Abstract
Purpose
Metastatic epidural spinal cord compression (MESCC) often requires anterior–posterior decompression and stabilization. To reduce approach-related complications, single-stage posterolateral vertebrectomy and 360° fusion is often performed. However, a sufficient reduction of kyphotic deformity through this approach has not been reported. The purpose of this study is to investigate the efficacy of kyphotic deformity reduction by this approach in MESCC.
Methods
A retrospective analysis and chart review was performed for 14 consecutive patients who underwent a vertebrectomy and decompression from a posterolateral approach. Anterior mesh stabilization of the ventral column is used as hypomochlion for the posterior compression manoeuvre, which leads to reduction of the kyphotic deformity.
Results
Pre-operative back pain was 7.2 on a visual analogue scale. Back pain was reduced to 4.4 at discharge and 2.0 at the latest follow-up with a mean follow-up of 12 months (p < 0.001). The Frankel score remains constant or improved from D to E. Radiological segmental kyphosis was corrected from a mean of 16° to 4° (p < 0.001) post-operatively with a loss of 3° at the final follow-up, but still with significant corrections compared with the pre-operative measurements (p < 0.003).
Conclusion
Single-stage posterolateral vertebrectomy and reconstruction is a safe and less invasive approach that allows a sufficient reduction of hyperkyphosis and preservation of neurological function in patients with MESCC. This approach is an efficient alternative to anterior–posterior fusion with good pain reduction and improved sagittal profile.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Objective
Spinal metastases frequently cause metastatic epidural spinal cord compression (MESCC) due to anterior or bilateral tumour growth and additional instability [6].
MESCC is estimated to occur in between 5 and 10 % of patients with cancer, most commonly of the breast, prostate and lung, and in up to 40 % of patients who have pre-existing bone metastases outside of the spine [3, 36].
Due to the ventral aspect of the pathology and to avoid neurological deficits [27], a surgical procedure was developed to obtain a circumferential decompression [33] by an anterior approach with the opportunity for immediate stabilization [20, 38]. Frequently, this procedure has to be combined with a posterolateral decompression and circumferential fixation with a subsequent surgery through a posterior approach.
Approximately 60 % of MESCCs are located in the thoracic spine [2, 31]. However, the ventral approach to the upper and midthoracic region is particularly challenging [17]. Therefore, a single-stage posterior approach to decompress the ventral aspect of the spinal cord has become more popular due to its less invasive nature [1, 4, 5, 9, 43]. A biomechanical stable fast and safe reconstitution with preservation of neurological function and complete resection is achievable, but in contrast to the ventral approach, the possibility of kyphotic deformity reduction has been less frequently reported [1, 9, 10, 20, 43].
Panjabi et al. noted in their biomechanical analysis that abnormal thoracic kyphosis caused a mechanical response that further increased thoracic kyphosis and that an abnormal thoracic configuration created a vicious cycle of abnormal tissue loading [21, 29, 30]. Hato et al. found in a finite-element analysis of closing-opening correction osteotomy for angular kyphosis of vertebral fractures that the lack of correction leads to an increased stress on the vertebral column [21, 25]. From the biomechanical point of view, the advantage of correcting the kyphotic angle as close as possible to physiological alignment in the thoracolumbar spine is proven. Otherwise, it will lead to clinical complications such as progressive back pain, instrument failure, and neurological deterioration.
Therefore, we investigated the efficacy and feasibility of the previously described single-stage approach with special attention to the reconstruction of kyphotic deformity in patients with MESCC.
Materials and methods
Study inclusion criteria
The study included patients who underwent posterior vertebral column resection with 360° osteosynthesis in MESCC and spinal instability with segmental kyphosis between December 2012 and April 2014.
Spinal instability was diagnosed on the basis of radiographic findings (pathological fractures with posterior element extension) combined with level-specific pain patterns that had been described previously in the Spine Instability Neoplastic Score criteria evaluation [15].
Patients also had to have at least one neurological sign or symptom (including pain) and were required not to have been paraplegic for longer than 24 h before study entry to improve neurologic deterioration [13]. The MESCC was restricted to a single thoracic area, which included only one vertebral body. Patients with certain radiosensitive tumours (e.g., lymphomas, leukaemia, and germ-cell tumours) and without extensive neurological deficits were excluded. The hyperkyphosis was defined as a pathologic accentuation of this normal curvature compared to cranial and caudal segments. In the case of plasmocytoma, single vertebral destruction is often osteolytic. This may produce mechanical instability with the risk of secondary fractures combined with kyphosis and spinal cord compression. Neurologic deterioration should be avoided in these cases, and therefore, these indications with kyphotic deformity already present were also included. Overall, a multidisciplinary tumour board involving specialists in radiology, oncology, pathology, and spinal surgery had to give the patient a minimal survival time of at least 2 years. The decision to treat the patient surgically was influenced by common scoring systems for the pre-operative evaluation of metastatic spine tumour prognosis [38, 39] to understand life expectancy and quality [7, 12].
Prospective documented medical records were retrospectively reviewed. For pain quantification, the visual analogue scale (VAS) was used [37]. Long-term outcome assessment was conducted with the Oswestry Disability Index (ODI), as described by Fairbank [11]. Long-term functional outcome was assessed using the Frankel score [18]. General well-being and activities of daily life were assessed by Karnofsky performance status [24]. The fusion rate was divided into five grades: grade 0, no healing; grade 1, minimal consolidation of bone graft; grade 2, bone graft consolidation; grade 3, bridging callus; and grade 4, bridging callus with trabeculations.
Segmental shortening of the spine was calculated from the height of the two adjacent vertebral bodies. The mean height was equalized to the supposed height of the affected vertebral body before metastatic involvement and was set in relation to the height between the two adjacent endplates after surgical treatment.
Technique
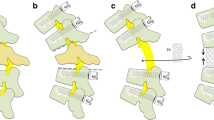
The single-stage posterolateral approach with posterior corpectomy and 360° decompression of the neuronal structures as well as anterior and posterior stabilization is shown in Fig. 1, and was previously described [9]. For sagittal alignment reconstruction, complete tissue removal was required to prevent spinal cord compression during the procedure and create enough space for re-lordosation. This mobilization of the upper against the lower levels simplifies re-lordosation during the reconstruction manoeuvre and avoids implant failure.
Schematic illustration of the surgical procedure. a The involved vertebral body with metastatic spinal cord compression (grey) and kyphotic deformity is shown. (b) The vertebrectomy with resection of adjacent discs. The temporary rod to avoid distraction is not shown. The titanium mesh cage is applied in the decompression cavity without distraction manoeuver (c), which provides the opportunity to perform the correction manoeuvre using the mesh as a hypomochlion and re-lordose by consecutive compression through the screw-rod system (d). The live operative procedure is performed with two mesh cages, as shown in (c)
Once resection of the vertebral body and adjacent discs was complete, the length and width of the cavity were measured using fluoroscopy and compared with the pre-operatively measured height and width dimensions on the CT/MRI-scan. Two length-adjusted mesh cages were created and filled with cancellous bone from the iliac crest. None of the locally excised bone was used for grafting.
The mesh cages were placed into the cavity on each side with fluoroscopic guidance. Due to the implantation of one mesh cage on each side, only a small space was necessary. To perform the re-lordosation manoeuvre, dorsal structures (i.e., facet joints, lamina, and partial pedicles) had to be removed in order to create enough space. The implanted mesh cages acted like a hypomochlion around the re-lordosation (Fig. 1). Length-adjusted rods precontoured to the former anatomic shape were finally fixed and step-by-step shortening was performed under fluoroscopic control with gentle manual palpation of the spinal cord. To avoid loosening or even break out of the screws due to a relatively high force on the screws during the step-by-step shortening and reconstruction of sagittal alignment, the caudal segments were fixed and the cranial segments were converged stepwise to the caudal segments because of higher counter bearing. Cranial pull-out of the screws can thus be avoided (Fig. 1d *,#). Neuroforaminal exits were checked to avoid nerve root entrapment. A posterior spondylodesis was performed at all levels. Standard wound drainage and stepwise suture closures of the fascia and skin completed the procedure (see Fig. 2).
Follow-up
Further oncology therapy was performed as determined by the interdisciplinary tumour board. Patients received early physiotherapeutic mobilization and required no extended post-operative bed rest.
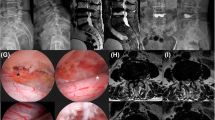
Exemplary documentation of patient # 4: a pre-operative CT-Scan in the lateral plane showing a severe deformity with 20° kyphosis, b sagittal pre-operative T2 MRI-Scan with compression of the spinal cord with metastatic destruction of the vertebral bodies, b pre-operative T2 MRI-Scan with intra-spinal mass in the axial plane causing stenosis of the spinal cord and acute walking ataxia at the patient’s clinical examination, d post-operative axial CT-Scan with decompression and two mesh grafts in place, e post-operative CT-Scan in the sagittal plane showing the reconstruction of the spine and the reduction of kyphotic deformity (4°). A.p. X-ray and lateral X-ray showing the reconstruction and improved kyphotic deformity in the follow-up examination (f, g)
Post-operative radiographs and clinical data were obtained after surgery, before discharge, and during follow-up visits that took place 3, 6, and 12 months after surgery, and annually after the first year.
Follow-up time was calculated from the time of surgery until the date of the last follow-up. Statistical analysis was carried out using Wilcox test for paired data. A significant difference between the groups was assumed when p < 0.05. Statistical Product and Service Solutions software version 15.0.1 (SPSS Inc., Chicago, IL) was used for computed analysis.
Results
A significant reduction of hyperkyphosis and preservation of neurological function could be observed in all 14 patients [8 male (57.1 %) and 6 female (42.9 %)].
The pre-operative Tomita Scores were evaluated with a mean of 4.1 (range 2–7), the Tokuhashi Score had a mean of 9.5 (range 8–11), and the Spinal Instability Neoplastic Score mean was 12 (range 10–14). The mean pre-operative Karnofsky performance status scale was 75 pre-operative (range 50–100) and 80 at latest follow-up (range 60–100).
The mean age at surgery was 63.6 years (range 51–78 years). The mean surgical time was 278 ± 64 min (Table 2). The mean follow-up time was 12 months (range 3–21 months).
Histological diagnoses of the primary tumours are shown in Table 1. The most common tumour types were plasmacytoma (35.7 %, n = 5), breast cancer (21.4 %, n = 3), and renal cell carcinoma (14.2 %, n = 2, patients #6 and #11, treated with pre-operative embolization). Anatomic localization as well as the length of decompression (shortening of the treated segment in comparison with the supposed height of the affected vertebral body before metastatic involvement) and instrumentation are shown in Tables 1 and 3.
On average, the patients reported pre-operative back pain of 7.2 on the VAS (range 4–10) with a reductions to 4.4 (range 2–6) at discharge and 2.0 (range 0–5) at the latest follow-up (p < 0.005).
On ODI at latest follow-up, 35.7 % of the patients scored minimal disability (0–20 %), 50.0 % reported moderate disability (21–40 %), 14.3 % had severe disability (41–60 %), and there were no reports of complete disability at the last follow-up. Initial ODI was not observed. The Frankel score improved from D to E (three patients) or was equal comparing pre- and post-operative scores. No neurological deterioration could be detected. The data are presented in Table 2.
No cases required further revision surgery due to persistent instability or neurological complications. No cases of chest drain placement, pulmonary complication or brace immobilization were encountered.
Radiographic data
Radiological segmental kyphosis was corrected from a mean of 16° (range 8°–26°) to 4° (1°–6°) (p < 0.001) post-operatively with a mean subsequent loss of 3° at the final follow-up (p < 0.003) according to the technique of Cobb [8] (Table 3). The angle was measured by CT-scan. However, the segmental kyphosis correction was still significant. The mean segmental shortening to the healthy segment was 0.94 cm on average (range 0.3–1.6 cm). In the radiological follow-up examinations, 20.0 % of the patients achieved a grade 1 bony fusion, 50.0 % had a grade 2, and 30.0 % reached a grade 3. No grade 0 healing was detected. No signs of screw loosening were observed (Table 4).
Discussion
This study found that the application of a posterior vertebral column resection with 360° osteosynthesis in MESCC constitutes a relatively fast, safe, and adequate surgical procedure, as described by others [1, 4, 5, 9, 43]. In contrast to others, we could further demonstrate that a significant reduction of kyphotic deformity is realizable.
Radiation therapy remains the first-line therapy in many patients with metastatic spinal tumours with confirmed effectiveness in avoiding neurological deterioration and long-term tumour control in patients without epidural compression [28]. However, decompression is indicated in patients with spinal metastases who have high-grade epidural compression (MESCC) with or without neurological deficit and three-column instability. One indication in this kind of patients is a fracture with spinal instability, spinal cord compression resulting from retropulsion of bony elements into the spinal canal. A second and different pathology is a metastatic mass lesion growing into the spinal canal mainly from anterolateral. In clinical results, both pathologies drive in the same result: a 360° decompression and stabilization in the established and described techniques [1, 4, 5, 9, 43].
A prospective clinical trial of direct decompressive surgical resection and stabilization with radiotherapy vs. radiotherapy alone for MESCC from Patchell et al. revealed a significant difference in the ability of surgical patients to walk compared to those treated by radiotherapy alone (84.0 vs. 56.9 %, p = 0.001) [31]. A meta-analysis conducted by Lee [26] also supported this finding.
Even without existing neurological deficits, the preservation of neurological function in three-column destruction, progressive deformity and pain may be considered for stabilization procedures.
Therefore, Fisher et al. have proposed the Spinal Instability Neoplastic Score (SINS), which encompasses the location of the tumour, the character and type of pain related to movement, the quality of the bone, spinal alignment, vertebral collapse and involvement of the posterior elements [15]. SINS is valuable in deciphering which patients require a stabilization procedure partially independent of the histological type (i.e., plasmocytoma as hematologic neoplasm with primary systemic treatment options if, for example, progressive deformity occurs) [16].
Stabilization can be achieved via anterior or posterior approaches. However, in the cases of a necessary resection of more than 50 % of the vertebral body diameter, a reconstruction of the vertebral body is usually required, as the maximal load is transmitted through the anterior column [40]. This can be achieved by an anterior approach as described by Harrington [20]. The advantage of this approach is that in addition to the complete ventral decompression, it provides an excellent opportunity for deformity correction, which is, in terms of hyperkyphosis, not explicitly described in sole dorsal stabilization procedures in MESCC.
However, this anterior approach frequently has to be combined with a posterolateral decompression and circumferential fixation via a subsequent surgery through a posterior approach and is accompanied by additional approach-related complications, such as reduction of vital capacity due to manipulation of pleura and temporary lung collapse [42]. It is also important to note that the regular anterior approach is technically very demanding above T 5 or 6 [19], depending on patient anatomy. Pure dorsal approaches, as described in this study and in previous publications, allow access to the entire thoracic vertebral column [9].
To avoid a two-stage procedure, many authors have performed a single-stage posterior or a posterolateral approach [1, 4, 5, 9, 43]. The surgical technique, as well as the indication of three-column tumour decompression and dorsal instrumentation via a single-stage dorsal or dorsolateral approach, is similar in the majority of published series [1, 4, 5, 9, 23, 43].
Amankulor, Bilsky, and Wang et al. recommended a surgical approach for MESCC with vertebral body involvement through a transpedicular resection of the vertebral body with anterior reconstruction using polymethyl methacrylate (PMMA) and Steinman pins (4,43, and 2). Studies suggest that PMMA reconstruction provides good mechanical stability and possible intrinsic antineoplastic properties [10, 32].
Eicker, Eleraky, Hofstetter, and Jandail modified this technique using expandable cages or titanium mesh cages with the advantage of less artefacts in follow-up imaging by magnetic resonance imaging (MRI) in comparison with the ferromagnetic artefacts produced by the Steinman pins and better ossification [9, 10, 22, 23].
Common in all publications is the missing characterization of the correction of hyperkyphotic deformity. Usually described is only the preservation of a pre-operative existing deformity without progression in follow-up examination [2, 4, 9, 22].
Eleraky et al. [10] reported a slightly improved lordosis for expandable cages compared to PMMA reconstruction after a single-stage lumbar posteroloteral approach, which did not reach significance. In contrast, this study supports that further deterioration and implant failure can be prevented with sufficient decompression and stabilization as well as hyperkyphosis reduction with reconstruction of the sagittal alignment.
Our described technique combined the idea of sufficient correction of a pathologic hyperkyphosis with the reduced invasiveness of a single-stage dorsal approach. A mean radiological proven segmental kyphosis correction of 12° was performed. This may reduce back pain and lead to lower hardware failure rates. From clinical point of view, this can also be detected in the improvement of quality of life [7, 12, 14, 26, 34].
One reason for the possible correction of deformity in the presented study is the opportunity of segmental shortening without neurological deterioration. According to Tomita, column shortening can be characterized into three phases: Phase 1 (safe range) involves spinal shortening within one-third of the vertebral segment, which is characterized by no deformity of the dural sac or the spinal cord. Phase 2 (warning range) has spinal shortening between one-third and two-thirds of the vertebral segment, which is characterized by shrinking and buckling of the dural sac and no deformity of the spinal cord. Phase 3 (dangerous range) is when spinal shortening occurs in excess of two-thirds of the vertebral segment, which is characterized by spinal cord deformity and compression by the buckled dura [41].
The results of this study showed a mean shortening of 37 % with seven cases of Phase 1 shortening and seven cases of Phase 2 shortening, with a maximum of 65 %. No neurological deterioration could be detected directly after surgery or on follow-up.
Second, a stepwise compression of the anterior titanium mesh between the vertebral body was performed, using the mesh as a hypomochlion. This procedure results in a reduction of kyphotic deformity with an accompanied compression and therefore, fixation of the implanted cages. This leads on to anterior vertebral column stability with low loss of correction on follow-up and no hardware failure.
Amankulor et al. also described a low rate of symptomatic hardware failure in patients with MESCC undergoing posterior decompression and instrumentation of 2.8 % [2]. His patient population had a limited life expectancy. Surgery was indicated regardless of the number of spinal column metastases or pathology. In this work, risk factors were detected for symptomatic hardware failure: iatrogenic chest wall destabilization after rib resection, construct lengths spanning six or more contiguous vertebral segments, and women with MESCC. In addition, the work of Quraishi [35] described lower complication rates in patients with lower epidural spinal cord compression (ESCC).
In this study, none of the patients had an iatrogenic chest wall destabilization due to a modified costotransversectomy with avoidance of the additional anterior approach. Furthermore, instrumented segments were always less than six levels, and the women in this study were in three of six cases patients with breast cancer with good prognosis compared with other tumour entities.
In addition to hardware failure, Akeyson et al. described in his group of patients a mean survival time of 29 weeks and a total of 13 complications in 25 patients [1]. Four of these complications were each cerebrospinal fluid fistulas or migration of hardware. In the present series, pedicle instrumentation was performed instead of Luque rectangle, which leads to higher primary stability. In combination with the compression/shortening technique, hardware failure could not be detected during the mean follow-up time of 12 months. In addition, no dural tear or fistula was detected.
In the work of Fehlings et al., two patients required a second spinal surgery: one for progressive neurologic deficits as a result of a spinal hematoma and one for screw malposition [14].
In the presented series with subselected patients (expected survival time above 2 years, not being paraplegic for longer than 24 h before study entry), the bias is obvious, but characterizes exactly the patients in which this procedure should be used.
Limitations
The limitations of this study include its retrospective nature and the relatively short follow-up time. Consequently, we were unable to account for other medical conditions that may have confounded the results. The small sample size as well as patient selection might have contributed to a certain selection bias.
Conclusion
Adequate tissue removal, custom tailored relative small titanium cages, and a stepwise reduction of the kyphotic deformity led to significant kyphotic correction 2 days after surgery as compared to the pre-operative measure. Although the kyphotic deformity slightly increased over the follow-up period, the degree of kyphotic deformity remained significant less than pre-operatively. In addition, patients reported sustained pain relief and there were no neurological deterioration post-operatively.
References
Akeyson E, McCutcheon IE (1996) Single-stage posterior vertebrectomy and replacement combined with posterior instrumentation for spinal metastases. J Neurosurg 85:211–220
Amankulor NM, Xu R, Iorgulescu JB, Chapman T, Reiner AS, Riedel E, Lis E, Yamada Y, Bilsky M, Laufer I (2014) The incidence and patterns of hardware failure after separation surgery in patients with spinal metastatic tumors. Spine J 14:1850–1859
Barron KD, Hirano A, Araki S, Terry RD (1959) Experiences with metastatic neoplasms involving the spinal cord. Neurology 9:91–106
Bilsky MH, Boland P, Lis E, Raizer JJ, Healey JH (2000) Single- stage posterolateral transpedicle approach for spondylectomy, epidural decompression, and circumferential fusion of spinal metastases. Spine 25:2240–2249
Bridwell K, Jenny A, Saul T, Rich KM, Grubb RL (1998) Posterior segmental spinal instrumentation (PSSI) with posterolateral decompression and debulking for metastatic thoracic and lumbar spine disease: Limitations and technique. Spine 13:1383–1394
Byrne TN, Benzel EC, Waxman SG (2000) Epidural tumors. In: Byrne TN, Benzel EC, Waxman SG (eds) Diseases of the spine and spinal cord. Oxford University Press, Oxford, pp 166–205
Choi D, Fox Z, Albert T, Arts M, Balabaud L, Bunger C, Buchowski JM, Coppes MH, Depreitere B, Fehlings MG, Harrop J, Kawahara N, Martin-Benlloch JA, Massicotte EM, Mazel C, Oner FC, Peul W, Quraishi N, Tokuhashi Y, Tomita K, Verlaan JJ, Wang M, Crockard HA (2015) Prediction of quality of life and survival after surgery for symptomatic spinal metastases: a multicenter cohort study to determine suitability for surgical treatment. Neurosurgery 77(5):698–708
Cobb J (1948) Outline for the study of scoliosis. In: Instructional course letters, vol 5. American Academy of Orthopaedic Surgeons, Ann Arbor
Eicker SO, Cornelius JF, Steiger HJ, Hänggi D (2012) 360-degree osteosynthesis via a posterolateral transpedicular approach in highrisk patients. Eur Spine J 21:1207–1213
Eleraky M, Papanastassiou I, Tran ND, Dakwar E, Vrionis FD (2011) Comparison of polymethylmethacrylate versus expand- able cage in anterior vertebral column reconstruction after pos- terior extracavitary corpectomy in lumbar and thoraco-lumbar metastatic spine tumors. Eur Spine J 20:1363–1370
Fairbank JC (1995) Use of oswestry disability index (ODI). Spine (Phila Pa 1976) 20:1535–1537
Falicov A, Fisher CG, Sparkes J, Boyd MC, Wing PC, Dvorak MF (2006) Impact of surgical intervention on quality of life in patients with spinal metastases. Spine (Phila Pa 1976) 31(24):2849–2856
Fan Y, Zhou X, Wang H, Jiang P, Cai S, Zhang J, Liu Y (2016) The timing of surgical intervention in the treatment of complete motor paralysis in patients with spinal metastasis. Eur Spine J. doi:10.1007/s00586-016-4406-7
Fehlings MG, Nater A, Tetreault L, Kopjar B, Arnold P, Dekutoski M, Finkelstein J, Fisher C, France J, Gokaslan Z, Massicotte E, Rhines L, Rose P, Sahgal A, Schuster J, Vaccaro A (2016) Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: results of the prospective multicenter AOSpine study. J Clin Oncol 34(3):268–276
Fisher CG, DiPaola CP, Ryken TC et al (2010) A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 35:E1221–E1229
Fourney DR, Gokaslan ZL (2003) Spinal instability and deformity due to neoplastic conditions. Neurosurg Focus 14:e8
Fourney DR, Gokaslan ZL (2003) Thoracolumbar spine: surgical treatment of metastatic disease. Curr Opin Orthop 14:144–152
Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ (1969) The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia 7:179–192
Guastella V, Mick G, Soriano C, Vallet L, Escande G, Dubray C, Eschalier A (2011) A prospective study of neuropathic pain induced by thoracotomy: incidence, clinical description, and diagnosis. Pain 152(1):74–81
Harrington KD (1984) Anterior cord decompression and spinal stabilization for patients with metastatic lesions of the spine. J Neurosurg 61:107–117
Hato T, Kawahara N, Tomita K, Murakami H, Akamaru T, Tawara D, Sakamoto J, Oda J, Tanaka S (2007) Finite-element analysis on closing-opening correction osteotomy for angular kyphosis of osteoporotic vertebral fractures. J Orthop Sci 12:354–360
Hofstetter CP, Chou D, Newman CB, Aryan HE, Girardi FP, Hartl R (2011) Posterior approach for thoracolumbar corpectomies with expandable cage placement and circumferential arthrodesis: a multicentre case series of 67 patients. J Neurosurg Spine 14:388–397
Jandial R, Kelly B, Chen MY (2013) Posterior-only approach for lumbar vertebral column resection and expandable cage reconstruction for spinal metastases. J Neurosurg Spine. 19(1):27–33. doi:10.3171/2013.4.SPINE12344
Karnofsky DA, Burchenal JH (1949) The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM (ed) Evaluation of chemotherapeutic agents. Columbia Univ Press, New York, p 196
Kawahara N, Tomita K, Baba H, Kobayashi T, Fujita T, Murakami H (2001) Closing-opening wedge osteotomy to correct angular kyphotic deformity by a single posterior approach. Spine 26:391–402
Lee CH, Kwon JW, Lee J, Hyun SJ, Kim KJ, Jahng TA, Kim HJ (2014) Direct decompressive surgery followed by radiotherapy versus radiotherapy alone for metastatic epidural spinal cord compression: a meta-analysis. Spine 39(9):pE587–pE592
Loblaw DA, Laperriere NJ, Mackillop WJ (2003) A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol) 15(4):211–217
Maranzano E, Latini P (1995) Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys 32:959–967
Panjabi MM (1988) Biomechanical evaluation of spinal fixation devices: I. A conceptual framework. Spine 13:1129–1134
Panjabi MM, Abumi K, Duranceau J, Crisco JJ (1988) Biomechanical evaluation of spinal fixation device: II. Stability provided by eight internal fixation devices. Spine 13:1135–1140
Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, Mohiuddin M, Young B (2005) Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 366:643–648
Piccioli A, Ventura A, Maccauro G, Spinelli MS, Del Bravo V, Rosa MA (2011) Local adjuvants in surgical management of bone metastases. Int J Immunopathol Pharmacol Jan-Mar 24(1 Suppl 2):129–132
Prasad D, Schiff D (2005) Malignant spinal-cord compression. Lancet Oncol 6(1):15–24
Quan GM, Vital JM, Aurouer N, Obeid I, Palussière J, Diallo A (2011) Pointillart V (2011), Surgery improves pain, function and quality of life in patients with spinal metastases: a prospective study on 118 patients. Eur Spine J 20(11):1970–1978
Quraishi NA, Arealis G, Salem KM, Purushothamdas S, Edwards KL, Boszczyk BM (2015) The surgical management of metastatic spinal tumors based on an Epidural Spinal Cord Compression (ESCC) scale. Spine J 15(8):1738–1743. doi:10.1016/j.spinee.2015.03.040
Schaberg J, Gainor BJ (1985) A profile of metastatic carcinoma of the spine. Spine 10:19–20
Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS (1995) When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 61:277–284
Sundaresan N, Galicich JH, Bains MS, Martini N, Beattie EJ (1984) Vertebral body resection in the treatment of cancer involving the spine. Cancer 53:1393–1396
Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J (2005) A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 30:2186–2191
Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T (2001) Surgical strategy for spinal metastases. Spine 26:298–306
Tomita K, Kawahara N, Murakami H, Demura S (2006) Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci 11:3–12
Vedantam R, Lenke LG, Bridwell KH, Haas J, Linville DA (2000) A prospective evaluation of pulmonary function in patients with adolescent idiopathic scoliosis relative to the surgical approach used for spinal arthrodesis. Spine 25(1):82–90
Wang JC, Boland P, Mitra N, Yamada Y, Lis E, Stubblefield M, Bilsky MH (2004) Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. J Neurosurg (Spine 1) 3:287–298
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This work is completed under compliance with Ethical Standards.
Funding
This study was not funded.
Conflict of interest
None of the authors has received research grants. Author MD has received a speaker honorarium from Medtronic, Stryker, and Spineart. Author SOE has received a speaker honorarium from Icotec, Stryker, and Spineart. The other authors declare that they have no conflict of interest.
Additional information
M. Dreimann and M. Hoffmann contributed equally to this study and therefore share first authorship.
Rights and permissions
About this article
Cite this article
Dreimann, M., Hoffmann, M., Viezens, L. et al. Reducing kyphotic deformity by posterior vertebral column resection with 360° osteosynthesis in metastatic epidural spinal cord compression (MESCC). Eur Spine J 26, 113–121 (2017). https://doi.org/10.1007/s00586-016-4805-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-016-4805-9