Abstract
Purpose
In this article, we aim to describe the presentation and management of a case of spontaneous intracranial hypotension caused by a dural tear from a ventral thoracic osteophyte at the T12 level that was refractory to non-surgical treatment modalities. A review of the literature has been performed. Also a proposal of diagnostic and treatment algorithm is presented. Intracranial hypotension and CSF leak as a result of dural tear is a common phenomenon. However, the detection of the source of CSF leak from a thoracic spinal osteophyte has rarely been reported.
Methods
Diagnostic workup including MRI and CT Myelogram as well as application of epidural blood patches and surgical technique of hemilaminectomy and osteophytectomy by transpedicular approach have been described. Literature review was conducted using relevant search terms in PubMed.
Results
The patient’s spontaneous intracranial hypotension symptoms resolved and this persisted on follow up visits. Review our experience as well as similar cases in the literature pointed us towards a diagnostic and treatment algorithm.
Conclusions
Spontaneous resolution is the norm for intracranial hypotension of most etiologies and management of all such cases begins with fluid resuscitation coupled with bed rest. On failure of conservative therapy, autologous epidural blood patches into the spinal epidural space should be tried, which often produce an immediate relief of symptoms. Osteophyte-induced dural tear and consequent intracranial hypotension may require surgical intervention if the symptoms are refractory to conservative treatment. Under all circumstances a careful step-wise approach for diagnosis and treatment of spontaneous intracranial hypotension needs to be followed, as we have proposed in our article.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intracranial hypotension (IH) is a syndrome of relative or absolute loss of cerebrospinal fluid (CSF) volume, manifesting as a spectrum of neurologic problems including orthostatic headache as the most common symptom. Although the majority of such volume losses result from iatrogenic over-drainage of CSF or from obvious trauma to the thecal sac, a variety of etiologies for spontaneous weakening or tearing of the thecal sac and resultant CSF leak has been proposed [1]. Since its first description by Schaltenbrand [2] in 1938, spontaneous intracranial hypotension (SIH), although underestimated, has remained to be a persistent problem, and to this day there exists no single management protocol to facilitate standardized care to all patients suffering from it. Extravasation of CSF generally does not cause local problems, but with continued leakage, the clinical presentation invariably includes an orthostatic headache due to downward displacement of the brain causing traction on pain-sensitive dural structures as well as possible compensatory dilation of the dural sinuses and meningeal vessels [3, 4]. Although the characteristic feature of orthostatic headaches in SIH is resolution on lying down for 15–30 min, paradoxical headache that occurs on lying down has also been reported [5]. Patients may report a sudden onset of symptoms (‘thunderclap headache’), which may be holocranial or localized to frontal or occipital regions. It is, thus, prudent to include SIH in the differential for a sudden onset of severe headache. Additional symptoms include neck stiffness, hypoacusis, photophobia, and nausea. Hypoacusis is postulated to be a result of intralabrynthine pressure changes exerting traction on the cochlear nerve causing hearing disturbances. Cranial nerves II (blurred vision), III, IV, and VI (diplopia), V (paresthesias in facial distribution), VII (facial spasm) and IX (dysgeusia) may also be affected [6]. Some patients may also exhibit radicular pain in the upper extremities, although at least some of this may be due to degenerative cervical spine disease when osteophytes are present. In severe cases, diencephalic compression due to severe brain sag, which may falsely suggest Chiari I, may cause a stuporous appearance [7]. Mokri et al. [8] reported one such case of tonsillar herniation down to C2 level in a 9 year chronic case of spinal CSF leak. Veeravagu et al. [9] suggest that, of all the effects of SIH the most severe are due to subdural hematoma, and they almost always require neurosurgical intervention. Also, diplopia has been demonstrated to be the strongest positive predictor of CSF leaks if an SIH is suspected [9]. In this article we propose the institution of a protocol which we practice to diagnose and treat SIH patients in our facility.
Case report
Presentation and pre-operative course
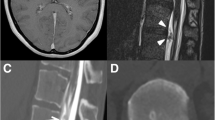
Our patient, a 38-year-old Caucasian female, presented with a 3 month history of severe positional headaches associated with nausea and vomiting, accompanied by a muffling noise in her ears and a progressively worsening gait. The patient became unable to stand for more than an hour as her headaches increased in severity, rendering her incapable of continuing with her daily chores and forcing her to lie supine and take pain medication frequently. She could not drive nor sit in a car except in a flat position. She had an extensive workup that included a brain magnetic resonance imaging (MRI) which was found to be normal, lacking the typical signs of SIH (see “Discussion”), a thoracolumbar MRI showing an anterior lumbar epidural fluid collection (Fig. 1), a lumbar puncture (LP) with an opening pressure of zero, and a computed tomography (CT) myelogram of the thoracolumbar spine that revealed a three mm T12–L1 osteophyte in the ventrolateral aspect of the of the thecal sac with CSF leaking into the epidural space (Fig. 2). This CSF leak was thought to be contributing to the patient’s symptoms. Multiple blood patches were attempted including a site-specific blood patch (Fig. 3). These provided mild temporary resolution of her symptoms, lasting for several days followed by resumption of her headaches. She was also treated with hyperhydration and caffeine products with no benefits. She was seen by several spine surgeons and no definitive treatment was offered to her. She was then referred to our clinic for consultation.
Post-myelography CT of the thoracolumbar spine with successive axial views from T12 to L1 (a through d) shows a 3 mm T12–L1 osteophyte on the left ventrolateral aspect of the thecal sac (arrow in c) with contrast leaking into the epidural space (arrowheads in b, c). The leak at this location was thought to be the source of this patient’s SIH
Physical examination revealed a well-developed 38 year old female, in severe distress due to severe headache, and unable to sit. She was lying on the examination table with positive photophobia and no diplopia. The rest of her neurological examination was non focal.
Operation and post-operative course
Since blood patches did not provide for a lasting resolution of the patient’s symptoms, she was offered the surgical option of osteophytectomy and dural repair. A T12–L1 left hemi-laminectomy and transpedicular approach were performed, and the osteophyte was resected. No spontaneous CSF leak was observed. However, when valsalva maneuver was performed, clear fluid was encountered. Given the location of the durotomy, a primary closure was not possible. A dural substitute (DuraGen, Integra) was placed with the addition of minimal dural sealant (Tisseel, Baxter). The wound was then closed in the usual fashion. Then a lumbar drain was placed. The patient was then placed flat for 24 h with CSF drainage at 10 ml per hour, then at 25° for 24 h with CSF draining at 10 ml per hour. The lumbar drain was weaned to off on post op day three. Patient was then allowed to ambulate. Once the headache did recur with upright posture, the drain was removed. Patient continued to do very well with the headache resolved completely. At her last follow-up, 4 years from surgery, the patient continued to be asymptomatic and have returned to baseline activities.
Discussion
Incidence
The increasing incidence of SIH in recent years, attributed to modern diagnostic modalities, suggests that it may no longer be a rare syndrome. The incidence, estimated to be 1:50,000 [10], has been measured in some studies to be half as common as subarachnoid hemorrhage with a female predominance of 2:1 [11]. The various causes of SIH are best categorized into primary and secondary causes. Primary pathologies include spontaneous tears due to weakness of the dural sac, spontaneous rupture of the nerve root sleeve, or problems causing meningeal diverticula such as abnormalities of connective tissues. Secondary causes include systemic hypovolemia, trauma, diagnostic lumbar punctures causing over-drainage of CSF (iatrogenic IH is associated more with larger lumbar puncture needles than with spinal anesthetic needles), spinal surgery, and chiropractic manipulation [12]. Degenerative lesions in the spine are a rare cause of secondary IH [12]; a review of literature shows less than ten reported cases of intracranial hypotension secondary to such lesions (Table 1), and SIH due to a thoracic column pathology has been only rarely reported in the literature. One case described a calcified T7–8 disc indenting the spinal cord, with an extradural collection of CSF at the same level [13]; one case involved a thoracic osteophyte associated with a CSF fistula [7], two other cases described single dorsal osteophytic spurs [9, 14], and one series illustrated two cases of intra-dural thoracic osteophyte with superimposed disc herniation [15]. Mechanical stressors are important for the pathogenesis of this particular secondary SIH. Although at varying degrees, each case necessitated surgical intervention. Amongst spinal bony pathologies, the lower cervical and upper thoracic spine sites have a higher predisposition for spontaneous CSF leak [16].
Diagnosis
Together with the clinical picture, the diagnosis of IH rests on a neuroradiological evaluation. A decrease in CSF volume, in accordance with the Monroe-Kellie doctrine, induces smooth dural contrast enhancement associated with cortical venous and venous plexus dilation, best seen on a contrast MRI [17]. However, this finding is non-specific and can be seen in meningitis, meningeal carcinomatosis, subarachnoid hemorrhage, superior sagittal sinus thrombosis, and post-craniotomy states. Additional findings include pituitary hyperemia, engorgement of venous sinuses, subdural fluid collections, pseudo-subarachnoid hemorrhage and sagging of the brain stem through the foramen magnum. These initial clues have been suggested in our diagnostic algorithm which we have created by compiling observations from case reports in the literature and our own experience (Fig. 4). Christoforodis et al. [17] pointed out that meningeal blush on external carotid artery angiography and anterior falcine artery enlargement were associated with SIH. Radioisotope cisternography may show limited ascent of tracer to the cerebral convexity and its early appearance in the bladder [10]. While not necessary to establish the diagnosis [7] biopsy of the meninges may show a layer of fibroblasts in the subdural zone with thin-walled blood vessels in an amorphous matrix in the absence of evidence of any inflammation or abnormal cells [8]. Although some patients may have normal CSF pressures, lumbar punctures may be useful in documenting intrathecal hypotension (<60 mmHg) along with the possibility of lymphocytic pleocytosis and xanthochromia. In general, however, lumbar punctures should be done very cautiously or avoided in the setting of imaging findings suggestive of low lying and particularly herniating tonsils. We consider this diagnostic modality to be purely optional. Diagnostic criteria [18] for SIH include the demonstration of extrathecal CSF on spinal imaging. In the absence of this particular imaging finding, cranial MRI demonstrating SIH is required with at least one of the following—(1) evidence of low CSF pressure, (2) spinal meningeal diverticulum, (3) improvement of symptoms following EBP administration. If extrathecal spinal CSF imaging findings nor MRI findings are present, two of the following are required—(1) low opening pressure, (2) spinal meningeal diverticulum, or (3) improvement of symptoms following EBP.
Therapeutic management
Once the diagnosis of SIH is suspected based on the clinical presentation along with any characteristic brain MRI findings, it is prudent to begin conservative measures. Spontaneous resolution is the norm for IH of most etiologies and management of all cases of SIH begins with ensuring fluid resuscitation coupled with bed rest [19]. Due to a relatively low incidence, no randomized controlled trials have been undertaken, but intravenous caffeine, steroids, and theophylline have been advocated as possible therapies. Elliot B solution (artificial CSF) can also be administered intrathecally to prevent impending transtentorial herniation [7]. Two cases of SIH from thoracic intervertebral disc prolapse have reportedly been treated with conservative management alone [13, 20]. Having failed conservative therapy, the mainstay of palliative treatment of SIH is autologous (and initially, undirected) blood patches into the spinal epidural space, which often produces an immediate relief of symptoms in as early as 2 h, starting with a 10–20 ml patch followed by a larger, 20–100 ml patch, followed by placing the patient in a Trendelenburg position. Acetazolamide can be used to reduce CSF pressure in conjunction with patches. Although there is limited data, many advocate that a uniform injection site in the lumbar region is as good as aiming for the site of the CSF leak, an idea that may expedite treatment [21]. Yokota et al. [14] reported the successful management of osteophyte-induced SIH by epidural blood patches (EBP) while Eross et al. [22] described the failure of targeted EBP in a similar patient. Hasiloglu et al. [15] also reported successful management by EBP alone, of two cases of SIH caused by thoracic osteophyte with superimposed disc herniation. The success rate of EBP depends on the severity of patient’s symptoms, timing of treatment, and the amount of blood injected [23, 24].
Failing a large volume blood patch, the ideal next step would be to locate the site of CSF leakage; the preferred non-invasive study being a fat-suppressed, fast-spin echo MRI, which may demonstrate extradural collections of CSF along a segment of the neuraxis. For more precise localization of the leak site, one will typically have to undergo the minimally invasive CT myelography, which often also demonstrates anatomical abnormalities such as bony spurs that are responsible for the leak. As denoted in our algorithm, if necessary, dynamic post-myelography CT scanning or dynamic myelography, where CT imaging is performed during the injection of diluted myelographic contrast material, can be used as they have a higher specificity for localizing the CSF leak site [25, 26]. In our case, CT myelography suited our patient better than MRI in precise localization of the CSF leak site. MRI showed anterior lumbar epidural fluid collection while the CT myelogram showed the causative osteophyte with signs of CSF leakage at the site.
Following the next step in our algorithm, if EBP fails to relieve SIH or there are frequent recurrences, surgery may be indicated or required to repair a known source of CSF leak. Veeravagu et al. [9] describe a case in which osteophyte-induced SIH led to formation of large bilateral holo-hemispheric subdural hematoma with tonsillar herniation and effacement of basilar cisterns. Craniotomy was performed for hematoma evacuation but collections recurred in merely 24 h of initial craniotomy. Surgery was then performed for osteophyte resection. If the precise location of the leak cannot be visualized by imaging studies, an exploratory surgery may be performed in the approximate location to assess the condition of the dura and nerve root sleeves [12]. Once the dura is exposed, if the thecal sac is not sufficiently full, intrathecal injection of saline while performing a Valsalva maneuver can help to locate the leakage site.
In our patient, blood patches predictably did not provide lasting relief and she required a transpedicular approach and resection of the ventral osteophyte given its proximity to the conus medullaris. Also, dural repair was attempted via duraplasty and application of fibrin glue. It is important to emphasize that CSF leak was not encountered during surgery except only upon Valsalva maneuver indicating the low pressure in the intrathecal compartment A lumbar drain was inserted to maintain a low CSF pressure and allow time for healing of the dural repair, and the patient was eased into an upright position with follow-ups showing complete resolution of symptoms.
Ideal treatment strategy for SIH is unknown. Table 1 identifies SIH cases secondary to degenerative spinal pathology reported in the literature, most of which resolved successfully with an EBP but some required surgical intervention. Our attempt at devising a diagnosis and treatment algorithm tries to reconcile various suggested approaches in the literature with our own experience. Larger case series may yield further insight into comparative effectiveness of various management strategies for this condition.
SIH is an underemphasized problem in neurosurgical practice. Most commonly presenting with an orthostatic headache, it can be a result of primary weaknesses in the meninges or secondary to various causes including degenerative spinal pathologies, in rare cases. Our patient presented with secondary SIH due to a thoracolumbar osteophyte and was managed with surgery after failure of conservative measures and EBP procedures. However, not all cases require surgery, and all patients need to be evaluated on a case-by-case basis.
References
Schievink WI, Meyer FB, Atkinson JL, Mokri B (1996) Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J Neurosurg 84(4):598–605. doi:10.3171/jns.1996.84.4.0598
Schaltenbrand G (1938) Neuere Anschauungen zur Pathophysiologie der Liquorzirkulation. Zentralbl Neurochir 3:290–299
Mokri B (2001) The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology 56(12):1746–1748
Mokri B, Posner JB (2000) Spontaneous intracranial hypotension: the broadening clinical and imaging spectrum of CSF leaks. Neurology 55(12):1771–1772
Mokri B, Aksamit AJ, Atkinson JL (2004) Paradoxical postural headaches in cerebrospinal fluid leaks. Cephalalgia Int J Headache 24(10):883–887. doi:10.1111/j.1468-2982.2004.00763.x
Schievink WI (2006) Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA J Am Med Assoc 295(19):2286–2296. doi:10.1001/jama.295.19.2286
Binder DK, Sarkissian V, Dillon WP, Weinstein PR (2005) Spontaneous intracranial hypotension associated with transdural thoracic osteophyte reversed by primary. dural repair. Case report. J Neurosurg Spine 2(5):614–618. doi:10.3171/spi.2005.2.5.0614
Mokri B, Parisi JE, Scheithauer BW, Piepgras DG, Miller GM (1995) Meningeal biopsy in intracranial hypotension: meningeal enhancement on MRI. Neurology 45(10):1801–1807
Veeravagu A, Gupta G, Jiang B, Berta SC, Mindea SA, Chang SD (2013) Spontaneous intracranial hypotension secondary to anterior thoracic osteophyte: resolution after primary dural repair via posterior approach. Int J Surg Case Rep 4(1):26–29. doi:10.1016/j.ijscr.2012.06.009
Ferrante E, Savino A, Sances G, Nappi G (2004) Spontaneous intracranial hypotension syndrome: report of twelve cases. Headache 44(6):615–622. doi:10.1111/j.1526-4610.2004.446012.x
Schievink WI, Roiter V (2005) Epidemiology of cervical artery dissection. Handbook of Cerebral Artery Dissection, Karger
Inamasu J, Guiot BH (2006) Intracranial hypotension with spinal pathology. Spine J Off J North Am Spine Soc 6(5):591–599. doi:10.1016/j.spinee.2005.12.026
Winter SC, Maartens NF, Anslow P, Teddy PJ (2002) Spontaneous intracranial hypotension due to thoracic disc herniation. Case report. J Neurosurg 96(3 Suppl):343–345
Yokota H, Yokoyama K, Noguchi H, Uchiyama Y, Iwasaki S, Sakaki T (2008) Thoracic osteophyte causing spontaneous intracranial hypotension. Cephalalgia Int J Headache 28(4):396–398. doi:10.1111/j.1468-2982.2008.01536.x
Hasiloglu ZI, Abuzayed B, Imal AE, Cagil E, Albayram S (2012) Spontaneous intracranial hypotension due to intradural thoracic osteophyte with superimposed disc herniation: report of two cases. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deformity Soc Eur Sect Cerv Spine Res Soc 21(Suppl 4):S383–S386. doi:10.1007/s00586-011-1828-0
Vishteh AG, Schievink WI, Baskin JJ, Sonntag VK (1998) Cervical bone spur presenting with spontaneous intracranial hypotension. Case report. J Neurosurg 89(3):483–484. doi:10.3171/jns.1998.89.3.0483
Christoforidis GA, Mehta BA, Landi JL, Czarnecki EJ, Piaskowski RA (1998) Spontaneous intracranial hypotension: report of four cases and review of the literature. Neuroradiology 40(10):636–643
Schievink WI, Maya MM, Louy C, Moser FG, Tourje J (2008) Diagnostic criteria for spontaneous spinal CSF leaks and intracranial hypotension. AJNR Am J Neuroradiol 29(5):853–856. doi:10.3174/ajnr.A0956
Allmendinger AM, Lee TC (2013) Spontaneous intracranial hypotension from calcified thoracic disc protrusions causing CSF leak successfully treated with targeted epidural blood patch. Clin Imaging 37(4):756–761. doi:10.1016/j.clinimag.2012.11.006
Rapport RL, Hillier D, Scearce T, Ferguson C (2003) Spontaneous intracranial hypotension from intradural thoracic disc herniation. Case report. J Neurosurg 98(3 Suppl):282–284
Cousins MJ, Brazier D, Cook R (2004) Intracranial hypotension caused by cervical cerebrospinal fluid leak: treatment with epidural blood patch. Anesth Analg 98(6):1794–1797 (table of contents)
Eross EJ, Dodick DW, Nelson KD, Bosch P, Lyons M (2002) Orthostatic headache syndrome with CSF leak secondary to bony pathology of the cervical spine. Cephalalgia Int J Headache 22(6):439–443
Sencakova D, Mokri B, McClelland RL (2001) The efficacy of epidural blood patch in spontaneous CSF leaks. Neurology 57(10):1921–1923
Paldino M, Mogilner AY, Tenner (2003) Intracranial hypotension syndrome: a comprehensive review. Neurosurg Focus 15(6):ECP2
Witiw CD, Fallah A, Muller PJ, Ginsberg HJ (2012) Surgical treatment of spontaneous intracranial hypotension secondary to degenerative cervical spine pathology: a case report and literature review. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deformity Soc Eur Sect Cerv Spine Res Soc 21(Suppl 4):S422–S427. doi:10.1007/s00586-011-1979-z
Luetmer PH, Mokri B (2003) Dynamic CT myelography: a technique for localizing high-flow spinal cerebrospinal fluid leaks. AJNR Am J Neuroradiol 24(8):1711–1714
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None of the authors has any potential conflict of interest.
Additional information
D. Dash and A. Jalali have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Dash, D., Jalali, A., Harsh, V. et al. Transpedicular surgical approach for the management of thoracic osteophyte-induced intracranial hypotension refractory to non-operative modalities: case report and review of literature. Eur Spine J 25 (Suppl 1), 209–215 (2016). https://doi.org/10.1007/s00586-016-4408-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-016-4408-5