Abstract
Purpose
Iatrogenic injuries to paraspinal muscles during the posterior lumbar surgery (PLS) cause a reduction in their cross-sectional areas (CSAs) and contractile densities over time post-surgery. This study aims to quantify such alterations.
Method
Pre- and postoperative CSAs (~6 months interval) of all paraspinal muscles were measured in six patients undergoing PLS using a 3-T magnetic resonance (MR) scanner to quantify the alterations in geometrical and tissue effective contractile (non-fatty) CSAs of these muscles at all lumbar levels. To examine the presence of any confounding effects on recorded changes within ~7-month period, measurements were also carried out on ten healthy volunteers.
Results
In the healthy population, an important (~22 %) portion of CSA of the erector spinae (ES) was noncontractile at the lower lumbar levels. Negligible variations over time in both the total geometrical (<1.7 % in average) and contractile (<1.2 %) CSAs of muscles were observed in the healthy group (i.e., no confounding effect). Following PLS, significant reductions were observed in the geometrical CSA of only multifidus (MF) muscle by ~14 and 11 % as well as in its contractile CSA by ~26 and 14 % at the L5–S1 and L4–L5 levels, respectively.
Conclusion
The total CSA of ES at lower lumbar levels shows substantial noncontractile contents in both healthy and patient populations. Biomechanical models of the spine should hence account for the noncontractile contents using only the effective contractile muscle CSAs. Postoperative variations in CSAs of paraspinal muscles may have profound effects on patterns of muscle activities, spinal loading, and stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Posterior lumbar surgeries (PLS) are common interventions for the treatment of low back disorders such as the degenerative disc disease, spinal tumor and vertebral fracture. During PLS, paraspinal muscles surrounding the surgical site are damaged due to ischemia, inadvertent dissection and denervation [1–3]. Such iatrogenic alterations decrease postoperative cross-sectional area (CSA) and tissue contractile density of back muscles [3–5, 7]. As a result, a significant reduction in the postoperative trunk strength has been reported in patients with longer retraction duration [4, 8]. To reduce such intraoperative injuries, minimally invasive approaches have been proposed [9]. Apart from the torque generation in different planes, paraspinal back muscles also play a crucial role in the equilibrium, control and stability of the ligamentous spine.
In the absence of noninvasive in vivo approaches to quantify muscle forces and spinal loads/stability, biomechanical modeling techniques are recognized as viable tools. Both optimization- and EMG-driven models use CSA of trunk muscles as input to partition external net moments among muscles. Any changes in CSA of back muscles as a result of intraoperative lesions may, therefore, alter the moment generation capacity of trunk, muscle activations and spinal loads/stability predicted by these models. Few modeling studies have estimated changes in postoperative pattern of muscle activations without subsequent quantification of the associated changes in the spinal loads and stability [10, 11]. In absence of detailed in vivo data on changes in CSA of individual back muscles following PLS, these models have either presumed some arbitrary values for the postoperative reduction in CSA of back muscles (i.e., 10, 30 and 40 %) [10] or removed some muscle fascicles in the model [11].
Several studies have measured CSAs of paraspinal muscles in healthy subjects using magnetic resonance (MR) or computed tomography (CT) techniques [12–14]. This is usually performed by digitizing muscles through their fascial boundaries using a mouse-guided tool. Following PLS, however, effective (i.e., contractile) CSA of muscles may diminish not only via the reduction of their geometrical CSAs but also by increases in the intramuscular noncontractile tissues (fatty infiltration) [3, 9, 15]. The fatty portion of muscle CSAs even in non-operated healthy population remains however unknown. By analyzing signal intensity of pixels, the MR imaging is an appropriate technique to distinguish the contractile (active tissues that are able to contract) from the noncontractile components (passive fat and connective tissues that do not contract). A number of studies have indicated reliability of MR imaging in the measurement of CSAs of human muscles as well as of their contractile and noncontractile contents [3, 12, 16–19].
To develop detailed postoperative models of the spine, the present study aims to measure pre- and postoperative (~6 months) CSAs of individual paraspinal muscles using MR imaging. This includes measurements of CSA of multifidus (MF), erector spinae (ES) (longissimus and iliocostalis as one muscle mass), quadratus lumborum (QL) and iliopsoas (IP) in six patients undergoing PLS. For the first time, pre- and post-operation CSAs of these muscles at all lumbar levels are separately investigated to quantify the alterations in their geometrical CSAs as well as noncontractile content. To examine the presence of any confounding effects on results within 6- to 7-month period as well as to quantify the noncontractile content in back muscles of healthy subjects, measurements are also carried out on ten healthy volunteers. Our future detailed biomechanical models [20, 21] studies will exploit such data to investigate the effect of intraoperative injuries to paraspinal muscles on postoperative muscle activations and lumbar loads/stability.
Materials and method
Subjects and surgical procedure
Six patients scheduled for a single-level degenerative lumbar disc disease by traditional (open) lumbar decompression laminectomy technique [22] at the L4–L5 or L5–S1 discs as well as ten healthy volunteers with no recent back complications were volunteered to undergo MR scans twice within an interval of about 6–7 months (Table 1). For patients, the initial MR scans were taken the day before operation. After routine physical and neurological tests, all patients were diagnosed with persistent low back pain as well as weakness and radiculopathy in the lower extremity (loss of motor power) caused by degenerative disc disease (herniated disc) as determined on MR images. All patients were operated on at a single institution by the same surgeon with more than 15 years of experience. The surgeon was not aware of the nature of this study so made no particular attempt to minimize muscle injuries. The subjects were positioned prone while skin incisions were made vertically along the center line. Paraspinal muscles were dissected and detached from the spinous process and lamina to make lamina visible (wound depth from skin to lamina). Bilateral decompression via vertebral lamina fenestration was accomplished with the muscles being pulled using a spinal retractor. After laminectomy, partial discectomy was performed by excision of herniated disc tissues. Each surgery lasted ~120 min with the muscle retraction time of ~100 min. Status of facet joints were normal and no denervation of these joints occurred. Patients were mobilized and were walking the day after surgery and no specific rehabilitation exercises were followed. The study was approved by the ethics committee and informed consent was read and signed by all subjects.
MR imaging protocol
A 3.0-T Siemens Trio Tim machine (Erlangen, Germany) was used for all scanning sessions by the same operator. T1-weighted images (repetition time, TR, of 767 ms and echo time, TE, of 14 ms) [3, 16, 18] with slice thickness of 3 mm were taken starting from the superior endplate of the S1 through slightly above the superior endplate of the T12 vertebra. Subjects laid on the MR table in a supine position with a pillow underneath their knees. All images were scanned with a matrix of 320 × 256 and a pixel size of 0.625 × 0.625 mm, thereby generating a field of view of 200 × 160 mm to encompass all back muscles. Identical imaging procedure was followed for both the initial and follow-up scans. Images were saved in the Digital Imaging and Communications in Medicine (DICOM) format.
Measurement of muscle CSAs
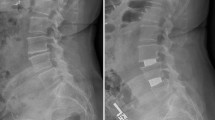
The DICOM files were imported into Mimics® (version 17.0, Materialise, Leuven, Belgium) to measure CSAs of the ES, QL and IP at each lumbar disc level from the T12–L1 through the L5–S1 (Fig. 1). Due to difficulties in discriminating between the multifidus (MF), longissimus and iliocostalis muscles on images, these muscles are usually considered as one muscle mass, i.e., erector spinae (ES) [23]. For patients and as needed in the detailed biomechanical models, the multifidus muscle was, however, carefully isolated using the fascial border separating it from the more laterally located longissimus [24]. No attempt was made to separate longissimus and iliocostalis muscles as their boundaries were less clear to identify in most images [25]. Three trained raters, blind to each other’s measurements, traced outer fascial boundaries of each muscle using a mouse-guided tool while excluding extramuscular fat and connective tissues. Subsequently, the software calculated the encompassed area within the traced boundary of each muscle (i.e., geometrical CSA including intramuscular noncontractile tissues).
Distinction of contractile and noncontractile muscle tissues
The PROFILE LINE (Fig. 2) and THRESHOLD (Fig. 3) functions in Mimics® allowed for the semi-automated identification of and thereby quantification of the CSAs of contractile (pure muscle) and intramuscular noncontractile (fat and connective tissues in case of the ES) components of muscles by analyzing signal intensity of pixels.
The PROFILE LINE and THRESHOLD functions used in Mimics® to determine lower and upper limits of pure normal muscle signal intensity for MF at the L4–L5 level of the same patient (Fig. 1) a before and b after surgery. The distinction between contractile and noncontractile components of each muscle at each level was made using the signal intensity of the pixels for each subject at each MRI session. For this, the PROFILE LINE function was initially used to measure signal intensity along several user-defined lines passing through different tissues including pure muscle, intramuscular fat, connective tissues and extramuscular fats (Fig. 2). From these, while varying for each subject at each MR session, the lower and upper limits of pure normal muscle signal intensity were determined to be, respectively, ~300 (lower limit) and 800 (upper limit). Subsequently, the THRESHOLD function was used within the border of each muscle to exclude tissues whose signal intensities were out of this range (i.e., noncontractile components such as intramuscular fat and connective tissues) (see Fig. 3)
Exclusion of intramuscular noncontractile components from the total CSA of MF at the L4–L5 level of the same patient (Fig. 1) a before and b after surgery (pixels corresponding to contractile components are highlighted)
Data and statistical analysis
Average coefficient of variation (CV value as standard deviation divided by mean) was calculated and paired correlation analyses for the three independent measurements (by three raters) of geometrical CSA of each muscle were carried out to investigate the inter-rater agreement [12, 26]. Mean of differences for the CSA of muscles (averaged over left and right muscles) measured under the two MR sessions (initial and follow-up) was calculated separately at each disc level (L5–S1 through T12–L1). For the patient group, for a given level and muscle if the mean of differences was larger than that of the control group, a paired t test analysis was performed to examine the statistical significance (p < 0.05). Moreover, for both patient and control groups and for each muscle at each level the CSA of the intramuscular noncontractile components (as percentage of the total geometrical CSA) were presented separately for the two MR scans. Similarly, paired t test analyses were carried out to determine statistical significance between initial and follow-up percentage of intramuscular noncontractile components at each level.
Results
Subject-specific data for the geometrical and intramuscular noncontractile CSAs for the initial and follow-up MR images are presented, separately for bilateral muscles, in the online supplementary data (SuppData.xlsx). Differences in the muscle CSAs measured over time were averaged over left and right muscles (Tables 2, 3). For the QL and IP muscles, CSAs of noncontractile components were found negligible compared to the associated total CSAs (less than 2 % in average for all 16 subjects at all levels for the two MR imaging scans). Therefore, results for the noncontractile CSAs are only presented for the ES in the control group and ES and MF in the patient group.
Control group
Average CVs for the three independent measurements for each muscle were smaller than 9.6 % (7.7 and 8.0 % for the ES, 9.1 and 9.6 % for the QL, and 6.1 and 6.9 % for the IP, respectively, for the 1st and 2nd MR sessions) with all R 2 > 0.97. Calculated geometrical CSAs of the muscles were therefore averaged over the three measurements at each vertebral level on each side. Mean of differences over time between the two MR sessions at each level remained smaller than 1.7 % (<1.1 %, <1.7 %, and <1.7 % for ES, QL, and IP, respectively) (Table 3). Percentage of intramuscular fat to the total CSA of ES was larger at lower levels (e.g., respectively, more than 22 and 12 % at the L5–S1 and L4–L5 levels which was statistically very significant when comparing these two lower levels to any of their upper levels) but only slightly changed over time between the two MR sessions (<1.2 % with no statistical significance) (Table 3). Any confounding parameter affecting CSAs of muscles in our patient group had, therefore, a negligible role.
Patient group
Average CVs for the three independent measurements for each muscle remained smaller than 10 % (4.4 and 5.2 % for the ES, 10.0 and 9.1 % for the MF, 6.3 and 6.5 % for the QL, and 3.0 and 2.9 % for the IP, respectively, for the 1st and 2nd MR sessions) with all R 2 > 0.96. Measured muscle CSAs were therefore averaged over the three measurements at each vertebral level and side. Average of differences in total CSA over time between the two MR sessions (at each level for all subjects) remained <5 % for the ES muscle, <3 % for the IP muscle and <4 % for the QL muscle (no statistical significance) (Table 3). On the other hand, larger and statistically significant reductions in the total CSA of the MF muscle at the L5–S1 (average reduction of ~13.7 % for left and right sides, p < 0.01) as well as L4–L5 (average reduction of ~10.8 % for left and right sides, p < 0.05) levels were observed (Table 3). No significant variations in CSA of the MF muscle at upper levels were, however, detected. Reductions in the intramuscular contractile CSA of the ES were within the range of those of control group except at the L4–L5 level where a reduction of 11.2 % was observed without any statistical significance (p = 0.07) (Table 3). On the other hand, larger and statistically significant reductions in the contractile CSA of the MF muscle at the L5–S1 (26.2 %, p < 0.001) and L4–L5 (14.2 %, p < 0.05) levels were recorded (Table 3).
Discussion
For the evaluation of CSAs of the ES and MF muscles, both total geometrical CSA and fatty infiltration content should be quantified while the latter can be overlooked for the QL and IP. A number of imaging studies have reported only the total CSA of the ES muscles (as the major lumbar extensor) in healthy populations [12–14]. As evident from the current measurements, a considerable portion of CSA of the ES muscle is, however, formed by noncontractile fatty components especially at the lower lumbar levels (average of ~22 and 12 % at the L5–S1 and L4–L5 levels, respectively) (Table 3). Even a greater portion of the preoperative CSA of the ES and MF muscles in our patients was infiltrated by fatty tissue (~18 % for the ES at the L4–L5 and ~28 and 21 % for the MF at the L5–S1 and L4–L5 levels, respectively) (Table 3). As for the MF muscle, the preoperative noncontractile CSAs were found large enough even at the upper lumbar levels (~22 and 32 % at the L1–L2 and T12–L1 levels, respectively). To improve accuracy, biomechanical models of the spine should hence account for the noncontractile contents using only the effective contractile muscle CSAs.
Both in vivo and modeling investigations have suggested an alteration in trunk muscle function, their activities and thus trunk strength following PLS [3, 4, 6, 10, 11]. Such alterations are assumed to occur as a result of intraoperative injuries to the erector spinae muscle (bulk of all posterior paraspinal muscles). For instance, Gille et al. [3] measured ~24 and 30 % reductions in the contractile CSA of the ES muscle (including MF) distal to the pedicle screw construct in patients undergoing pedicle screw-enhanced L4–L5 arthrodesis, respectively, with and without intraoperative cholinergic blockade (6 months follow-up). Results of the current study using high-resolution 3.0-T MR Images as well as careful isolation of multifidus (MF) muscle from longissimus and iliocostalis through their fascial border demonstrated that only the geometrical CSA of more medially located multifidus (MF) muscle was significantly reduced (by ~14 and 11 % at the L5–S1 and L4–L5 levels, respectively) while that of the ES (longissimus and iliocostalis) remained unchanged (Table 3). The contractile CSAs of both the MF and ES muscles were, however, reduced following PLS without any statistical significance for the ES (Table 3) thus indicating the case for minimally invasive approaches to preserve muscles [9]. In accordance with Gille et al. [3], no significant changes in the contractile CSAs of muscles were measured proximal to the surgical sites (i.e., at T12/L3 levels).
Likewise, evaluation of the postoperative CSA of the ES and MF muscles based solely on their geometrical CSA could underestimate the total muscle atrophy. For instance, a degree of atrophy of 0.88 (ratio of the postoperative to preoperative CSA of back muscles) has been reported in eight patients undergoing posterior lumbar inter-body fusion at L4–L5 or L5–S1 levels while digitizing muscles through their fascial boundaries [8]. Some other MR studies have similarly evaluated changes in CSAs of trunk muscles in patients with back pain or disc herniation based solely on their geometrical CSA by drawing polygons around outer borders [27, 28]. Results of the present study, however, clearly suggest that both the geometrical and fatty-infiltrated CSAs of muscles should be taken into account when evaluating changes in effective CSA of these muscles following interventions (Tables 2 and 3).
Inter-rater agreement for the measurement of geometrical CSA of muscles was assessed. All the average CVs remained smaller than 10 % (R 2 > 0.96) demonstrating an acceptable inter-rater agreement. Higher inter-rater variabilities (i.e., larger CV) of CSA measurement for QL and MF compared to other muscles are consistent with their relatively smaller areas. Intra-rater agreement was also assessed randomly and as expected smaller variabilities for one rater on different occasions were observed when compared to three raters at any given occasion [16]. Small changes in CSAs of muscles in our healthy group (Table 3) within a period of about 7 months measured by the three raters (measurements on two occasions) also indirectly confirm the intra-rater agreement.
Small changes in the muscle CSAs of our healthy group over time were likely due to different lumbar positions (thus different lines of action of muscles with respect to the MR scan plane), changes in the image positions along the lumbar spine, natural alterations within ~7 months period, and intra-rater variabilities. While the distinction between contractile and noncontractile components of each muscle was made through a semi-automated approach in Mimics®, choice of the lower and upper thresholds of pure normal muscle signal intensity could affect the results. These limits were set by two trained users through the PROFILE LINE function in Mimics® while ensuring (through eye inspections) proper separation of fatty components from contractile tissues (Fig. 3). We acknowledge the relatively low number of patients but we are confident that this would not compromise our conclusions regarding the significant postoperative reductions in the geometrical and contractile CSA of multifidus at the L4–L5 and L5–S1 levels. This is because such reductions were observed in almost every single patient (see supplementary data). Moreover, our findings agree with other studies showing a reduction in geometrical CSA of ES (Motosuneya et al. [8] with eight subjects) as well as in their contractile CSA (Gille et al. [3] with ten subjects).
References
Kawaguchi Y, Matsui H, Tsuji H (1994) Back muscle injury after posterior lumbar spine surgery. Part 2: histologic and histochemical analyses in humans. Spine 19:2598–2602
Kawaguchi Y, Matsui H, Tsuji H (1996) Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine 21:941–944
Gille O, Jolivet E, Dousset V, Degrise C, Obeid I, Vital JM, Skalli W (2007) Erector spinae muscle changes on magnetic resonance imaging following lumbar surgery through a posterior approach. Spine 32:1236–1241
Gejo R, Matsui H, Kawaguchi Y, Ishihara H, Tsuji H (1999) Serial changes in trunk muscle performance after posterior lumbar surgery. Spine 24:1023–1028
Kim DY, Lee SH, Chung SK, Lee HY (2005) Comparison of multifidus muscle atrophy and trunk extension muscle strength: percutaneous versus open pedicle screw fixation. Spine 30:123–129
Mayer TG, Vanharanta H, Gatchel RJ, Mooney V, Barnes D, Judge L, Smith S, Terry A (1989) Comparison of CT scan muscle measurements and isokinetic trunk strength in postoperative patients. Spine 14:33–36
Airaksinen O, Herno A, Kaukanen E, Saari T, Sihvonen T, Suomalainen O (1996) Density of lumbar muscles 4 years after decompressive spinal surgery. Eur Spine J 5:193–197
Motosuneya T, Asazuma T, Tsuji T, Watanabe H, Nakayama Y, Nemoto K (2006) Postoperative change of the cross-sectional area of back musculature after 5 surgical procedures as assessed by magnetic resonance imaging. J Spinal Disord Tech 19:318–322
Min SH, Kim MH, Seo JB, Lee JY, Lee DH (2009) The quantitative analysis of back muscle degeneration after posterior lumbar fusion: comparison of minimally invasive and conventional open surgery. Asian Spine J. 3:89–95
Bresnahan L, Fessler RG, Natarajan RN (2010) Evaluation of change in muscle activity as a result of posterior lumbar spine surgery using a dynamic modeling system. Spine 35:E761–E767
Gatton ML, Pearcy MJ, Pettet GJ (2011) Computational model of the lumbar spine musculature: implications of spinal surgery. Clin Biomech 26:116–122
Marras WS, Jorgensen MJ, Granata KP, Wiand B (2001) Female and male trunk geometry: size and prediction of the spine loading trunk muscles derived from MRI. Clin Biomech 16:38–46
Anderson DE, D’Agostino JM, Bruno AG, Manoharan RK, Bouxsein ML (2012) Regressions for estimating muscle parameters in the thoracic and lumbar trunk for use in musculoskeletal modeling. J Biomech 45:66–75
Seo A, Lee JH, Kusaka Y (2003) Estimation of trunk muscle parameters for a biomechanical model by age, height and weight. J Occup Health 45:197–201
Hu ZJ, Fang XQ, Zhou ZJ, Wang JY, Zhao FD, Fan SW (2013) Effect and possible mechanism of muscle-splitting approach on multifidus muscle injury and atrophy after posterior lumbar spine surgery. J Bone Joint Surg Am 95(24):e192(1–9)
Holmbäck AM, Askaner K, Holtås S, Downham D, Lexell J (2002) Assessment of contractile and noncontractile components in human skeletal muscle by magnetic resonance imaging. Muscle Nerve 25:251–258
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R (1998) Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 85:115–122
Kent-Braun JA, Ng AV, Young K (2000) Skeletal muscle contractile and noncontractile components in young and older women and men. J Appl Physiol 88:662–668
Hu ZJ, He J, Zhao FD, Fang XQ, Zhou LN, Fan SW (2011) An assessment of the intra- and inter-reliability of the lumbar paraspinal muscle parameters using CT scan and magnetic resonance imaging. Spine 36:E868–E874
Arjmand N, Shirazi-Adl A (2006) Model and in vivo studies on human trunk load partitioning and stability in isometric forward flexions. J Biomech 39:510–521
Hajihosseinali M, Arjmand N, Shirazi-Adl A, Farahmand F, Ghiasi MS (2014) A novel stability and kinematics-driven trunk biomechanical model to estimate muscle and spinal forces. Med Eng Phys. doi:10.1016/j.medengphy.2014.07.009
Epstein NE (2011) Lumbar Spine Stenosis. In: Winn HR (ed) Youmans neurological surgery. Saunders, Philadelphia, pp 2932–2933
Käser L, Mannion AF, Rhyner A, Weber E, Dvorak J, Müntener M (2001) Active therapy for chronic low back pain: part 2. Effects on paraspinal muscle cross-sectional area, fiber type size, and distribution. Spine 26:909–919
Belavý DL, Bansmann PM, Böhme G, Frings-Meuthen P, Heer M, Rittweger J, Zange J, Felsenberg D (2011) Changes in intervertebral disc morphology persist 5 mo after 21-day bed rest. J Appl Physiol 111:1304–1314
Ranson CA, Burnett AF, Kerslake R, Batt ME, O’Sullivan PB (2006) An investigation into the use of MR imaging to determine the functional cross sectional area of lumbar paraspinal muscles. Eur Spine J 15:764–773
Keller A, Gunderson R, Reikerås O, Brox JI (2003) Reliability of computed tomography measurements of paraspinal muscle cross-sectional area and density in patients with chronic low back pain. Spine 28:1455–1460
Barker KL, Shamley DR, Jackson D (2004) Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain: the relationship to pain and disability. Spine 29:E515–E519
Dangaria TR, Naesh O (1998) Changes in cross-sectional area of psoas major muscle in unilateral sciatica caused by disc herniation. Spine 23:928–931
Acknowledgments
This work was supported by grants from the Iran National Science Foundation (INSF), Sharif University of Technology (Tehran, Iran), Tehran University of Medical Sciences (Tehran, Iran), Institut de recherche Robert-Sauvé en santé et en sécurité du travail, IRSST, (Montréal, Canada), and Natural Sciences and Engineering Research Council of Canada (NSERC). Assistance of Mr. Saman Jamshidnejad is greatly appreciated.
Conflict of interest
The authors have no conflicts of interest concern.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghiasi, M.S., Arjmand, N., Shirazi-Adl, A. et al. Cross-sectional area of human trunk paraspinal muscles before and after posterior lumbar surgery using magnetic resonance imaging. Eur Spine J 25, 774–782 (2016). https://doi.org/10.1007/s00586-015-4014-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-4014-y