Abstract
Background
Mobile health (mHealth) is emerging as the most convenient way to deliver rehabilitation services remotely, and collect outcomes in real time, thus contributing to disease management by transferring care from hospital to home. It facilitates accessibility to healthcare, enhances patients’ understanding of their condition, and their willingness to engage in self-management, giving way to high-quality care to the satisfaction of both patients and healthcare professionals.
Purpose
The purpose of this study was to examine the effect of using a smartphone app (called Snapcare) on pain and function in patients suffering from chronic low back pain.
Methods
Ninety-three patients with chronic low back pain were recruited and randomly allocated to either the Conventional group (n = 48) receiving a written prescription from the Physician, containing a list of prescribed medicines and dosages, and stating the recommended level of physical activity (including home exercises) or the App group (n = 45) receiving Snapcare, in addition to the written prescription. Pain and disability were assessed at baseline and after 12 weeks of treatment.
Results
Both the groups showed significant improvement in pain and disability (p < 0.05). The App group showed a significantly greater decline in disability (p < 0.001).
Conclusion
Health applications are promising tools for improving outcomes in patients suffering from various chronic conditions. Snapcare facilitated increase in physical activity and brought about clinically meaningful improvements in pain and disability in patients with chronic low back pain.
Graphical abstract
These slides can be retrieved under Electronic Supplementary Material.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low back pain (LBP) is considered a major health problem due to its high prevalence [1, 2], high probability of recurrence [3], and associated disability [4]. It is the leading cause of activity limitation and work absence throughout much of the world, imposing a high economic burden on individuals, families, communities, industry, and governments [5, 6]. Low back pain which lasts for more than 3 months is defined chronic low back pain (CLBP) [7], and accounts for 75% to 90% of the societal costs of back pain [8]. An analysis of the Global Burden of Disease Study 2010 revealed that LBP has been the leading contributor to overall YLDs (Years Lived with Disability), through the past decade [9], with CLBP as one of the primary reasons for work loss, healthcare use, and disability benefits [10,11,12]. CLBP causes disability by reducing a patient’s ability to keep up with Activities of Daily Living (ADLs) [13, 14]. Patients are usually unable to undertake employment [15], and spend a large proportion of their time lying down [16, 17]. They also go through significant emotional despair [18, 19], and become restricted in their social behavior [20]. Reduced activity as measured by numerous life function and disability scales, and significant levels of physical deconditioning, have been documented in this population [21,22,23,24,25,26,27,28]. Along with the considerable impact of CLBP on daily functioning [11], it has been observed that while improvements occur in sleep disturbance and psychological distress after treatment for CLBP; sitting, standing, and lifting still remain notable problems despite treatment [29].
Intensive rehabilitation programs for patients with CLBP incorporate progressive training in endurance, flexibility, and strength in order to reverse deconditioning and improve life function [30]. During the last 15 years, numerous studies have concluded that exercise helps alleviate chronic low back pain [31,32,33,34,35,36,37] and CLBP patients who engage in adequate levels of physical activity have better prognosis in terms of pain, disability, and quality of life [38, 39]. Despite the benefits, adherence to exercise programs is often suboptimal, with dropout rates ranging from 10 to 36% [40]. Follow-up data suggests that from one-third to two-thirds of patients are noncompliant with exercises [41]. This result is particularly true regarding unsupervised exercise at home [42,43,44]. It has also been proposed that many recurrent cases of low back pain could have been avoided if patients had adhered to their home exercise programs [45, 46]. Geographical and transportation barriers, socio-economic factors, and financial constraints might be important determinants of this non-adherence [47]. The most commonly cited reasons are the lack of time to exercise, and the inability to fit the exercises into their daily routine [48]. It is thus important to look for alternative models of health service delivery that could better meet patients’ preferences, and in so doing, enhance exercise treatment compliance [49].
mHealth is an innovative way to approach healthcare; it involves use of the core utility of mobile phones, along with their more complex applications and functionalities [50]. The advantage of using mobile technology for healthcare is that mobile devices are personal, and thus constantly accessible to patients [51, 52]. Since mobile devices today integrate features that previously researchers needed to specifically incorporate into these devices to allow their use for monitoring and other healthcare purposes [53], mHealth is emerging as the most convenient way to deliver rehabilitation services remotely, and collect outcomes in real time, thus contributing to disease management by transferring care from hospital to home. Moreover, the proliferation of smartphones, has culminated in an abundance of healthcare applications which enable patients to self-monitor their health data and share it with their healthcare professionals, thus reducing healthcare consumption and cost [54,55,56]. Clearly, the deployment of mHealth facilitates accessibility to healthcare, enhances patients’ understanding of their condition, and their willingness to engage in self-management, giving way to high-quality care to the satisfaction of both patients and care professionals.
The Snapcare App is one such platform designed to monitor patient’s daily activity levels and symptomatic profile. The smartphone application disseminates personalized care in terms of activity goals and home exercise program based on patient’s baseline health data and pain levels after each activity session. These goals are advanced based on patient’s comfort level with physical activity and exercise at various intervals, which are gauged via Patient Reported Outcome Measures (PROMs) and exercise data collected via the app. Enough evidence exists to establish that use of mobile technology effectively promotes certain desired behaviors including physical activity [57]. However, to the best of our knowledge, there exist no studies as yet which focused on reducing disability by improving physical activity and exercise adherence in CLBP patients, using a smartphone-based intervention. Hence, the purpose of this single blind RCT was to evaluate the effect of using Snapcare on pain and function in patients suffering from CLBP.
Methods
Study design
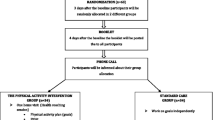
We conducted a single-blinded RCT (with blinding of the outcome assessor) to evaluate the efficacy of a patient-centered smartphone-based intervention (in addition to standard written prescription), in reducing pain and disability, as compared to standard written treatment prescription provided by the Physician. This trial has been designed according to the CONsolidated Standards of Reporting Trials (CONSORT) statement [58] and is reported according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) statement [59] (Fig. 1).
Participants
We recruited 93 patients with chronic LBP from the outpatient Spine Department in a private hospital in New Delhi, India, between September 2016 and December 2016. Consenting participants were randomly allocated to either the Conventional group (n = 48) and received a written prescription from the Physician, containing a list of prescribed medicines and dosages, and stating the recommended level of physical activity (including home exercises), or the App group (n = 45) and received Snapcare, in addition to the written prescription.
The number of subjects was determined using Software G. Power 3.192. An effect size of 0.37 was obtained from the data relative to changes in Oswestry Disability Index (ODI) in a study by Multani et al. [60], which examined the effects of telemedicine services integrated with physiotherapeutic care on pain, muscle strength, and function in patients with chronic LBP. The effect size was calculated using t tests (difference between two dependent means). A prior analysis of sample size using F-tests (ANOVA: Repeated measures, within-between interaction) showed that the total sample size required would be 79 to have 90% power at effect size of 0.37 and alpha level of 0.05. Considering a dropout rate of 12%, the total sample size obtained was 90, or 45 subjects per group.
Inclusion criteria
Adults over 18 years of age with mechanical LBP persisting for over 12 weeks with or without radicular symptoms and undergoing treatment for the same, who were prescribed at least some level of daily physical activity (including home exercises), along with regular medicines, reported consistent pain (at least 5 in the Numerical Rating Scale), and with regular use of an Android mobile device with internet access and fluency in English (verbal and written) were recruited for the study.
Exclusion criteria
Participants with any history of spinal surgery, tumor, infection, systemic rheumatological disease, ankylosing spondylitis; evidence of neuromuscular disorders, cardiorespiratory illnesses, moderate to severe cognitive impairment; active cancer therapy in the past 12 months; any major surgery in the last 3 months; non-ambulatory (wheelchair bound) or any health conditions limiting mobility and preventing active participation in physical activities; inability to comply with study requirements per investigator’s judgment, were excluded.
Recruitment method
Healthcare providers screened (all) potential participants from the outpatient Spine Department of the Indian Spinal Injuries Centre, New Delhi, India, and informed them about the study. Potential participants interested in participating in the study received a Participant Information Sheet and were referred to the clinical research team. Patients with CLBP who were prescribed conservative treatment (including medicines, physical activity, and home exercises) and met the inclusion criteria were invited to participate in the trial, post Physician encounter. A research assistant then discussed the study purpose, methodology, and possible risks of the study, and offered participation to those patients. If they agreed to participate, written consent was obtained, and baseline data collected.
The study was approved by the Institutional Ethical Committee, ISIC. All procedures followed were in accordance with the institutional ethical standards for human experimentation and with the Helsinki Declaration.
Group allocation
Random allocation to App or Conventional group was done after confirming eligibility and baseline assessment. Allocation was blinded and performed using a computer-generated random allocation schedule operated by a remote researcher. The allocation of participants was concealed by assigning a number to each subject. All identifying information on consent forms, along with demographic history, was kept confidential.
Procedures
Potential participants undergoing treatment for CLBP at outpatient Spine Department, ISIC, New Delhi, India, were screened by their healthcare providers to determine eligibility, inform about the trial objectives, and invite participation. Patients who expressed interest were given a Participant Information Sheet to decide if they wanted to participate in the study. A clinical researcher was responsible for booking potential participants by explaining all study relevant details and receive informed consent from patients that agreed to participate. A research assistant then proceeded to collect demographic and baseline data related to the study outcomes. Snapcare was given to all participants in the App group at baseline with clear instructions for use. Participants were informed that their access to Snapcare App will be deactivated at the end of the study period. A record of the provider’s prescription, specific to each participant, was obtained and personalized activity goals were generated in accordance with the baseline data collected. The app collected daily activity data (distance walked and exercises performed) over a 12-weeks period, and displayed day-to-day variation in activity levels. Patients received notifications and surveys, triggered based on analysis of physical activity data captured through inbuilt sensors and patients’ use of the app. This data was automatically synced to a secure server where machine learning algorithms analyzed the daily physical activity data and generated recommendations for next day’s session. Most study participants comprised of patients visiting from different states and distant locations who relied on remote contact with the physician office after initial consultation. Owing to these accessibility constraints, the follow-up data could not be recorded in person. Therefore, at the end of 12 weeks, a telephone interview was conducted for both groups to assess the treatment outcomes (pain and disability). Outcome data was extracted and forwarded for analysis to a research assistant who remained blinded to group assignment throughout the trial.
Interventions
The App group received Snapcare, along with a regular written prescription from the doctor. Through the Snapcare App, the patients received daily activity goals (including back and aerobic exercises), which were developed based on their health status, ADLs, and daily activity progress. Participants were also advised to continue with their medicines as usual. The app intervention was aimed at motivating, promoting, and guiding the participants to increase their level of physical activity and exercise adherence.
Snapcare addressed the following
-
1.
Increase in physical activity: Participants were guided in increasing their physical activity level in a way that suited their individual lifestyle preferences. The World Health Organization (WHO) in its 2010 Global Recommendations on Physical Activity for Health, recommends 150 min of moderate-intensity or 75 min of vigorous-intensity aerobic physical activity, or an equivalent combination of both throughout the week [61].
For the App group, daily achievable physical activity goals (including home exercises) were set, as against a general long-term goal of 4 km daily walk in a single stretch and 2 sets daily of 7 back exercises which were set for all patients by the advising physician. Goals were set in a way that increase in physical activity was gradual. Activity goals consisted of aerobic exercises (walking/running), and a set of home exercises customized according to each individual participant’s health, nature of LBP, and functional status at baseline. The goals were modified and adjusted based on participants’ daily physical activity performance and functional progress. Performance against goals was monitored, and intelligent reinforcement provided via auto-generated app notifications and reminders prompted by data aberrations. Goal attainment was assessed by comparing the records of actual daily physical activity with the target set. This information was also available to the patients at the end of each session of aerobic and home exercises.
-
2.
Improvement in function: Each day, participants were encouraged to try and perform as much of their routine activities independently as possible. The aim was to see their progress toward normality in terms of performing basic tasks such as walking, sitting, standing, and self-care activities, without pain. The ultimate goal of the whole intervention program was to reverse disability or at least prevent further disability in patients, by making them more active and mobile.
-
3.
Increase in engagement and compliance: Despite the numerous benefits of physical activity (including home exercises), patients’ physical activity adherence level is often suboptimal [62]. The most common factors cited for noncompliance include lack of time, and the inability to fit physical activity into daily routine. It has also been shown that CLBP patients tend to avoid activities believed to produce discomfort. To modify this behavior, Snapcare used gamification to increase engagement as well as compliance with prescribed activity plan, through a system of rewards for each action completed and every milestone achieved. The prescribed home exercise plan was broken into Levels and Stages (See “Appendix 1”); each Level consisting of 3 Stages. Patients could access next Level exercises only when they had successfully completed all Stages of the previous Level. This introduced an aspect of gamification, which helped keep patients excited about home exercises. With each action that patients completed, they were given reward points. Snapcare’s user interface was aesthetically designed in a way that it served to generate a sense of instant gratification that kept the patients engaged (See “Appendix 2”).
Outcomes
Primary outcomes, i.e., pain and disability for both the groups were collected at baseline and at the end of 12 weeks, using Numeric Pain Rating Scale (NPRS) and Modified Oswestry Disability Index (MODI), respectively. The modified version developed by Fritz and Irrgang [63] comprises of a section regarding employment/home-making ability substituted for the section related to sex life because the sex life item is frequently found to be left blank. MODI comprises of 10 items and each item is scored from 0 to 5 where higher score represents greater disability. The total score is multiplied by two and expressed as a percentage.
In addition, the App group was also assessed for secondary outcomes including their daily physical activity (distance measured through an activity tracker built within the app) and progress in symptoms through the CSS (Current Symptom Score). The CSS was obtained via administration of a self-reported questionnaire, created by a research team specifically for this study (Table 1) as the available tools were either limited in value, measuring just one aspect of the condition (LBA: Roland-Morris Disability Questionnaire, Quebec Back Pain Disability Scale; Sleep: Pittsburg Sleep Quality Index; Mood: BDI; Quality of life: SF-36, etc.), or were too lengthy and technical to warrant daily engagement. Hence, the need to develop a tool which was easy to comprehend, took under 2 min to fill, and yet provided relevant information about the various aspects of patient’s daily progress (pain, sleep, mood, mobility, and function). CSS was developed and pilot tested on 20 patients to gauge the effect of backache on patients’ overall well-being.
Data analyses
The Intension-To-Treat (ITT) principle was used for analyses which included all subjects who were randomized to the study and used the app for at least a week. The baseline values of the participants lost to follow-up were carried forward to replace their missing values at subsequent measurement. Data were assessed by Kolmogorov–Smirnov test for the normality of the distribution scores. The demographic characteristics and the baseline criterion measures were compared between the Conventional group and the App group at the study entry by an independent t test. A 2 × 2 mixed model ANOVA was employed to assess the difference between groups (Conventional, App), time (baseline and post-12 weeks of intervention) and interaction effect (Group X Time). To account for the difference in scores between the 2 groups at baseline, ANCOVA was used with the pre-values taken as covariates. The change in the CSS and activity level in the App group, following 12-weeks of app usage, was examined using paired t-test. Significance level was set at p < 0.05.
Results
There was no significant difference for the demographic characteristics between the groups (Table 2). The App group showed slightly greater disability score at baseline.
Pain
A 2 × 2 mixed model ANOVA yielded a main effect for time, F (1, 90) = 148.8, p < 0.001. However, the main effect of group was non-significant, F (1, 90) = 1.443, p = 0.233 as was the interaction effect F (1, 90) = 0.84, p = 0.362, indicating that although both groups showed a significant decrease in the pain score at 12 weeks, there was no significant difference between the Conventional and App groups (Table 3; Fig. 2).
Disability
The MODI scores at baseline were found to be significantly different between the groups. Therefore, ANCOVA was used with the baseline score set as the covariate. A 2 × 2 mixed model ANCOVA yielded a main effect for time, F (1, 90) = 4.739, p = 0.032 and a significant interaction effect F (1, 90) = 9.053, p = 0.003, indicating that although both groups recorded a decline in disability index, the drop for the App group was significantly greater (Table 3; Fig. 3).
Symptom score and physical activity
The paired t-test was used to assess the change in symptom scores and activity levels in the App group, from baseline to 12-weeks of app usage. The results indicated a significant drop in the CSS with an improvement in each of its components (p < 0.05). The improved general mobility was also reflected in the daily distance walked by the patients which gradually approached the physician’s recommended target, i.e., 4 km/day (Table 4). Table 5 elucidates the frequency distribution of respondents for CSS subscales.
Discussion
CLBP is one of the primary reasons for work loss, healthcare use, and disability benefits, and is known to have a profound impact on a patient’s life by causing disability and reducing their functional capacities [10,11,12, 64]. Further, even though physical activity and exercise therapy are an integral part of musculoskeletal rehabilitation in LBP, adherence to home exercises and engaging in routine physical activity remains a problem for patients [62]. Since mobile devices are personal and constantly accessible to patients, the use of mHealth, specifically smartphone applications, is considered a potentially useful way for patients to monitor their condition, as it enhances patients’ ability and their willingness to engage in self-management, thus improving treatment compliance [65].The purpose of the present study was to reduce disability in CLBP patients, by offering them structured daily activity goals aimed at gradually increasing their functional capacity.
The present study is the first to examine the impact of mHealth interventions on treatment outcomes in CLBP patients. Our results were consistent with similar investigations that examined the impact of mHealth in other chronic conditions and showed a positive impact on condition related outcomes [66,67,68,69,70]. The study used pain intensity and disability as the treatment outcomes and follow-up data clearly revealed marked improvement in both the indices after 12 weeks of treatment. Moreover, the App group demonstrated a significant decline in disability index, when compared to conventional prescription.
To date, a number of studies have examined the efficacy of mHealth interventions in improving clinical outcomes in several chronic diseases. A systematic review [66] investigated the impact of mHealth for chronic disease management on treatment adherence and patient outcomes. Of the 27 studies that measured impact on treatment adherence, 15 studies reported significant improvements, while 16 of the 41 RCTs that measured disease-specific clinical outcomes, found differences between groups on account of mHealth intervention. However, most of these studies focus on chronic conditions such as chronic obstructive pulmonary disease (COPD), diabetes, and asthma. Beratarrechea et al., [67] reviewed 163 articles, including 9 randomized controlled trials, to study the impact of mHealth interventions on chronic disease outcomes and found that four (2 diabetes, 1 heart disease, and 1 asthma) out of five trials reported that mobile interventions improved outcomes—improved glycemic control was reported in diabetic patients; a study of usual heart failure showed improvement in symptoms, physical impairment, functional capacity, and quality of life; while improved asthma control was reported owing to transmission of daily spirometry readings and feedback from physician. Williams et al., [68] showed that telehealth interventions supported self-monitoring behavior in COPD patients. Kirwan et al. [69] examined the efficacy of a diabetes smartphone application in improving glycemic control and other diabetes-related outcomes and reported significant improvements in HbA1c levels from baseline. A recent review [70] of 12 RCTs revealed that use of app-based interventions that support diabetes self-support, yields a clinically significant HbA1c reduction among adult population with diabetes, especially type 2 diabetes.
Interventions for CLBP at present focus on activity and level of functioning, with particular focus on limitations and restrictions in the performance of ADLs [11]. Although function can be assessed using various instruments, self-reported outcomes reveal patient’s own perception of limitations [71], and so these patient-based outcome measures are gaining importance as indicators of physical function [13, 71]. The modified version of ODI used in the present study has demonstrated excellent reliability (ICC = 0.84–0.92) and validity in previous psychometric analyses [63, 72]. While the normative score for ODI is estimated to be 8.73 [73], a normative value for MODI has not been reported. Also, Tonosu et al. [73] observed that ODI of the LBP group with and without disability were 22.07 and 11.88, respectively. Using ROC curve, they estimated the optimal cut-off to be 12 points. For MODI, Hicks and Manal [74] observed the scores of patients with and without high functional limitation in chronic LBP to be 41.7 and 24.2 points, respectively. In accordance with the findings of Hicks and Manal, both the groups demonstrated restricted function at baseline. After 12 weeks, the disability scores as assessed by MODI, were found to be significantly reduced in both the groups, with a difference of 12 points recorded in the conventional group and 30 points in the app group. However, the presence of statistical significance does not attest to the clinical importance of the change. Therefore, it becomes imperative to consider the minimal clinically important difference (MCID) which is defined as the smallest change that best distinguishes the patients who have improved and those remaining stable. Knowledge of the MCID enables clinicians to determine whether the patient has actually improved an amount that is likely to be perceived as important to the patient. The MCID reported for MODI by previous studies range from 10.66 to 15 points [63, 72, 74]. The present study recorded a difference of 30 points in MODI scores post-intervention deeming this change clinically significant.
It has further been asserted [75] that sedentary individuals should gradually move to continuous bouts of activity, beginning only with shorter bouts of activity. In addition, ACSM/AHA currently recommend [76] a minimum of 30 min moderate-intensity aerobic activity on 5 days each week or a minimum of 20 min vigorous-intensity activity 3 days per week, which can be accumulated in sessions of 10 min or more. In line with these guidelines, patients who were found to be deconditioned to physical activity were started off with 10 min of activity (walking), which was gradually increased depending on patients’ comfort and adaptation. Further, the results of other trials and reviews [77, 78] suggest that providing incremental real-time feedback during progress toward a goal, along with the use of activity monitors, helps patients adjust their behavior in time to make better progress, and thus may be more helpful compared to traditional feedback. Use of such activity monitors has been shown to improve level of physical activity, thus enhancing improvement in function and decrease in pain in CLBP patients [79]. In the present study, not only were physical activity goals set based on individual’s health and daily progress in function, but real-time feedback was also provided through graphs and CSS scores (See “Appendix 3”); in-app activity monitors were also used to keep patients motivated to improve physical function and to enable them to adapt to improved levels of physical activity faster (See “Appendix 4”).
The finding that increased physical activity is associated with improvement in functional capacity is consistent with other studies. Durstine et al., [80] suggested that physical activity improves physical functioning and psychological well-being compromised in people due to poor health, and that people with chronic diseases and disabilities can derive similar benefits from increase in physical activity. This claim was corroborated by results from clinical trials [81, 82] among older persons, which demonstrated that exercise programs improve aerobic capacity, walking speed, muscle strength, and self-reported functional scores. In 2003, Keysor [82] reviewed the existing scientific evidence on the impact of late-life physical activity and exercise on functional limitations and disability, and concluded that physical exercise, especially walking, increases muscle strength and aerobic capacity and reduces functional limitations. Another review of literature undertaken in 2009 [75], provided evidence that not only did aerobic exercises have a positive effect on endurance, which transferred to daily activities such as walking, stair-climbing, and sit-to-stand, but also resulted in moderate improvements in disability, overall physical function, and pain. This deduction was further substantiated by Gretebeck et al. [83] and Pahor et al. [84] who established that among those with existing disability, physical activity and exercise programs increased functional ability, and are effective in reducing disability even among vulnerable older people suffering functional decline in late life. The increase in physical activity of CLBP patients, brought on by Snapcare, not only improved daily functioning (ADLs as reported via CSS questionnaire), but also produced significant decline in their existing disability. The results of the current study indicated a significant improvement in patients’ daily reported symptoms (CSS) with a drop in each of its components. The percentage of respondents that reported poor sleep reduced from 33 to 4%, those reporting poor mood decreased from 31 to 9%, and restricted physical activity was reported by only 4% respondents after 12 weeks as opposed to 64% at baseline. Among the ADLs, lifting, sitting, and prolonged standing were found to be the most frequently restricted. While an improvement in overall function was observed, lifting was reported to be difficult by a large number of responders (80%) even after 12 weeks of intervention. Premises already laid down by various researchers explain this effect of increase in physical activity on ADLs and disability index in patients with CLBP.
The increase in physical activity levels of the App group can be attributed to the many unique features offered by Snapcare. The application and the intervention strategy were designed keeping in mind the limitations of other healthcare applications as well as suggestions for better results, outlined in various researches. Anderson et al., [85] investigated consumers’ use of apps for health monitoring and perceived benefits, and based on their exhaustive analysis, provided suggestions for improvement of healthcare apps. Their research indicated that persistence with self-monitoring is quite poor in patients suffering from chronic conditions, and self-management through an application depends upon constant stimulation which can be achieved via use of gamification and rewards for achieved milestones [86]. These suggestions were taken into account and functionalities like gamification and reward points were seamlessly integrated into the intervention process (See “Appendix 2”). This engaging design and interface helped build up engagement, sustained usage, and ultimately compliance.
Moreover, Snapcare emphasized dissemination of individualized activity goals. As suggested by Durstine et al., [80] although principles for exercise prescription for people with disability are scientific information based, expected adaptations observed in a general population may not apply to other populations. Generalized goals do not carry equal weight when it comes to outcome improvement, since patient interests, health needs, clinical status, and level of disability are subjective; so while guidelines can be used as starting points, particular exercise mode, intensity, frequency, and duration should be modified keeping in mind this subjectivity.
Limitations
The present study had some limitations. Daily activity and functional progress data was collected through the Snapcare App, and so this data was available only for the App group, since without the application there was no way of collecting the same for the Conventional group. In addition, owing to accessibility constraints, the follow-up data for both groups was collected through telephonic interview and this difference in mode of administration of questionnaire at baseline and post-intervention may be a confounder. There is a chance that factors other than increase in physical activity affected the disability index of the participants. One factor could be adherence to prescribed treatment, in terms of medicine intake and consistency with prescribed physical activity (including home exercises), which was not measured in the present study. Further, since all primary outcome data was self-reported, a response bias could have led participants to overestimate or underestimate the severity of their condition. Future investigations must utilize larger sample population, longer intervention duration, and tracking of progress in both groups, to substantiate the impact of Smartphone application-based interventions in reducing disability in CLBP patients. Moreover, there is a dearth of economic evidence for m-health solutions, which is primarily limited by disparate estimation methods, lack of RCTs and long-term evaluation studies, small sample sizes, and absence of quality data and appropriate measures. Future studies must explicitly explore the most crucial determinant of m-health services, i.e., cost-effectiveness, including costs when using the app, frequency of visits to different care-givers, sick leaves, etc.
Conclusion
This study speaks in favor of the efficacy of a smartphone application (called Snapcare) in increasing physical activity and function of patients with CLBP, thereby reducing their disability index. Snapcare interface met the requirements set for an engaging healthcare app, and patients who used the application found that reaching their daily physical activity goals was stimulating and rewarding. Our findings suggest that a combination of engaging interface, along with an individualized approach to goal-setting, is likely to be the most effective strategy for maximizing physical activity level and compliance to home exercise programs.
References
Loney PL, Stratford PW (1999) The prevalence of low back pain in adults: a methodological review of the literature. Phys Ther 79(4):384–396
Walker BF, Muller R, Grant WD (2004) Low back pain in Australian adults. Prevalence and associated disability. J Manip Physiol Ther 27(4):238–244
Oleske DM, Lavender SA, Andersson GB, Morrissey MJ, Zold-Kilbourn P, Allen C, Taylor E (2006) Risk factors for recurrent episodes of work-related low back disorders in an industrial population. Spine 31(7):789–798
Frymoyer JW (1992) Predicting disability from low back pain. Clin Orthop Relat Res 279:101–109
Andersson GBJ (1997) The epidemiology of spinal disorders. In: Frymoyer JW (ed) The adult spine: principles and practice. Lippincott-Raven, Philadelphia, pp 93–141
Taimela S, Kujala UM, Salminen JJ, Viljanen T (1997) The prevalence of low back pain among children and adolescents: a nationwide, cohort-based questionnaire survey in Finland. Spine 22(10):1132–1136
Charlton JE (2005) Core Curriculum for Professional Education in Pain, 3rd edn. International Association of the Study of Pain (IASP) Press, Seattle
Schoppink LE, van Tulder MW, Koes BW, Beurskens SA, de Bie RA (1996) Reliability and validity of the Dutch adaptation of the Quebec back pain disability scale. Phys Ther 76(3):268–275
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Abraham J et al (2013) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2163–2196
Beurskens AJ, Henrica C, Köke AJ, van der Heijden GJ, Knipschild PG (1995) Measuring the functional status of patients with low back pain: assessment of the quality of four disease-specific questionnaires. Spine 20(9):1017–1028
Beurskens AJ, De Vet HC, Köke AJ (1996) Responsiveness of functional status in low back pain: a comparison of different instruments. Pain 65(1):71–76
Beurskens AJ, Henrica C, Kökeb AJ, Lindeman E, van der Heijden GJ, Regtope W et al (1999) A patient-specific approach for measuring functional status in low back pain. J Manip Physiol Ther 22(3):144–148
Ostelo RW, de Vet HC (2005) Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol 19(4):593–607
Smith JA, Osborn M (2007) Pain as an assault on the self: an interpretative phenomenological analysis of the psychological impact of chronic benign low back pain. Psychol Health 22(5):517–534
Painter JR, Seres JL, Newman RI (1980) Assessing benefits of the pain center: why some patients regress. Pain 8(1):101–113
Sanders SH (1983) Automated versus self-monitoring of ‘up-time’ in chronic low-back pain patients: a comparative study. Pain 15(1–4):399–405
Follick MJ, Ahern DK, Laser-Wolston N (1984) Evaluation of a daily activity diary for chronic pain patients. Pain 19(4):373–382
Bradley LA, Prokop CK, Margolis R, Gentry WD (1978) Multivariate analyses of the MMPI profiles of low back pain patients. J Behav Med 1(3):253–272
Armentrout DP, Moore JE, Parker JC, Hewett JE, Feltz C (1982) Pain-patient MMPI subgroups: the psychological dimensions of pain. J Behav Med 5(2):201–211
Fordyce WE (1976) Behavioral Concepts in Chronic Pain and Illness. CV Mosby, St. Louis
McNeill TH, Warwick DA, Andersson GU, Schultz AL (1979) Trunk strengths in attempted flexion, extension, and lateral bending in healthy subjects and patients with low-back disorders. Spine 5(6):529–538
Addison R, Schultz A (1980) Trunk strength in patients seeking hospitalization for chronic low-back disorders. Spine 5:539–544
Mayer TG, Tencer AF, Kristoferson S, Mooney V (1984) Use of noninvasive techniques for quantification of spinal range-of-motion in normal subjects and chronic low-back dysfunction patients. Spine 9(6):588–595
Kishino ND, Mayer TG, Gatchel RJ, Parrish MM, Anderson C, Gustin L et al (1985) Quantification of lumbar function: Part 4: isometric and isokinetic lifting simulation in normal subjects and low-back dysfunction patients. Spine 10(10):921–927
Mayer TG, Smith SS, Keeley J, Mooney V (1985) Quantification of lumbar function: part 2: sagittal plane trunk strength in chronic low-back pain patients. Spine. 10(8):765–772
Keeley J, Mayer TG, Cox R, Gatchel RJ, Smith J, Mooney V (1986) Quantification of lumbar function: part 5: reliability of range-of-motion measures in the sagittal plane and an in vivo torso rotation measurement technique. Spine. 11(1):31–35
Mayer TG, Barnes D, Nichols G, Kishino ND, Coval K, Piel B et al (1988) Progressive Isoinertial Lifting Evaluation. II. A comparison with isokinetic lifting in a disabled chronic low-back pain industrial population. Spine 3(9):998–1002
Curtis L, Mayer TG, Gatchel RJ (1994) Physical progress and residual impairment quantification after functional restoration. Part III: Isokinetic and isoinertial lifting capacity. Spine 19:401–405
Hägg O, Fritzell P, Nordwall A (2003) The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J 12(1):12–20
Hartigan C, Rainville JA, Sobel JB, Hipona MA (2000) Long-term exercise adherence after intensive rehabilitation for chronic low back pain. Med Sci Sports Exerc 32(3):551–557
Mayer TG, Gatchel RJ, Mayer H, Kishino ND, Keeley J, Mooney V (1987) A prospective two-year study of functional restoration in industrial low back injury: an objective assessment procedure. JAMA 258(13):1763–1767
Spitzer WO (1987) Scientific approach to the assessment and management of activity-related spinal disorders: a monogram for clinicians. Report of the Quebec task force on spinal disorders. Spine 12:S1–S60
Waddell G (1987) Volvo Award in Clinical Sciences: A new clinical model for the treatment of low-back pain. Spine 12(7):632–644
Manniche C, Bentzen L, Hesselso̸e G, Christensen I, Lundberg E (1988) Clinical trial of intensive muscle training for chronic low back pain. Lancet 332(8626–8627):1473–1476
Koes BW, Bouter LM, Beckerman H, Van Der Heijden GJ, Knipschild PG (1991) Physiotherapy exercises and back pain: a blinded review. BMJ 302(6792):1572–1576
Nordin M, Campello M (1999) Physical therapy. Neurol Clin 17(1):75–89
van der Velde G, Mierau D (2000) The effect of exercise on percentile rank aerobic capacity, pain, and self-rated disability in patients with chronic low-back pain: a retrospective chart review. Arch Phys Med Rehabil 81(11):1457–1463
Pinto RZ, Ferreira PH, Kongsted A, Ferreira ML, Maher CG, Kent P (2014) Self-reported moderate-to-vigorous leisure time physical activity predicts less pain and disability over 12 months in chronic and persistent low back pain. Eur J Pain 18(8):1190–1198
Vuori IM (2001) Dose-response of physical activity and low back pain, osteoarthritis, and osteoporosis. Med Sci Sports Exerc 33(6 Suppl):S551–S586
Blanchard CM, Courneya KS, Rodgers WM, Fraser SN, Murray TC, Daub B, Black B (2003) Is the theory of planned behavior a useful framework for understanding exercise adherence during phase II cardiac rehabilitation? J Cardiopulm Rehabil Prev 23(1):29–39
Sluijs EM, Kok GJ, Van der Zee J (1993) Correlates of exercise compliance in physical therapy. Phys Ther 73(11):771–782
Reilly K, Lovejoy B, Williams R, Roth H (1989) Differences between a supervised and independent strength and conditioning program with chronic low back syndromes. J Occup Environ Med 31(6):547–550
Sluijs EM, Knibbe JJ (1991) Patient compliance with exercise: different theoretical approaches to short-term and long-term compliance. Patient Educ Couns 17(3):191–204
Nelson BW, Miller M, Hogan M, Wegner JA, Kelly C (1995) The clinical effects of intensive, specific exercise on chronic low back pain: a controlled study of 895 consecutive patients with 1-year follow up. Orthopedics 18(10):971–981
Linton SJ, Hellsing AL, Bergström G (1996) Exercise for workers with musculoskeletal pain: does enhancing compliance decrease pain? J Occup Rehabil 6(3):177–190
Middleton A (2004) Chronic low back pain: patient compliance with physiotherapy advice and exercise, perceived barriers and motivation. Phys Ther Rev 9(3):153–160
Rimmer JH, Wang E, Smith D (2008) Barriers associated with exercise and community access for individuals with stroke. J Rehabil Res Dev 45(2):315
Bassett SF (2003) The assessment of patient adherence to physiotherapy rehabilitation. New Zealand Journal of Physiotherapy. 31(2):60–66
Cranen K, Drossaert CH, Brinkman ES, Braakman-Jansen AL, IJzerman MJ, Vollenbroek-Hutten MM (2012) An exploration of chronic pain patients’ perceptions of home telerehabilitation services. Health Expect 5(4):339–350
Kay M, Santos J, Takane M (2011) mHealth: New horizons for health through mobile technologies. World Health Organ 64(7):66–71
Whittaker R (2012) Issues in mHealth: findings from key informant interviews. J Med Internet Res 14(5):e129
Fogg BJ, Adler R (2009) Texting 4 Health: a simple, powerful way to change lives. Captology Media, Palo Alto
Fiordelli M, Diviani N, Schulz PJ (2013) Mapping mHealth research: a decade of evolution. J Med Internet Res 15(5):e95
Mosa AS, Yoo I, Sheets L (2012) A systematic review of healthcare applications for smartphones. BMC Med Inform Decis Mak 12(1):67
De La Torre-Díez I, López-Coronado M, Vaca C, Aguado JS, de Castro C (2015) Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemed e-Health 21(2):81–85
Iribarren SJ, Cato K, Falzon L, Stone PW (2017) What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS ONE 12(2):e0170581
Evans WD, Abroms LC, Poropatich R, Nielsen PE, Wallace JL (2012) Mobile health evaluation methods: the Text4baby case study. J Health Commun 17(sup1):22–29
Schulz KF, Altman DG, Moher D (2010) CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother 1(2):100–107
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K et al (2013) SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 158(3):200–207
Multani NK, Singh B, Garg S (2006) Effectiveness of telemedicine services integrated into physiotherapeutic health care system. J Exerc Sci Physiother 2:87–91
Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010
Schaller A, Froboese I (2014) Movement coaching: study protocol of a randomized controlled trial evaluating effects on physical activity and participation in low back pain patients. BMC Musculoskelet Disord 15(1):391
Fritz JM, Irrgang JJ (2001) A comparison of a modified Oswestry low back pain disability questionnaire and the Quebec back pain disability scale. Phys Ther 81(2):776–788
Follick MJ, Smith TW, Ahern DK (1985) The Sickness Impact Profile: a global measure of disability in chronic low back pain. Pain 21(1):67–76
Machado GC, Pinheiro MB, Lee H, Ahmed OH, Hendrick P, Williams C, Kamper SJ (2017) Smartphone apps for the self-management of low back pain: a systematic review. Best Pract Res Clin Rheumatol 30(6):1098–1109
Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS (2015) Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res 17(2):e52
Beratarrechea A, Lee AG, Willner JM, Jahangir E, Ciapponi A, Rubinstein A (2014) The impact of mobile health interventions on chronic disease outcomes in developing countries: a systematic review. Telemed e-Health 20(1):75–82
Williams V, Price J, Hardinge M, Tarassenko L, Farmer A (2014) Using a mobile health application to support self-management in COPD: a qualitative study. Br J Gen Pract 64(624):e392–e400
Kirwan M, Vandelanotte C, Fenning A, Duncan MJ (2013) Diabetes self-management smartphone application for adults with type 1 diabetes: randomized controlled trial. J Med Internet Res 15(11):e235
Wu Y, Yao X, Vespasiani G, Nicolucci A, Dong Y, Kwong J, Li L, Sun X, Tian H, Li S (2017) Mobile App-Based Interventions to Support Diabetes Self-Management: A Systematic Review of Randomized Controlled Trials to Identify Functions Associated with Glycemic Efficacy. JMIR Mhealth Uhealth 5(3):e35
Brouwer S (2004) Disability in Chronic Low Back Pain: psychometric properties of ADL-and work-related instruments. Groningen: s.n. 107p
Davidson M, Keating JL (2002) A comparison of five low back disability questionnaires: reliability and responsiveness. Phys Ther 82(1):8–24
Tonosu J, Takeshita K, Hara N, Matsudaira K, Kato S, Masuda K, Chikuda H (2012) The normative score and the cut-off value of the Oswestry Disability Index (ODI). Eur Spine J 21(8):1596–1602
Hicks GE, Manal TJ (2009) Psychometric properties of commonly used low back disability questionnaires: are they useful for older adults with low back pain? Pain Med 10(1):85–94
Manini TM, Pahor M (2009) Physical activity and maintaining physical function in older adults. Br J Sports Med 43(1):28–31
Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC et al (2007) Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 116(9):1094
Conroy MB, Yang K, Elci OU, Gabriel KP, Styn MA, Wang J et al (2011) Physical activity self-monitoring and weight loss: 6-month results of the SMART trial. Med Sci Sports Exerc 43(8):1568
Mansi S, Milosavljevic S, Baxter GD, Tumilty S, Hendrick P (2014) A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskelet Disord 15(1):231
McDonough SM, Tully MA, Boyd A, O’Connor SR, Kerr DP, O’Neill SM et al (2013) Pedometer-driven walking for chronic low back pain a feasibility randomized controlled trial. Clin J Pain 29(11):972
Durstine JL, Painter P, Franklin BA (2001) Erratum: physical activity for the chronically ill and disabled (Sports Medicine (2000) 30: 3 (207–2191)). Sports Med 31(8):627
Ettinger WH, Burns R, Messier SP, Applegate W, Rejeski WJ, Morgan T et al (1997) A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis: the Fitness Arthritis and Seniors Trial (FAST). JAMA 277(1):25–31
Keysor JJ (2003) Does late-life physical activity or exercise prevent or minimize disablement?: a critical review of the scientific evidence. Am J Prev Med 25(3):129–136
Gretebeck RJ, Ferraro KF, Black DR, Holland K, Gretebeck KA (2012) Longitudinal change in physical activity and disability in adults. Am J Health Behav 36(3):385–394
Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS et al (2014) Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 311(23):2387–2396
Anderson K, Burford O, Emmerton L (2016) Mobile health apps to facilitate self-care: a qualitative study of user experiences. PLoS ONE 11(5):e0156164
Zichermann G, Cunningham C (2011) Gamification by design: Implementing game mechanics in web and mobile apps. O'Reilly Media Inc, Sebastopol, CA
Acknowledgements
The authors would like to thank Ranjeet Randhawa (CEO, Snapcare Technologies) for providing the app free of cost for patient use. The authors would also like to acknowledge the work of our language editor, Ms. Shivani Verma and finally, the participants for their time and cooperation.
Funding
The study was supported by Snapcare Technologies Pvt. Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The corresponding author is a research consultant for Snapcare Technologies Pvt. Ltd. The authors derived no financial benefit from sales or from publication of this research. The data from the app were obtained from two independent observers not related to Snapcare Technologies Pvt. Ltd.
Ethical standards
The study was approved by the Institutional Ethical Committee, Indian Spinal Injuries Centre and an informed consent obtained from all participants. (Date: 28 July 2016; IEC reference No: ISIC/RP/2015/047).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1

Appendix 2

Appendix 3

Appendix 4

Rights and permissions
About this article
Cite this article
Chhabra, H.S., Sharma, S. & Verma, S. Smartphone app in self-management of chronic low back pain: a randomized controlled trial. Eur Spine J 27, 2862–2874 (2018). https://doi.org/10.1007/s00586-018-5788-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-018-5788-5