing in spine surgery, this issue has been examined in the broader surgical population. Using the National Anesthesia Clinical Outcomes Registry, Whitlock et al. [14] observed that perioperative mortality was associated with a start time after 16h00. Similarly, increased surgical complications have been reported in general surgery, plastic surgery, orthopedic and maxilla-facial surgery when surgery is performed out of regular hours [6, 15,16,17,18,19,20].
Several factors may explain the relationship between ‘after-hours’ surgery and poorer patient outcomes. Longer fasting periods and induction of catabolic metabolism have been demonstrated following hip fracture in the elderly where surgical delays beyond 48 h and was associated with increased mortality [21]. Another possible explanation for the higher occurrence rate of AEs may relate to the circadian rhythm. In the cardiac surgery literature, percutaneous coronary intervention performed at night is associated with an increased mortality likely due to biological circadian variation [5]. Provider fatigue may also contribute to poorer perioperative outcomes. Among orthopedic surgeons and residents, O’Brien et al. [22] demonstrated that sleep deprivation altered attention, working memory, and concentration, which can lead to intraoperative adverse events. Lastly, patients operated ‘after hours’ are more likely to encounter obstacles in their care pathway with delays/errors due to inexperienced/insufficient nursing or medical staff.
Our study results demonstrated that patients operated ‘after-hours’ were twice as likely to die during their admission compared to patients operated during regular hours. However, this finding did not persist in adjusted analysis. Our results demonstrated that in-hospital mortality was strongly correlated to a history of active malignancy, neurologic injury and age. Interestingly, neither traumatic injury and oncologic indication were predictive of mortality in our population. Our ability to conclusively detect a relationship between ‘after-hours’ status and postoperative mortality was most likely due to the small number of outcomes in our dataset. Furthermore, the small number of outcomes precludes further subgroup analyses, although there are likely groups in which surgical timing has more importance. In the overall spine population, Street et al. [11] reported a mortality rate of 2.1% but over 90% of the deaths reported occurred in the emergent population, mainly in the traumatic spinal cord injury population. With an in-hospital mortality ranging from 6.5 to 7.5% [23], the impact of AEs on mortality in the traumatic spinal cord injury population should not be overlooked.
Adverse events were reported in 67% of the patients. The vast majority could be overlooked as minor AEs, however, even minor AEs can contribute to patient satisfaction, length of stay and cost. Using the same AE collection system on 1 815 patients who underwent spine surgery, Hellsten et al. [24] reported that AEs accounted for $8.38 million of a single institution’s expenses over a 4 years period, with grade 1 and 2 AEs representing 43% of the aggregate cost. Pulmonary complications, our second most frequent AE, have been associated with a major increase in the cost of care by Whitmore et al. [25] ($7233 per complication). Lastly, at our institution, Street et al. [26] demonstrated an increased length of stay associated with decubitus ulcers, delirium, pneumonias and urinary tract infection. These data emphasize the potential significance of even minor AEs.
There are inherent limitations to this study. First, our definition of the ‘after-hours’ designation is arbitrary, as no clear definition exists although our definition of after-hours was consistent with multiple prior publications [6, 14, 17, 18, 20, 27]. In addition, the characteristics of the two groups were different and, although we adjusted for many confounders in our analysis, residual confounding was possible due to unmeasured confounders. For example, the use of SII to control for surgical complexity may have not completely accounted for all aspects of complexity and led to residual confounding. Furthermore, due to a low incidence and possibly lack of statistical power, we were not able to detect difference between the ‘after-hours’ and ‘in-hours’ groups in the occurrence of any specific intraoperative AEs nor are we able to determine the precise reasons for the increase in adverse events after hours. Similarly, our results do not answer the question of whether modifying surgical timing will result in improved outcomes. In addition, due to the retrospective nature of the study, we were unable to report patient-reported outcomes, which would have added to the importance of our findings. Finally, our data represents patterns of practice and a patient population from only a single academic center and may not be reflective of all institutions or practices. We believe the patient population and spectrum of spine pathology served at our institution likely reflects many other tertiary referral hospitals.
‘After-hours’ surgery is a common practice among spinal surgeons and many reasons dictate surgical timing, such as pathologies with acute neurological deterioration/deficit that require rapid intervention to optimize neurologic recovery. At our institution, limited access to the operative room is an issue. In this study, 46% of the cases were performed ‘after-hours’ with half of these done during weeknight/evenings. About 53.89% of the cases performed ‘after-hours’ were neurologically intact or had a nerve root injury. Therefore, we can assume that a significant percentage of the surgeries that were performed ‘after-hours’ could have been done in the regular daytime hours and potentially resulted in better patient outcomes. The risk of operating ‘after-hours’ and the pathologically driven urgency of a case should be weighted when booking a spinal emergency to the operative room. The lack of access to ‘In-hours’ surgery time, and the associated delays in access, has myriad implications for patient care. Delays can lead to prolonged fasting of patients, omission of normal medications, and often prolonged immobilization in ‘spinal precautions’, which can result in pulmonary atelectasis among other consequences. The interrelationship between all these factors remains speculative and beyond the scope of this study. However, we believe that our results provide a foundation for further study into this challenging but important subject. Dedicated, daytime operating room time for spine surgery is a potential solution to improve patient outcomes in high volume centers.
Lastly, there are many other intangible aspects of performing ‘after-hours’ surgery that was not captured in this study. A study published in 2014 demonstrated that there was a significant increase in cost for open tibial fracture operated ‘after-hours’ [28]. This association has never been studied in spine surgery. ‘After-hours’ surgery is potentially associated with additional resource consumption, cost and detrimental to health care professionals. At some centers, operative room professionals are a scarce resource and over-utilization of these resources ‘after-hours’ may lead to team fatigue and apathy. Limitation in ‘after-hour’ surgery in general with increased daytime operating room access could result in wiser spending and better staff morale.
Conclusion
Non-elective spine surgery performed outside the usual daytime operating hours is associated with increased perioperative adverse events and hospital length of stay, even after adjusting for potential confounders. Unadjusted mortality doubles with ‘after-hours’ surgery although this finding was not robust on adjusted analysis. Our findings raise important questions about the timing of emergency spine surgery in high volume centers.
References
Hendee WR (2001) To err is human: building a safer health system. J Vasc Interv Radiol 12:P112–P113. https://doi.org/10.1016/S1051-0443(01)70072-3
Eastridge BJ, Hamilton EC, O’Keefe GE et al (2003) Effect of sleep deprivation on the performance of simulated laparoscopic surgical skill. Am J Surg 186:169–174. https://doi.org/10.1016/S0002-9610(03)00183-1
Aya AGM, Mangin R, Robert C et al (1999) Increased risk of unintentional Durai puncture in night-time obstetric epidural anesthesia. Can J Anesth/J Can d’anesth 46:665–669. https://doi.org/10.1007/BF03013955
Lonze BE, Parsikia A, Feyssa EL et al (2010) Operative start times and complications after liver transplantation. Am J Transplant 10:1842–1849. https://doi.org/10.1111/j.1600-6143.2010.03177.x
Lairez O, Roncalli J, Carrié D et al (2009) Relationship between time of day, day of the week and in-hospital mortality in patients undergoing emergency percutaneous coronary intervention. Arch Cardiovasc Dis 102:811–820. https://doi.org/10.1016/j.acvd.2009.09.010
Ricci WM, Gallagher B, Brandt A et al (2009) Is after-hours orthopaedic surgery associated with adverse outcomes? J Bone Joint Surg Am 91:2067–2072. https://doi.org/10.2106/JBJS.H.00661
Roberts TT, Vanushkina M, Khasnavis S et al (2015) Dedicated orthopaedic operating rooms. J Orthop Trauma 29:e18–e23. https://doi.org/10.1097/BOT.0000000000000154
Bhattacharyya T, Vrahas MS, Morrison SM et al (2006) The value of the dedicated orthopaedic trauma operating room. J Trauma Inj Infect Crit Care 60:1336–1341. https://doi.org/10.1097/01.ta.0000220428.91423.78
Araujo RLC, Karkar AM, Allen PJ et al (2014) Timing of elective surgery as a perioperative outcome variable: analysis of pancreaticoduodenectomy. HPB (Oxford) 16:250–262. https://doi.org/10.1111/hpb.12107
Switzer JA, Bennett RE, Wright DM et al (2013) Surgical time of day does not affect outcome following hip fracture fixation. Geriatr Orthop Surg Rehabil 4:109–116. https://doi.org/10.1177/2151458513518344
Street JT, Lenehan BJ, DiPaola CP et al (2012) Morbidity and mortality of major adult spinal surgery. A prospective cohort analysis of 942 consecutive patients. Spine J 12:22–34. https://doi.org/10.1016/j.spinee.2011.12.003
Mirza SK, Deyo RA, Heagerty PJ et al (2008) Development of an index to characterize the “invasiveness” of spine surgery: validation by comparison to blood loss and operative time. Spine 33:2651–2661. https://doi.org/10.1097/brs.0b013e31818dad07 (Discussion 2662)
Rampersaud YR, Neary MA, White K (2010) Spine adverse events severity system: content validation and interobserver reliability assessment. Spine 35:790–795. https://doi.org/10.1097/BRS.0b013e3181bf25a3
Whitlock EL, Feiner JR, Chen L-L (2015) Perioperative mortality, 2010–2014: a retrospective cohort study using the national anesthesia clinical outcomes registry. Anesthesiology 123:1312–1321. https://doi.org/10.1097/ALN.0000000000000882
Phatak UR, Chan WM, Lew DF et al (2014) Is nighttime the right time? risk of complications after laparoscopic cholecystectomy at night. J Am Coll Surg 219:718–724. https://doi.org/10.1016/j.jamcollsurg.2014.05.009
Kelz RR, Tran TT, Hosokawa P et al (2009) Time-of-day effects on surgical outcomes in the private sector: a retrospective cohort study. J Am Coll Surg 209:434.e2–445.e2. https://doi.org/10.1016/j.jamcollsurg.2009.05.022
Bertram A, Hyam D, Hapangama N (2013) Out-of-hours maxillofacial trauma surgery: a risk factor for complications? Int J Oral Maxillofac Surg 42:214–217. https://doi.org/10.1016/j.ijom.2012.11.001
Lee K-T, Mun G-H (2013) Is after-hours free-flap surgery associated with adverse outcomes? J Plast Reconstr Aesthet Surg 66:460–466. https://doi.org/10.1016/j.bjps.2012.12.007
Vimalesvaran S, Ayis S, Krasemann T (2013) Balloon atrial septostomy performed “out-of-hours”: effects on the outcome. Cardiol Young 23:61–67. https://doi.org/10.1017/S1047951112000364
Yeung A, Butterworth SA (2015) A comparison of surgical outcomes between in-hours and after-hours tracheoesophageal fistula repairs. J Pediatr Surg 50:805–808. https://doi.org/10.1016/j.jpedsurg.2015.02.036
Shiga T, Wajima Z, Ohe Y (2008) Is operative delay associated with increased mortality of hip fracture patients? systematic review, meta-analysis, and meta-regression. Can J Anesth/J Can d’anesth 55:146–154. https://doi.org/10.1007/BF03016088
O’Brien MJ, O’Toole RV, Newell MZ et al (2012) Does sleep deprivation impair orthopaedic surgeons’ cognitive and psychomotor performance? J Bone Joint Surg Am 94:1975–1981. https://doi.org/10.2106/JBJS.K.00958
Jain NB, Ayers GD, Peterson EN et al (2015) Traumatic spinal cord injury in the United States, 1993–2012. JAMA 313:2236–2243. https://doi.org/10.1001/jama.2015.6250
Hellsten EK, Hanbidge MA, Manos AN et al (2013) An economic evaluation of perioperative adverse events associated with spinal surgery. Spine J 13:44–53. https://doi.org/10.1016/j.spinee.2013.01.003
Whitmore RG, Stephen J, Stein SC et al (2012) Patient comorbidities and complications after spinal surgery: a societal-based cost analysis. Spine 37:1065–1071. https://doi.org/10.1097/BRS.0b013e31823da22d
Street JT, Noonan VK, Cheung A et al (2015) Incidence of acute care adverse events and long-term health-related quality of life in patients with TSCI. Spine J 15:923–932. https://doi.org/10.1016/j.spinee.2013.06.051
Forbes C, Butterworth SA (2014) Perioperative outcomes of primary renal tumour resections: comparison of in-hours to out-of-hours surgery. Pediatr Surg Int 30:1003–1007. https://doi.org/10.1007/s00383-014-3560-4
Schenker ML, Ahn J, Donegan D et al (2014) The cost of after-hours operative debridement of open tibia fractures. J Orthop Trauma 28:626–631. https://doi.org/10.1097/BOT.0000000000000078
Acknowledgements
We are grateful to the local clinical research personnel, support staff and medical students for their active participation: Juliet Batke, Leilani Reichl, Allan Aludino Angela Tsang, Lise Bélanger, Leanna Ritchie, Eryck Moskven and Samuel Vijayan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has relevant conflicts of interest related to this work. Dr. Fisher and Dr. Dvorak have received royalties from Medtronic. Dr. Fisher and Dvorak have received consulting fees from Medtronic. Dr. Fisher has received consulting fees from Nuvasive. Dr. Street and Dr. Fisher received an OREF Grant paid to the institution and AOSPINE provides a fellowship support paid to the institution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Charest-Morin, R., Flexman, A.M., Bond, M. et al. ‘After-hours’ non-elective spine surgery is associated with increased perioperative adverse events in a quaternary center. Eur Spine J 28, 817–828 (2019). https://doi.org/10.1007/s00586-018-5848-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-018-5848-x