Abstract
Purpose
If surgery for thoracolumbar incomplete cranial burst fractures (Magerl A3.1.1) is necessary, the ideal stabilization strategy still remains undetermined. To justify posterior–anterior stabilization, which generates higher costs and potentially higher morbidity vs. posterior-only stabilization, clinical trials with sufficient power and adequate methodology are required. This prospective randomized single-centre pilot trial was designed to enable sufficient sample-size calculation for a randomized multicentre clinical trial (RASPUTHINE).
Methods
Patients with a traumatic thoracolumbar (Th11–L2) incomplete burst fracture (Magerl A3.1.1) were randomly assigned either to the interventional group (posterior–anterior) or to the control group (posterior-only). Primary endpoint of the study was the clinical outcome measured using the Oswestry Disability Index (ODI) at 24 months. Radiological outcome was assessed as secondary endpoint by evaluation of mono- and bisegmental kyphotic angulation and monosegmental fusion.
Results
21 patients were randomly assigned to interventional group (n = 9) or control group (n = 12). One posterior-only treated patient showed a severe initial loss of correction resulting in a crossover to additional anterior bisegmental fusion. The ODI measures at the primary study endpoint showed less but insignificant (p = 0.67) disability for the interventional group over the control group (13.3 vs. 19.3%). Comparison of preoperative bisegmental kyphosis in supine position with the bisegmental kyphosis at 24-month FU in upright position showed a worsened kyphosis for the control group (10.7° → 15.6°), whereas an improved kyphosis (11° → 8.3°) was detectable for the interventional group.
Conclusion
The results of this pilot RCT showed less disability for the posterior–anterior group linked with a significant better restoration of the sagittal profile in comparison with the posterior-only group. To detect a clinically significant difference using the ODI and assuming a 20% loss of FU rate, a total of 266 patients have to be studied in the multicentre trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic incomplete cranial burst fractures (Magerl A3.1.1) without neurological deficit located in the thoracolumbar junction represent the highest subgroup of fractures within the thoracolumbar spine in working populations across all industrialized countries. The incidence in Germany is estimated to be 6/100,000 per year. Especially, in Germany, most of these fractures are treated surgically aiming to restore the spinal sagittal balance, preventing neurological deficits, and maintaining function and quality of life. Surgical treatment options for this fracture entity include posterior-only stabilization (PO), combined posterior stabilization and anterior fusion (PA), and anterior-only fusion (AO) [1,2,3].
Posterior bisegmental (short segment) stabilization for Magerl A3.1.1 fractures is widely used, technically easy, and associated with a low access-related morbidity [4, 5]. PO may lead to loosening of the instrumented construct, since the anterior column contributes 80% to the spinal stability [6,7,8]. Therefore, the rate of additional anterior stabilization has increased in Germany from 29% in 2000 [9] to 44% in 2009 [10] leading on the other hand to increased perioperative morbidity and treatment costs [11, 12]. However, according to Schnake et al., additional anterior fusion with modern implants can be a safe procedure leading to a satisfactory long-term radiological and clinical outcome [13].
So far, only five clinical observational studies [5, 9, 14,15,16] have compared the clinical and radiological outcomes after PA or PO for burst fractures (Magerl A3) within the thoracolumbar junction (T11–L2). Theses results were reviewed and summarized by Oprel et al. who concluded that a better radiographic correction and maintenance of fracture-related kyphotic angulation can be achieved with PA [17]. However, a clinical difference for PA in comparison with PO was not detectable. Reviewing the up to date evidence, the ideal strategy for stabilization of thoracolumbar burst fractures still remains undetermined [18], particularly if the clinical outcome is the parameter of main importance. Furthermore, there are no clinical studies available analysing especially the subgroup of incomplete cranial burst fractures (Magerl A3.1.1), while all available studies do not differentiate between incomplete burst fractures (A3.1.1), burst–split fractures (A3.2), or complete burst fractures (A3.3).
To justify the increasing rate of PA with given higher costs and potentially higher perioperative morbidity, a proof of clinical benefit for PA over PO by a randomized controlled trial with an adequate number of patients is necessary. Furthermore, due to the lack of clinical data for Magerl A3.1 fractures comparing both treatment strategies, this randomized monocentric pilot study was determined to analyse clinical and radiological differences between both treatment strategies (PO vs. PA) to perform sample-size calculation for a planned large multicentre trial. Due to increased segmental stability with reduced loss of correction after additional anterior fusion, a better clinical and radiological outcome for interventional group (additional anterior monosegmental fusion) was hypothesized.
Methods
Patient population
The pilot study received approval by the local ethics committee. Between January 2010 and December 2011, all patients between 18 and 65 years with a singular traumatic thoracolumbar (Th11–L2) incomplete cranial burst fracture (A3.1.1) without neurological deficit were screened for trial participation after primary posterior fixation. Primary operation was performed by open short segment (bisegmental, 4 screw) stabilization, using a monoaxial Schanz-screw-based stabilization system (USS Fracture, Synthes GmbH, Switzerland) without fracture-level screw insertion. In addition, a monosegmental fusion was performed by decortication of the fracture-level facet joint and applying 2.5 cc of demineralized bone matrix (DBM, Synthes GmbH, Switzerland). Trial screening was performed 2 or 3 days after the primary posterior operation according to the study inclusion and exclusion criteria (Table 1). Minor accompanying injuries other than the spine (e.g., mild head injury and limb bruise) did not interfere with trial participation. If a patient meets the study criteria, an informed consent was obtained and the patient was enrolled according to the trial protocol (Fig. 1). Patients were randomized by a sealed envelope system either to the intervention group (additional anterior fusion) or to the control group (no additional anterior fusion). All patients from the interventional group received an endoscopic assisted mini-thoracotomy to perform a standardized anterior monosegmental fusion [19]. Anterior stabilization/fusion was achieved by the use of an autologous tricortical iliac crest bone graft and an angle-stable plate with four screws (MACS, Aesculap Inc., Tuttlingen, Germany).
Patients from both groups were informed about the necessity of posterior implant removal after confirmation of anterior bony healing to release the temporarily stabilized uninjured caudal motion segment. To minimize performance bias, only experienced surgeons (minimum of 20 posterior–anterior stabilizations/year) were eligible to operate study patients anteriorly.
Randomization
Thirty patients were planned to be included for pilot trial participation. Hence, a 1:1 randomization table for 30 patients was prepared using the RANDList software (DatInf GmbH, Tübingen, Germany). To blind the surgeon regarding the potential patient allocation while obtaining informed consent for trial participation, only the study nurse was allowed to assess the randomization table.
Clinical outcome
An internationally accepted and validated spinal trauma specific score is still missing. Thus, an adapted German version of the Oswestry Disability Index (ODI) [20] was used as spine specific primary outcome criteria to detect spinal function and pain. ODI scores were obtained at 3 and 12 months after primary operation and at study closure after 24 months. Baseline ODI value was obtained at the timepoint of randomization. As secondary outcome criteria length of inpatient stay, complications and Odom’s criteria were analysed in a descriptive fashion. To minimize measurement bias, an independent evaluation of patients’ questionnaire forms with pseudonymized data was performed.
Radiological outcome
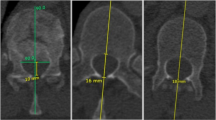
Mono-, bisegmental kyphotic angulation, and bisegmental scoliosis angulation using the validated Cobb angle method [21, 22] were preoperatively routinely obtained on sagittal computed tomography (supine position), and within postoperative FU (post surgery, after 3, 12, and 24 months after randomization) using a–p and lateral X-rays in upright position (Fig. 2a–c). Prior to implant removal computed tomography analysis and X-ray in upright position was routinely used in each patient, to determine anterior bony fusion (intervention group) or fracture consolidation (control group).
Case example showing a Magerl A3.1.1 fracture of Th12. a Radiographic evaluation: A bisegmental scoliosis angulation, B monosegmental kyphotic angulation and C bisegmental kyphotic angulation. b X-ray 3 months after posterior bisegmental fixation and anterior monosegmental fusion. c X-ray after posterior implant removal at final FU
Statistical analysis
Only patients who completed the 24-month FU were included for confirmatory analysis of the primary efficacy endpoint. Following the intention-to-treat principle, patients were analysed according to their primary group randomization. The confirmatory test for treatment group difference with respect to the primary endpoint was performed by Mann–Whitney U test or fisher exact test using SPSS 20.0 for Mac (SPSS Inc., Chicago, IL, USA). The two-sided type I error rate was set to 5%. Descriptive methods were used for all secondary efficacy and safety variables, using statistical methods that suit the empirical distribution of data. The homogeneity of treatment groups was described by comparison of the demographic data and baseline values.
Results
Patient population
Twenty-one patients fulfilled inclusion criteria (Table 1) and gave informed consent for study participation. These patients were randomly assigned either to the intervention group (n = 9) or to the control group (n = 12). Both groups were comparable regarding baseline demographic, clinical, and radiological data (Table 2). At 24 months, seven patients from interventional group and ten patients from control group were available for the final FU (FU rate 81%).
Surgical-related findings
Primary posterior fixation was performed by mean of 4 days (range 0–15 days) after trauma. In case of randomization to the interventional group, additional anterior monosegmental fusion was performed 9 days after primary posterior stabilization in average (range 5–13 days). Due to the additional anterior procedure, patients of the control group (21 days; range 8–34 days) had a shorter hospitalization time in comparison with patients of the interventional group (31 days; range 18–40 days). One delayed wound healing without indication for revision was recorded in the posterior-only group. One patient from control group showed a severe loss of correction after mobilization with a crossover need to an additional anterior bisegmental fusion. No serious adverse events were recorded after additional anterior fusion procedure. There was no evidence of vascular or neurologic complications in both groups. None of the interventional patients and none of the control patients needed a blood transfusion during surgery and within the postoperative course.
Clinical results
Analysing the ODI as primary outcome criteria, a high impairment was evident post-trauma. Comparing both groups within further FU, no significant difference was detectable until 12 months after the primary operation. After implant removal, posterior-only group showed a slight worsening of ODI, while a further improvement was detectable for interventional group (Fig. 3). At the primary endpoint of the study (24-month FU), a lower but insignificant ODI score (p = 0.67) for interventional group (13.3 ± 10.6%) in comparison with the control group (19.3 ± 15.9%) was detectable. Calculating the general improvement from preoperative timepoint until the endpoint of the study, interventional group showed an overall ODI improvement of 67.7%, whereas an overall ODI improvement of 54.4% was detectable in the control group.
According to the postoperative obtained ODOM’s criteria, no differences between the both groups were noticeable with mainly excellent/good results prior posterior implant removal. At the final FU, all patients of the interventional group were comfortable with the result. In contrast, two patients of the control group showed only fair results. Comparing the ODOM’s criteria at the final FU with the 3-month FU timepoint, only one patient in the posterior–anterior group stated worsened results (excellent → good) compared to three patients in the posterior-only group (excellent/good → fair).
Radiological results
Comparing baseline kyphotic and scoliotic alignment (Table 2), no relevant differences were detectable between control and interventional groups. After primary posterior bisegmental stabilization, bisegmental kyphosis (Fig. 4) was reduced to 4.9° ± 7.8° (control group) and 6.4° ± 9.1° (interventional group). After additional monosegmental anterior fusion, a further reduction of bisegmental kyphosis to 3.6° ± 8.5° was achievable in the interventional group. In both groups, a loss of correction was detectable within further FU. Comparing baseline bisegmental kyphotic angulation in supine position with kyphotic angulation in standing position at the study endpoint, posterior-only group showed a worsening (10.7° ± 6.4° → 15.6 ± 5.9°) in contrast to the interventional group, which showed an improvement of kyphotic misalignment (11.0° ± 8.6° → 8.3° ± 9.5°). Analysing the overall loss of bisegmental correction (kyphosis after posterior stabilization vs. 24-month FU), a higher loss of correction in the posterior-only group (10.9°) was detectable in comparison with the interventional group (1.9°). This leads to a significantly better restoration of the sagittal profile (kyphosis) for the interventional group in comparison with the control group (p = 0.04). Analysing monosegmental kyphotic angulation (Fig. 5), similar findings were detectable for the control group. Analysing monosegmental kyphotic angulation of interventional group before and after implant removal, monosegmental kyphotic angulation remained stable until final FU (Fig. 5).
Comparing the correction of bisegmental scoliotic deformity between control and interventional group showed no relevant differences at preoperative timepoint (2.7° ± 2.3° vs. 2.7° ± 2.2°) and the endpoint at 24-month FU (1.8° ± 2.3° vs. 2.0° ± 1.8°).
Sample-size calculation
Sample-size calculation for the planned large multicentre trial was performed using the given means and standard deviation of ODI measures at 24 months. Effect size of ODI at 24-month FU was calculated with d = 0.44. To detect a significant clinical difference with a significance level of 5% and a power of 95%, a total of 222 patients are required when applying a t test (this holds also true for an analysis of variance), and loss of FU rate in this study was assumed to be 20%. This is accounted by total randomizing of 266 (133/133) patients.
Discussion
The aim of this randomized controlled trial was primary to evaluate the clinical benefit and secondarily the radiological advantages of an additional monosegmental fusion after bisegmental posterior reduction and fixation for thoracolumbar incomplete cranial burst fractures (Magerl A3.1.1). Due to the lack of clinical data comparing treatment strategies for Magerl A3.1.1 fractures, this study was designed as a feasibility study to perform sample size and power calculation for a planned multicentre study. A better clinical and radiological outcome of the interventional group (additional anterior monosegmental fusion) due to increased segmental stability with reduced loss of correction after additional anterior fusion was hypothesized.
Analysing burst fractures (Magerl A3) within the thoracolumbar junction, incomplete cranial burst fractures (Magerl A3.1.1) are the most common fracture type. These fractures might be treated conservatively if no severe instability is detected [23]. However, there is evidence supporting the idea of surgical treatment in case of unstable burst fractures based on a RCT from Siebenga et al. [24]. Especially, in German speaking countries, A3.1.1 fractures are predominantly operated to restore the spinal sagittal balance, preventing patients from neurological deficits, and to restore function and quality of life. A prospective multicentre observational study (MCS II) conducted by the spine section of the German Trauma Society, evaluated treatment of thoracolumbar burst fractures (Magerl A3) in 588 patients. 63% (373) of these fractures were classified as Magerl-type A3.1 fractures. 91.5% (341) of these Magerl A3.1 fractures were treated operatively, while 8.5% (32) were treated conservatively [10].
Systematic literature reviews show that the choice of treatment is predominantly based on surgeons or institutional preferences [1,2,3]. Comparing given data from observational multicentre study I (MCS I) of the spine section of the German Trauma Society [9] and MCS II [10], an increasing rate of combined posterior–anterior fusion procedures from 25% (MCS I) to 41% (MCS II) and a decreasing rate from 67 to 51% for posterior-only procedures are evident.
So far, only a small number of observational studies have compared posterior–anterior vs. posterior-only stabilization of burst fractures within the thoracolumbar junction. Danisa et al. [14] evaluated in a retrospective case control study 49 patients with a singular unstable thoracolumbar burst fracture treated anterior-only (16), posterior-only (27), and posterior–anterior (6). Due to clinical similarity for all strategies but higher complications and costs for anterior or combined treatment, the authors concluded that posterior-only surgery should be preferred. Been and Bouma [5] were able to show in a retrospective analysis of 46 patients a similar clinical outcome but a significantly higher loss of correction on X-rays in case of posterior-only treatment. Briem et al. [15] compared in a retrospective matched pair analysis ten vs. ten patients with thoracolumbar burst fractures. Although the patients with posterior-only stabilization showed a significantly higher radiographic loss of correction during follow-up, quality of live parameters evaluated by SF-36 did not differ between the groups. Due to similarity of clinical results, both authors advocated to use posterior-only treatment.
Beside the fact that these three observational studies did not especially analyse the subgroup of incomplete cranial burst fractures, our present randomized study confirmed the radiological results of Been and Bouma [5] and Briem et al. [15] describing an insufficient capability to maintain postoperative correction by posterior-only stabilization. Particularly, posterior-only group demonstrated an increasing loss of correction from 3-month FU until final FU at 24 months. At study endpoint, bisegmental/monosegmental kyphotic angulation of posterior-only group was worse compared to the preoperative baseline data due to insufficient anterior column support. Contrary, a significantly better overall reduction was achieved and in the interventional group with an improved bisegmental/monosegmental kyphotic angulation at the final FU. The additional anterior procedure led to a stable fusion of the injured segment proved by constant values of treatment group monosegmental kyphosis before and after implant removal. Hence, the detected treatment group bisegmental loss of correction after implant removal occurred only in the inferior healthy motion segment. This finding might be explained by a temporary overcorrection of the inferior motion segment due to the primary bisegmental stabilization and normalisation to segmental physiological values after implant removal and “inferior segment liberation”.
Highest evidence for comparison of PO vs. PA treatment strategy was provided by Korovessis et al. [12] analysing 40 patients with lumbar burst fractures in a prospective randomized controlled trial. Beside the fact that this study addressed only the lumbar spine, clinical results analysed by VAS–Pain measurement were insignificantly better in the posterior-only group. However, due to the inability to maintain the restoration of sagittal alignment, the authors recommended not to use posterior-only stabilization. Radiological results of Korovessis et al. [12] are similar to the results of our present study. However, contrary to the data presented by Korovessis et al. [12] and recently published reviews from Scheer et al. [25] and Zhu et al. [18], our results tend to a better clinical outcome for PA over PO. Potential explanations may arise from a better comparability of patient cohort by focusing to a subgroup of burst fractures, as well as the use of a disability score (ODI) as primary outcome criteria, even though the used ODI score is not ideal to analyse trauma patients. However, ODI has already proven its ability to detect outcome differences in several studies evaluating trauma patients, and therefore, its use is widely accepted.
Most concerns regarding combined posterior–anterior treatment for thoracolumbar fractures are based on an increased rate of complications, longer OR time, and higher cost without proof of clinical benefit [17]. In a recent published review about the operative management of thoracolumbar burst fractures, Scheer et al. stated a lower complication rate for posterior-only approach in comparison with anterior or combined approaches [25]. In contrast to the data presented by Scheer et al., we only observed one delayed wound healing in the posterior group, but no deep wound infection nor any vascular or neurologic complications occurred in either group. No complications were detected in the interventional group. Of course, a two-staged operation results in a longer overall operation time and potentially in a higher loss of blood as Danisa et al. [14] and Wood et al. [26] have stated. However, none of the interventional patients needed an additional transfusion after study intervention.
Limitations
This randomized pilot study has methodical strengths and weaknesses. The analysed fractures were highly comparable as only incomplete cranial burst fractures (Magerl A3.1.1) were included. Individuals in both groups were comparable due to insignificant difference comparing baseline demographic, clinical, and radiological parameters. However, the week point of this single center feasibility study was the limited number of patients and a significant loss of FU. Hence, only seven patients from interventional group and ten patients from control group were available for the final FU.
Furthermore, postoperative radiographic analysis in spinal fractures is difficult to compare, because the trauma X-ray normally is done in a horizontal supine position. The follow-up X-rays are performed in an upright position. It can be assumed that the overall values for reduction might be better than measured in this study. Yet, comparability of results is not affected, because the situation was similar for both groups.
The short-term FU is an additional limitation of the present study. At study endpoint, most of the patients did well, even with a significant thoracolumbar kyphotic misalignment. Especially, young patients have the ability to use cranial and caudal compensation, e.g., pelvic retroversion, lumbar hyperlordosis, to maintain sagittal balance. However, if patients are getting older, they might loose the ability to use these compensatory mechanisms. In these circumstances, the patient reported outcome might become worse, due to spinal imbalance. Hence, a longer FU might potentially detect an increase of disability in the posterior-only group, while worse radiological results were already obvious at 24 months.
Conclusion
This feasibility pilot study was designed to allow a sample-size calculation for a multicentre trial (RASPUTHINE). Analysing the given 24-month FU data, our results showed slightly less disability for the posterior–anterior group linked with a significant better restoration of the sagittal profile in comparison with the posterior-only group. However, the results of this randomized feasibility study should be confirmed by a large multicentre trial with sufficient amount of patients. To detect a clinical significant difference by calculation of ODI means and standard deviation including 20% loss of FU rate, a total of 266 patients will be needed. If the planned multicentre study is able to demonstrate a clinical superiority in addition to an already proven radiological superiority, the trend towards a combined posterior–anterior intervention for Magerl A3.1.1 fractures could be justified.
References
Alpantaki K, Bano A, Pasku D et al (2010) Thoracolumbar burst fractures: a systematic review of management. Orthopedics 33:422–429. doi:10.3928/01477447-20100429-24
Dai L-Y, Jiang S-D, Wang X-Y, Jiang L-S (2007) A review of the management of thoracolumbar burst fractures. Surg Neurol 67:221–231. doi:10.1016/j.surneu.2006.08.081 (discussion 231)
Verlaan JJ, Diekerhof CH, Buskens E et al (2004) Surgical treatment of traumatic fractures of the thoracic and lumbar spine: a systematic review of the literature on techniques, complications, and outcome. Spine (Phila Pa 1976) 29:803–814
Yue JJ, Sossan A, Selgrath C et al (2002) The treatment of unstable thoracic spine fractures with transpedicular screw instrumentation: a 3-year consecutive series. Spine 27:2782–2787. doi:10.1097/01.BRS.0000035727.46428.BE
Been HD, Bouma GJ (1999) Comparison of two types of surgery for thoraco-lumbar burst fractures: combined anterior and posterior stabilisation vs. posterior instrumentation only. Acta Neurochir (Wien) 141:349–357
Knop C, Blauth M, Bastian L et al (1997) Fractures of the thoracolumbar spine. Late results of dorsal instrumentation and its consequences. Unfallchirurg 100:630–639
Lakshmanan P, Jones A, Mehta J et al (2009) Recurrence of kyphosis and its functional implications after surgical stabilization of dorsolumbar unstable burst fractures. Spine J 9:1003–1009. doi:10.1016/j.spinee.2009.08.457
Wang X-Y, Dai L-Y, Xu H-Z, Chi Y-L (2008) Kyphosis recurrence after posterior short-segment fixation in thoracolumbar burst fractures. J Neurosurg Spine 8:246–254. doi:10.3171/SPI/2008/8/3/246
Knop C, Blauth M, Bühren V et al (2000) Surgical treatment of injuries of the thoracolumbar transition. 2: operation and roentgenologic findings. Unfallchirurg 103:1032–1047
Reinhold M, Knop C, Beisse R et al (2010) Operative treatment of 733 patients with acute thoracolumbar spinal injuries: comprehensive results from the second, prospective, internet-based multicenter study of the Spine Study Group of the German Association of Trauma Surgery. Eur Spine J 19:1657–1676. doi:10.1007/s00586-010-1451-5
Knop C, Kranabetter T, Reinhold M, Blauth M (2009) Combined posterior–anterior stabilisation of thoracolumbar injuries utilising a vertebral body replacing implant. Eur Spine J 18:949–963. doi:10.1007/s00586-009-0970-4
Korovessis P, Baikousis A, Zacharatos S et al (2006) Combined anterior plus posterior stabilization versus posterior short-segment instrumentation and fusion for mid-lumbar (L2–L4) burst fractures. Spine 31:859–868. doi:10.1097/01.brs.0000209251.65417.16
Schnake KJ, Stavridis SI, Kandziora F (2014) Five-year clinical and radiological results of combined anteroposterior stabilization of thoracolumbar fractures. J Neurosurg Spine 20:497–504. doi:10.3171/2014.1.SPINE13246
Danisa OA, Shaffrey CI, Jane JA et al (2009) Surgical approaches for the correction of unstable thoracolumbar burst fractures: a retrospective analysis of treatment outcomes. Eur Spine J 83:977–983. doi:10.3171/jns.1995.83.6.0977
Briem D, Lehmann W, Ruecker AH et al (2004) Factors influencing the quality of life after burst fractures of the thoracolumbar transition. Arch Orthop Trauma Surg 124:461–468. doi:10.1007/s00402-004-0710-5
Schnee CL, Ansell LV (1997) Selection criteria and outcome of operative approaches for thoracolumbar burst fractures with and without neurological deficit. J Neurosurg 86:48–55. doi:10.3171/jns.1997.86.1.0048
Oprel PP, Tuinebreijer WE, Patka P, den Hartog D (2009) Combined anterior-posterior surgery versus posterior surgery for thoracolumbar burst fractures: a systematic review of the literature. Open Orthop J 4:93–100. doi:10.2174/1874325001004010093
Zhu Q, Shi F, Cai W et al (2015) Comparison of anterior versus posterior approach in the treatment of thoracolumbar fractures: a systematic review. Int Surg 100:1124–1133. doi:10.9738/INTSURG-D-14-00135.1
Kandziora F, Pingel A, Hoffmann C (2014) Incomplete cranial burst fracture of L1 treated by mini-open thoracoscopically-assisted anterior vertebral column reconstruction. Eur Spine J 23:2018–2019. doi:10.1007/s00586-014-3494-5
Osthus H, Cziske R, Jacobi E (2006) Cross-cultural adaptation of a German version of the Oswestry Disability Index and evaluation of its measurement properties. Spine 31:E448–E453. doi:10.1097/01.brs.0000222054.89431.42
Keynan O, Fisher CG, Vaccaro A et al (2006) Radiographic measurement parameters in thoracolumbar fractures: a systematic review and consensus statement of the spine trauma study group. Spine (Phila Pa 1976) 31:E156–E165. doi:10.1097/01.brs.0000201261.94907.0d
Sadiqi S, Verlaan J-J, Lehr AM et al (2017) Measurement of kyphosis and vertebral body height loss in traumatic spine fractures: an international study. Eur Spine J 26:1483–1491. doi:10.1007/s00586-016-4716-9
Wood KB, Buttermann GR, Phukan R et al (2015) Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit: a prospective randomized study with follow-up at sixteen to twenty-two years. J Bone Joint Surg Am 97:3–9. doi:10.2106/JBJS.N.00226
Siebenga J, Leferink VJM, Segers MJM et al (2006) Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine 31:2881–2890. doi:10.1097/01.brs.0000247804.91869.1e
Scheer JK, Bakhsheshian J, Fakurnejad S et al (2015) Evidence-based medicine of traumatic thoracolumbar burst fractures: a systematic review of operative management across 20 years. Global Spine J 5:73–82. doi:10.1055/s-0034-1396047
Wood KB, Bohn D, Mehbod A (2005) Anterior versus posterior treatment of stable thoracolumbar burst fractures without neurologic deficit: a prospective, randomized study. J Spinal Disord Tech 18(Suppl):S15–S23
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Scholz, M., Kandziora, F., Tschauder, T. et al. Prospective randomized controlled comparison of posterior vs. posterior–anterior stabilization of thoracolumbar incomplete cranial burst fractures in neurological intact patients: the RASPUTHINE pilot study. Eur Spine J 27, 3016–3024 (2018). https://doi.org/10.1007/s00586-017-5356-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-017-5356-4