Abstract
Purpose
This retrospective study determined the rate of osteoarthritis and spontaneous facet joint fusion and analyzed risk factors related to patient characteristics, fracture type or surgical technique on pre- and postoperative CT after percutaneous instrumentation in thoracolumbar fractures.
Methods
1050 facet joints adjacent to screws in 148 patients (15–85 years) with thoracolumbar fractures were analyzed with an average time between CTs of 12.3 months. Screw diameters, lengths and cement augmentation were recorded. Facet joint violation by screw trajectory and by insertion depth was classified in three grades. Pre- and postoperative osteoarthritis was graded as absent, minor or severe and postoperative facet joint fusion as absent, partial or complete.
Results
The facet violation rate was moderate in 15.4% and severe in 0.6% according to screw trajectory, and 11.0 and 0.6%, respectively, according to insertion depth. Osteoarthritis was preoperatively rated moderate in 9.6% and severe in 1.2%. A progression was evidenced in 79 facet joints (7.5%). Screw cement augmentation was the main predictive factor (p < 0.0001). Partial fusion was evidenced in 2.6% and complete fusion in 1% of facet joints. Risk factors were: BMI (p = 0.0002), age (p = 0.0013), preoperative osteoarthritis (p = 0.0005), time between 2 CTs (p = 0.0001), B-type fractures (p = 0.0005), concomitant anterior fusion (p = 0.0034).
Conclusions
Occurrence or worsening of osteoarthritis was mainly observed in elderly patients with cement-augmented screws and spontaneous facet fusion in elderly patients with high BMI and preoperative osteoarthritis, or in anteriorly fused B-type injuries. Thus, percutaneous instrumentation can safely be removed after fracture consolidation in younger patients while preserving facet joints.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Percutaneous instrumentation is currently recognized as safe and efficient treatment method of non-neurologic thoracolumbar fractures [1, 2]. Long-term clinical outcomes seem similar when comparing conservative treatment and classic surgical treatment of incomplete burst fractures [3,4,5]. Percutaneous instrumentation represents a valuable alternative to bracing as it provides immediate stabilization and quick mobilization of the patient. In unstable fractures, percutaneous instrumentation has some benefits over open surgery: the avoidance of paravertebral muscle dissection is associated with lower intraoperative blood loss, risk of infection, less postoperative pain, reduction of operative time and hospitalization [6, 7].
Posttraumatic kyphosis correction can be achieved and maintained over time by combining percutaneous instrumentation and kyphoplasty in burst fractures [8, 9]. Anterior fusion might be considered in major anterior column defects and disco-ligamentous three-column injuries [10, 11]. This aspect is crucial since percutaneous osteosynthesis does not enable bone grafting like open posterior fusion. Additionally, percutaneous instrumentation can be used as temporary internal fixator, which is removed through minimal skin incisions once consolidation is obtained at the anterior column [11, 12]. This approach differs from traditional open surgery as the paravertebral musculature is preserved while remobilizing non-fused levels. This seems particularly interesting in younger patients who require a longer construct at the thoracolumbar junction or in the lumbar spine.
However, facet joint degeneration or even spontaneous fusion might be a concern when considering implant removal. Postoperative facet deterioration has been evidenced on computed tomography (CT) at 1-year follow-up [13]. It remains unclear to what extent the type of injury with involvement of the posterior column or screw misplacement exerting a pressure on facet joints might play a role. Moreover, spinal instrumentation might increase stress at its cranial end, which carries a risk of developing osteoarthritis at adjacent segments.
The aim of this study was to determine the rate of facet joint degeneration such as osteoarthritis and spontaneous fusion caused by percutaneous instrumentation in thoracolumbar fractures. Risk factors related to patient characteristics, fracture type and surgical technique were analyzed.
Materials and methods
A retrospective study was conducted on CT images of patients who underwent posterior percutaneous instrumentation for thoracolumbar fractures at our institution between January 2009 and December 2014 by three senior spine surgeons. Patients with ankylosing spondylitis or diffuse idiopathic skeletal hyperostosis were excluded. Two independent experienced spine surgeons reviewed CT images together and ratings relied on a consensus. Preoperative and postoperative thin-cut CT images were performed at a minimum follow-up of 6 months. Most patients underwent a CT after one year to check consolidation prior to implant removal. If implant removal was not indicated (elderly patients, short construct without functional impairment), a follow-up CT was usually performed within the second year. Images were available on our Picture Archiving and Communication System (PACS) for 148 consecutive patients. The average time between CT-scans was 12.3 (6–28) months. There were 96 males and 52 females. The average age at the time of surgery was 46.3 (15–85) years. The average body mass index (BMI) was 25.8 (16.5–39.4) kg/m2.
Twenty-seven patients had more than one vertebral fracture. Fracture levels were located between T2 and L5, predominantly at the thoracolumbar junction or in the cranial lumbar spine: T12 (15.5%), L1 (38.5%), L2 (11.5%) and L3 (10.8%). The AO-classification [14] was used and A, B and C-types without neurology were present in this cohort. The most common types were: incomplete burst fractures A3.1 or A3.2 (43.9%), pincer type fractures A2.3 (18.9%) and flexion-distraction fractures B2.1 or B2.2 (15.5%).
In 20 patients, percutaneous instrumentation was combined with kyphoplasty in A3.1 fractures. Major anterior column defects and disco-ligamentous three-column injuries were completed by select one-level anterior fusion in 60 patients and by corpectomy in 45 patients. Short constructs (4 screws) were used in 34 patients and long constructs (6–12 screws) in 114 patients. Cement augmentation of pedicle screw was performed in 34 elderly patients with osteopenia or osteoporosis. Screw diameters ranged from 4.5 to 7.5 mm and lengths ranged from 35 to 55 mm depending on vertebral levels and dimensions.
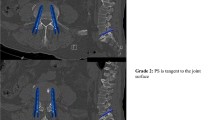
In total, 1050 facet joints adjacent to a screw were reviewed. This included facet joints at the cranial mobile junction between the non-instrumented and the instrumented spine, as well as facet joints within the construct. The screw position relative to the facet joint was analyzed with regard to screw trajectory and insertion depth. Thoracic facet joints were analyzed on sagittal CT-reconstructions according to their anatomical orientation (374 screws from T1 to T11) and the cranio-caudal screw position classified in three grades (Fig. 1): extraarticular (grade 1), intraarticular involving the caudal third of the facet joint (grade 2) or transarticular (grade 3). At the thoracolumbar junction and in lumbar facet joints (676 screws from T12 to S1) the mediolateral screw position was analyzed on axial CT-reconstructions (Fig. 2): extraarticular (grade 1), intraarticular involving the lateral third of the facet joint (grade 2) or transarticular (grade 3). Furthermore, the insertion depth was assessed on axial CT-reconstructions to quantify the extent of facet depression caused by the screw head. Three categories were determined (Fig. 3): no depression (grade 1), depression <1/3 (grade 2) and depression >1/3 of facet joint (grade 3).
Facet joints adjacent to a pedicle screw were then compared pre- and postoperatively on axial CT-reconstructions to assess signs of osteoarthritis and postoperative fusion. Osteoarthritis was divided into three grades (Fig. 4): absent (grade 1), minor osteoarthritis characterized by cartilage thinning, subchondral sclerosis and/or presence of intraarticular gas (grade 2), or severe osteoarthritis if the joint space was very narrow and osteophytes present (grade 3). Postoperative occurrence of osteoarthritis was defined a transition from grade 1 to 2, and worsening of osteoarthritis as transition from grade 2 to 3. Furthermore, spontaneous facet joint fusion was assessed postoperatively (Fig. 5): absent (grade 1), partial fusion with remaining joint visible (grade 2), or complete fusion (grade 3).
Statistical evaluation was performed with R software 3.1.0. Risk factors for the development of facet osteoarthritis or fusion were analyzed using a Chi-squared test of homogeneity when application conditions were assumed. If not, a non-parametric Fisher’s exact test was used. The Gaussian assumption on the distribution of quantitative variables was assessed using the Shapiro–Wilk test. A Student t test was used to compare quantitative values depending whether osteoarthritis progressed or fusion occured according to application conditions. Otherwise, the non-parametric Mann–Whitney test was used. Univariate analyses were completed by multivariate logistic regression models to check which risk factors were predominant. The significance level was set at 0.05.
Results
Screw positioning
An overall assessment was made with regard to pedicle screws trajectory and possible damage caused to the facet joint: 882 screws (84.0%) were extraarticular (grade 1), 162 screws (15.4%) were intraarticular <1/3 of the facet joint (grade 2) and 6 screws (0.6%) were transarticular (grade 3). Table 1 details the distribution by grades and vertebral levels.
Insertion depth of screws was analyzed to assess possible damage to the facet joint by pressure of the screw head: 928 screws (88.4%) did not cause facet depression (grade 1), 116 screws (11.0%) caused a depression by <1/3 (grade 2) and 6 screws (0.6%) caused a depression by >1/3 (grade 3). Table 2 demonstrates the distribution of insertion depth grades by vertebral levels.
Facet joint osteoarthritis
In 65 of 148 patients (46.1%) osteoarthritis (grade 2 or 3) was already evidenced on preoperative CT at more than one facet joint. Preoperatively, minor osteoarthritis (grade 2) was present in 101 of 1050 facet joints (9.6%) and severe osteoarthritis (grade 3) in 13 facet joints (1.2%). Postoperatively, minor osteoarthritis was observed in 172 facet joints (16.4%) and severe osteoarthritis in 17 facet joints (1.6%).
When comparing osteoarthritis pre- and postoperatively, an occurrence (transition from grade 1 to 2) or worsening (transition from grade 2 to 3) was noticed in 79 facet joints (7.5%). Facet degeneration occurred more frequently at lumbar instrumented levels and at the thoracolumbar junction (p = 0.0211). Adjacent segment degeneration at the cranial transition between instrumented and non-instrumented levels was evidenced in 9 of 296 mobile facet joints (3.0%).
Table 3 summarizes the influence of factors related to patient characteristics, fracture type and surgical technique on the occurrence or worsening of osteoarthritis. A significant influence was evidenced for the use of bigger screw diameters (p = 0.0164) and cement augmentation of pedicle screws (p < 0.0001). A multivariate analysis confirmed that bigger screw diameters and cement augmentation were linked. A longer time of instrumentation in situ (between pre- and postoperative CT) increased the rate of osteoarthritis (p = 0.0027). Screw positioning had no statistically significant influence, but this result must be interpreted with caution as the rate of screws harming the facet joint (grade 3) was 0.6% for screw trajectory and insertion depth.
Facet joint fusion
Postoperatively, partial fusions were observed in 27 facet joints (2.6%) and complete fusions in 10 facet joints (1.0%). None of these fusions were located at the transition between instrumented and non-instrumented levels. Patient-related factors that influenced fusion were: higher age (p = 0.0013), preoperative presence of osteoarthritis (p = 0.0005) and higher BMI (p = 0.0002). A multivariate analysis showed that these factors were linked and that preoperative osteoarthritis was the predominant factor.
The fracture type influenced the occurrence of partial fusion (B3) and complete fusion (B2) significantly (p = 0.0005). Facet joint fusion was associated with concomitant anterior interbody fusion (p = 0.0034). Facet joint fusion increased as the time between pre- and postoperative CT-scans increased (p = 0.0001). Facet joint fusion was more frequent in the thoracic spine and at the thoracolumbar junction (T12–L1) compared to lumbar instrumented level (p = 0.0198). Pedicle screw dimensions, trajectory, insertion depth and cement augmentation had no statistically significant influence on facet joint fusion (Table 4).
Discussion
Percutaneous instrumentation can be used as a temporary internal fixator without fusion or with select anterior fusion in the management of thoracolumbar fractures. After consolidation, removal of the fixation system through previous skin incisions aims to restore motion at immobilized levels. This is beneficial for young adult patients, particularly in the lumbar spine where the range of motion is larger than in the thoracic spine [11, 15, 16]. This strategy implies that facet joints should be preserved at non-fused levels. However, Proietti et al. [13] described facet joint degeneration in patients who underwent removal of instrumentation at the thoracolumbar junction and in the lumbar spine. This observation raises the need to evaluate the accuracy of percutaneous instrumentation and to analyze factors that might promote facet deterioration.
Violation of cranial facet joints by pedicle screws is recognized as a potential risk factor for the development of adjacent segment degeneration [17]. Babu et al. [18] classified facet violation in three grades, related to the mediolateral screw position on axial CT-reconstructions, which were comparable to our classification used in the lumbar spine. These authors reported a rate of 7.1% grade 2 and 8.5% grade 3 violations in 306 percutaneous pedicle screws. Knox et al. [19] reported an incidence of 11.48% violation of cranial facet joints after minimal invasive placement of 282 lumbar pedicle screws. Park et al. [20] described a violation rate of 31.5% in 184 lumbar percutaneous pedicle screws. Violation was predominantly found at L4–L5 facet joints. Jones-Quaidoo et al. [21] compared open and percutaneous pedicle screw placement: the facet violation rate was 13.6% in 264 percutaneous screws versus 6% in 263 open screws. This difference was attributed to the fact that the percutaneous technique does not allow direct visualization of anatomical landmarks of the facet joint. Wang et al. [22] published a systematic review and meta-analysis of four studies with a cumulative sample size of 1755 pedicle screws. The facet violation rates were similar for open (18.72%) and percutaneous (18.18%) techniques. Several studies analyzed the potential benefit of the intraoperative three-dimensional CT reconstruction and computer-assisted navigation compared to simple fluoroscopy in percutaneous pedicle screw placement. When using navigation, Tian et al. [23] reported a 3.7% violation rate (136 screws), Yson et al. [24] found a 4% violation rate (125 screws), and Ohba et al. [25] demonstrated a 5.1% violation rate (79 screws). Fluoroscopy only was used in our cohort, and the rates of moderate (15.4%) and severe (0.6%) facet violation are in line with previous findings. However, there is a paucity of literature concerning facet violation and insertion depth of the pedicle screw. The screw head might exert a pressure on the facet. We, therefore, tend to choose longer screws in younger patients to avoid possible damage to the facet joint if we intend to remove the instrumentation after fracture consolidation.
Proietti et al. [13] analyzed the facet joints of 30 patients who underwent percutaneous osteosynthesis (4 screws) without fusion for lumbar fractures. Progressive signs of osteoarthritis were observed on CT in 21.42% of facet joints at 8 months postoperatively, and in 76.92% of cases at 12 months. A traumatic injury of facet joints was present in 10 patients, and facet degeneration occurred at non-instrumented middle levels. The incidence of facet violation was 12% and comparable to our results. However, it was not possible to elucidate if degenerative changes were caused by the fracture itself rather than the fixation. In our study, occurrence or worsening of osteoarthritis was mainly observed at lumbar levels and at the thoracolumbar junction. A multivariate analysis showed that cement augmentation of pedicle screws and the use of larger screw diameters influenced facet joint degeneration. Large cement-augmented screws are used in elderly patients with osteopenic or osteoporotic bone. The screw pullout strength and resistance to the bending moments increases significantly with this technique [26, 27]. In vitro, the effect of toggle migration is lowered with augmentation techniques under cranio-caudal cyclic loading [28]. In vivo, cement-augmented instrumentation withstands a combination of axial compression, antero-posterior shear, and flexion–extension moments, but loading is probably transmitted through non-fused facet joints. This might possibly explain why cement-augmented screws could accelerate facet degeneration in elderly patients that already present osteoarthritis. However, the clinical relevance of this observation is secondary, as implant removal would not be considered in a geriatric trauma population.
Spontaneous facet joint fusions were occasionally observed in our cohort. Patient-related factors were age, preexisting degenerative changes and higher BMI. On the other hand, fracture related factors were demonstrated; B-type injuries with disrupted facet joints were predictive of degeneration and spontaneous fusion. As the combination between anterior column and posterior tension band injuries represents an unstable situation, a combined approach including posterior percutaneous instrumentation and anterior fusion can be recommended in B-type fractures [29, 30]. Facet fusion was, therefore, significantly associated with concomitant anterior fusion and tended to increase over time. In clinical practice, fracture consolidation might be checked on CT after 6–12 months. Instrumentation might then be removed percutaneously in younger patients without harming facet joints. This approach allows consolidating the fracture level by select approach, while motion is restored at non-fused levels. One of this studies’ drawback is that the range of motion of non-fused facet joints has not been quantified after implant removal. It might be interesting to analyze their kinematic behavior at long-term follow-up.
Conclusion
This study reported a low rate of severely misplaced screws harming facet joints after percutaneous instrumentations in thoracolumbar trauma. Occurrence or worsening of facet joint osteoarthritis was mainly observed when using cement-augmented pedicle screws with large diameters. The rate of spontaneous facet fusion was also low, and observed in elderly patients with high BMI and preoperative facet osteoarthritis. A traumatic involvement of facet joints in B-type injuries and concomitant anterior fusion also influenced spontaneous facet fusion. Thus, percutaneous instrumentation can safely be removed after fracture consolidation in younger patients while preserving facet joints.
References
Court C, Vincent C (2012) Percutaneous fixation of thoracolumbar fractures: current concepts. Orthop Traumatol Surg Res 98:900–909
Scheer JK, Bakhsheshian J, Fakurnejad S et al (2015) Evidence-based medicine of traumatic thoracolumbar burst fractures: a systematic review of operative management across 20 years. Global Spine J 5:73–82
Gnanenthiran SR, Adie S, Harris IA (2012) Non-operative versus operative treatment for thoracolumbar burst fractures without neurologic deficit: a meta-analysis. Clin Orthop Relat Res 470:567–577
Shen WJ, Liu TJ, Shen YS (2001) Non-operative treatment versus posterior fixation for thoracolumbar junction burst fractures without neurological deficit. Spine (Phila Pa 1976) 26:1038–1045
Wood K, Buttermann G, Mehbod A et al (2003) Operative compared with non-operative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Jt Surg Am 85:773–781
Vanek P, Bradac O, Konopkova R et al (2014) Treatment of thoracolumbar trauma by short-segment percutaneous transpedicular screw instrumentation: prospective comparative study with a minimum 2-year follow-up. J Neurosurg Spine 20:150–156
Lee JK, Jang JW, Kim TW et al (2013) Percutaneous short-segment pedicle screw placement without fusion in the treatment of thoracolumbar burst fractures: is it effective? Comparative study with open short-segment pedicle screw fixation with posterolateral fusion. Acta Neurochir 155:2305–2312
Fuentes S, Blondel B, Metellus P et al (2010) Percutaneous kyphoplasty and pedicle screw fixation for the management of thoraco-lumbar burst fractures. Eur Spine J 19:1281–1287
Rahamimov N, Mulla H, Shani A et al (2012) Percutaneous augmented instrumentation of unstable thoracolumbar burst fractures. Eur Spine J 21:850–854
Eck JC (2011) Minimal invasive corpectomy and posterior stabilization for lumbar burst fracture. Spine J 11:904–908
Charles YP, Walter A, Schuller S et al (2012) Thoracolumbar fracture reduction by percutaneous in situ contouring. Eur Spine J 21:2214–2221
Takami M, Yamada H, Nohda K et al (2014) A minimally invasive surgery combining temporary percutaneous pedicle screw fixation without fusion and vertebroplasty with transpedicular intracorporeal hydroxyapatite blocks grafting for fresh thoracolumbar burst fractures: prospective study. Eur J Orthop Surg Traumatol 24:159–165
Proietti L, Scaramuzzo L, Schirò GR et al (2015) Degenerative facet joint changes in lumbar percutaneous pedicle screw fixation without fusion. Orthop Traumatol Surg Res 101:375–379
Magerl F, Aebi M, Gertzbein SD et al (1994) A comprehensive classification of thoracic and lumbar injuries. Eur Spine J 3:184–201
Wild MH, Glees M, Plieschnegger C et al (2007) Five-year follow-up examination after purely minimally invasive percutaneously and conventionally treated patients. Arch Orthop Trauma Surg 127:335–343
Heintel TM, Berglehner A, Meffert R (2013) Accuracy of percutaneous pedicle screws for thoracic and lumbar spine fractures: a prospective trial. Eur Spine J 22:495–502
Park P, Garton HJ, Gala VC et al (2004) Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine (Phila Pa 1976) 29:1938–1944
Babu R, Park JG, Mehta AI et al (2012) Comparison of superior-level facet joint violations during open and percutaneous pedicle screw placement. Neurosurgery 71:962–970
Knox JB, Dai JM 3rd, Orchowski JR (2011) Superior segment facet joint violation and cortical violation after minimally invasive pedicle screw placement. Spine J 11:213–217
Park Y, Ha JW, Lee YT et al (2011) Cranial facet joint violations by percutaneously placed pedicle screws adjacent to a minimally invasive lumbar spinal fusion. Spine J 11:295–302
Jones-Quaidoo SM, Djurasovic M, Owens RK 2nd et al (2013) Superior articulating facet violation: percutaneous versus open techniques. J Neurosurg Spine 18:593–597
Wang L, Wang Y, Yu B et al (2015) Comparison of cranial facet joint violation rate between percutaneous and open pedicle screw placement: a systematic review and meta-analysis. Medicine (Balitmore) 94:e504
Tian W, Xu Y, Liu B et al (2014) Lumbar spine superior-level facet joint violations: percutaneous versus open pedicle screw insertion using intraoperative 3-dimensional computer-assisted navigation. Chin Med J (Engl) 127:3852–3856
Yson SC, Sembrano JN, Sanders PC et al (2013) Comparison of cranial facet joint violation rates between open and percutaneous pedicle screw placement using intraoperative 3-DCT(O-arm) computer navigation. Spine (Phila Pa 1976) 38:E251–E258
Ohba T, Ebata S, Fujita K et al (2016) Percutaneous pedicle screw placements: accuracy and rates of cranial facet joint violation using conventional fluoroscopy compared with intraoperative three-dimensional computed tomography computer navigation. Eur Spine J 25:1775–1780
Choma TJ, Frevert WF, Carson WL et al (2011) Biomechanical analysis of pedicle screws in osteoporotic bone with bioactive cement augmentation using in vivo multicomponent loading. Spine (Phila Pa 1976) 36:452–454
Charles YP, Pelletier H, Hydier P et al (2015) Pullout characteristics of percutaneous pedicle screws with different cement augmentation methods in elderly spines: an in vitro biomechanical study. Orthop Traumatol Surg Res 101:369–374
Bostelmann R, Keiler A, Steiger HJ et al (2015) Effect of augmentation techniques on the failure of pedicle screws under cranio-caudal cyclic loading. Eur Spine J. doi:10.1007/s00586-015-3904-3
Vaccaro AR, Oner C, Kepler CK et al (2013) AOSpine thoracolumbar spine injury classification system: fracture description, neurological status, and key modifiers. Spine (Phila Pa 1976) 38:2028–2037
Vaccaro AR, Schroeder GD, Kepler CK et al (2016) The surgical algorithm for the AOSpine thoracolumbar spine injury classification system. Eur Spine J 25:1087–1094
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declared that they have no potential conflict of interest.
Funding
No source of funding for this study.
Rights and permissions
About this article
Cite this article
Tromme, A., Charles, Y.P., Schuller, S. et al. Osteoarthritis and spontaneous fusion of facet joints after percutaneous instrumentation in thoracolumbar fractures. Eur Spine J 28, 1121–1129 (2019). https://doi.org/10.1007/s00586-017-5173-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-017-5173-9