Abstract
Purpose
To investigate the difference of intra-dural space for spinal cord in magnetic resonance imaging between patients with and without developmental cervical stenosis and its clinical significance.
Methods
445 patients with cervical spondylotic myelopathy who had decompression surgeries were recruited. Based on their lateral radiographs, they were divided into stenosis and non-stenosis groups. On the magnetic resonance images, the ratio of the sagittal diameter of the dural sac to that of the vertebral body was measured and calculated as MRI Pavlov ratio at mid-vertebral level on sagittal images, and the ratio of the transverse area of the spinal cord to that of the dural sac was measured and calculated as occupation ratio on axial images from C3 to C7. The two ratios were compared between the two groups. We examined the correlations of the Pavlov ratio and the MRI Pavlov ratio between different vertebral levels. The correlation between the Pavlov ratio of each level and its corresponding MRI Pavlov ratio was also examined. The stenosis group was further divided into space-reserving and non-space-reserving subgroups based on their occupation ratios; then, clinical parameters were compared between the two subgroups to determine the clinical significance of the reserving space.
Results
The MRI Pavlov ratio of the stenosis group was significantly smaller at C3–C7 (P < 0.001), while the occupation ratio was larger without significance. The Pavlov and MRI Pavlov ratios were correlated significantly at different levels (P < 0.001). The Pavlov ratio correlated significantly with its corresponding MRI Pavlov ratio at each level (P < 0.001). For space-reserving subgroup, the recovery rate was lower (P < 0.05) than that for non-space-reserving group, and was higher in anterior approach than that in posterior approach (P < 0.05).
Conclusions
Developmental cervical stenosis is associated with a smaller sagittal diameter of dural sac, but does not lead to a significant decrease of intra-dural space available for the cord. For patients with normal intra-dural space, the recovery after anterior decompression surgery was better.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Developmental canal stenosis (DCS), one of the main precipitating factors of cervical spondylotic myelopathy (CSM), was diagnosed if the Pavlov ratio, the sagittal diameter of the middle spinal canal to the sagittal diameter of the middle vertebral body, was 0.82 or less on plain radiographs [1].
However, problems of this diagnostic criterion seemed to emerge with the development of magnetic resonance imaging (MRI). For example, whether the existence of the reserving space for the spinal cord was correlated with the stenosis of the bony canal and the optimal surgical approach for patients with DCS is not yet clear [2].
In this study, we measured and calculated the morphological values in MRI images in patients with or without DCS, and tried to reveal the influence of the intra-dural reserving space on the spinal cord, as well as the difference in their clinical manifestations.
Materials and methods
A cohort of 445 (252 males, 193 females) patients with CSM who received operation at our hospital between February 2006 and December 2010 underwent the recruitment retrospectively for the study. The average age was 56.63 years (ranging from 20 to 88).
Patients with simple CSM were included in our research. Patients with the following manifestations were excluded: any type of the ossification of posterior longitudinal ligament (continuous, limited or mixed), disc herniation or osteophyte with the occupation rate >50 % [3], ossification of ligamentum flavum, cervical kyphosis deformity (the angle from C2 to C7 < 0°or local Cobb angle >5°) [4, 5], and trauma history.
There were 230 (146 males, 84 females) patients included in this study. The average age was 55.78 years (ranging from 24 to 85). The average age of onset was 53.84 years (ranging from 23 to 84), and the average duration was 23.24 months (1–120 months). The average preoperative modified JOA score (mJOA) was 12.14 (4–16.5).
The radiographic data included the lateral images on X-ray, axial and sagittal T2-weighted magnetic resonance images in neutral position of the cervical spine from C3 to C7 in 230 patients, 1150 levels. The measurements were recorded as minimum 0.1 mm using the length measurement device of Centricity Enterprise Web 3.0 on plain radiographs. The MRI images were produced on a 1.5T Scanner (Siemens Sonata; Siemens Medical Systems, South Iselin, NJ). The sequences used were sagittal T2-weighted (TE 130 ms and TR 4481 ms) and axial T2-weighted (TE 112 ms and TR 3587 ms) and flip angle 6° with MTC. The parameters were measured by two senior spine surgeons independently, and the average value of each parameter at each level was taken for independent calculation. The definitions of the parameters were as follows:
-
1.
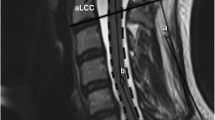
Pavlov ratio = the sagittal diameter of the spinal canal/the sagittal diameter of the vertebral body (Fig. 1a).
Fig. 1 a A The sagittal diameter of the vertebral body (the sagittal distance from the middle of the anterior surface to the middle of the posterior surface), B the sagittal diameter of the spinal canal (the sagittal distance measured from the middle of the posterior surface of the vertebral body to the closest point on the spino-laminar line), C the dynamic sagittal diameter of the spinal canal (the sagittal distance measured from the posterior inferior edge of the vertebral body to the closest point on the inferior adjacent spinous process)on X-rays. b D The sagittal diameter of the vertebral body, E the sagittal diameter of the dural sac (the sagittal distance between the midpoint of the anterior surface and the posterior surface of the dural sac) on T2-weighted sagittal MRI. c F The transverse area of the spinal cord, G the transverse area of the dural sac on T2-weighted axial MRI
-
2.
All cases were categorized into two groups: developmental cervical stenosis group (stenosis group, the ratio was smaller than 0.82) and non-developmental cervical stenosis group (non-stenosis group, the ratio was 0.82 or larger).
-
3.
MRI Pavlov ratio = the sagittal diameter of the dural sac/the sagittal diameter of the vertebral body (Fig. 1b).
-
4.
Occupation ratio = the transverse area of the spinal cord/the transverse area of the dural sac in axial image in T2-weighted MRI (Fig. 1c).
-
5.
Reserving space = the space surrounding the spinal cord filled with cerebrospinal fluid (CSF) in axial image in MRI.
-
6.
Segments with or without reserving space: the average of the occupation ratio in each segment of the non-stenosis group was considered as the standard. A segment whose occupation ratio was no more than the standard value was defined as with reserving space, otherwise without reserving space.
-
7.
Space-reserving/non-space-reserving subgroup: the cases who had three or more segments with “reserving space” were classified as space-reserving subgroup; otherwise classified as non-space-reserving subgroup.
Anterior cervical decompression and fusion was carried out on patients with ventral compression of the spinal cord caused by one or two segmental intervertebral disc herniation or osteophytes. Posterior single-door laminoplasty was carried out on patients with multi-segmental compression of the spinal cord due to severe DCS or degenerative cervical stenosis. The symptoms, physical signs and radiologic measurements such as the number of compression levels, the degree of compression, surgical trauma and general health status were also taken into consideration to facilitate the surgical option.
The cases were followed up for 29 months (24–72 months) on average. Patients with complications were ruled out at the final follow-up, such as spinal cord injury, postoperative epidural tear, cerebrospinal fluid fistula, or epidural hematoma. The severity of myelopathy was evaluated by the modified Japanese Orthopaedic Association (mJOA) score, and recovery rate (RR) was determined with Hirabayashi’s formula [(24-month postoperative score – preoperative score)/(17 – preoperative score) × 100 %)] [6].
The MRI Pavlov ratio and the occupation ratio were compared between the stenosis and non-stenosis group before surgery. We examined the correlations of the Pavlov ratio and the MRI Pavlov ratio between different vertebral levels. The correlation between the Pavlov ratio of each level and its corresponding MRI Pavlov ratio was also examined. The age, age of onset, duration of disease, pre- and postoperative mJOA score and RR were also compared between space-reserving and non- space-reserving subgroups.
Data were analyzed with SPSS 19.0 software (SPSS Inc, Chicago, IL, USA). Statistical significance was set at 0.05. Descriptive statistics in the form of mean ± SD for all spine parameters were obtained from all patients. All populations were confirmed to approximate the normal distribution by probability plots. Pearson’s correlation coefficient was used to calculate the correlation between MRI and clinical parameters. Equal variance criterion of all groups was upheld by Mauchly’s test. Then, Paired or Group Student t test was utilized to evaluate the difference among different groups.
Results
The intra-observer and inter-observer reliability was 0.86 and 0.75, respectively. There were 182 patients in stenosis group and 48 patients in non-stenosis group. Comparisons of MRI Pavlov ratio and occupation ratio between stenosis and non-stenosis group are shown in Table 1. The MRI Pavlov ratio was smaller significantly in stenosis group than that in non-stenosis group at all levels (P < 0.001). However, the occupation ratio was larger with no significance. The occupation ratio had the largest value at C5, and the smallest value at C7 in both groups.
The Pavlov ratios were correlated significantly between different levels (P < 0.001, Table 2), so as to the MRI Pavlov ratios (P < 0.001, Table 3). The MRI Pavlov ratio correlated with its corresponding Pavlov ratio at each level (P < 0.001, Table 4). The sagittal and dynamic sagittal diameters of the vertebral canal correlated significantly at C3–C7 (P < 0.01, Table 5).
The comparisons of the age, age of onset, duration of disease, pre- and postoperative mJOA score, RR in 24 months between space-reserving and non-space-reserving subgroups were shown in Table 6. The patients with shorter duration of the disease for clinics had a better RR in non-space-reserving subgroup (P < 0.05).
The pre- and postoperative mJOA score and the RR in space-reserving and non-space-reserving subgroups were shown in Tables 7 and 8, respectively. The surgical approach of the anterior decompression and fusion was significantly more effective than posterior single “opening-door” laminoplasty in space-reserving subgroup (P < 0.05). However, the results of the two approaches were similar in non-space-reserving subgroup.
Discussion
The bony cervical canal stenosis was thought to be one of the main precipitating factors that cause CSM [7, 8]. Pavlov et al. used the ratio of the sagittal diameter of the spinal canal to the sagittal diameter of the vertebral body from plain radiographs (Pavlov ratio) as a reliable determinant instead of the true diameter of the cervical spinal canal. They concluded that a value ≤0.82 represented stenosis in 92 % of cases with a 6 % false-positive rate [1].
However, evidence concerning the correlation between the Pavlov ratio and the true sagittal diameter of the cervical spinal canal were still controversial. Herzog et al. [9] reported a good correlation between the sagittal diameter of the spinal canal on plain films and sagittal CT images. Blackley [10] showed that the canal-to-body ratio was of limited value in the assessment of the true diameter of the canal. With a wide use of magnetic resonance images, Hulcelle et al. [11] made a direct measurement of the dural sac, spinal cord and other soft tissues, and found that an individual cervical spine bony measurement was unable to accurately assess the degree of compression and the reserving space for the spinal cord. After the measurement of the sagittal diameter of the dural sac and the spinal cord directly on T2-weighted sagittal MRI images, we utilized the MRI Pavlov ratio, a parameter reflecting the relationship between the vertebral body, and the dural sac and its surrounding soft tissues to evaluate the exact structure in the spinal canal to prevent the phenomenon that the dural sac still had adequate space around it, despite the canal was relatively narrow. Moreover, in this study, we found the MRI Pavlov ratio was smaller significantly in patients with DCS in every segment, which suggested that the vertebral canal bony stenosis was probably accompanied by a smaller sagittal diameter of the dural sac, and this could be a pathogenic factor influencing spinal cord compression. We also found that the Pavlov ratios were correlated significantly between different levels (P < 0.001, Table 2), so as to the MRI Pavlov ratios (P < 0.001, Table 3), suggesting morphological correlations between different cervical segments. The MRI Pavlov ratio also correlated with the Pavlov ratio at each level (P < 0.001, Table 4), indicating the morphological correlation between soft and bony structures. Dynamic stenosis was also a main precipitating factor for CSM [12]. In this study, we found that the sagittal and dynamic sagittal diameters of the vertebral canal correlated significantly (P < 0.01, Table 5), suggesting a steady stenosis situation in different postures.
In all cases, the spinal cord could be compressed from all directions, and its oval structure depended on both the sagittal and transverse structures. Therefore, the application of MRI to measure the soft tissues directly was meaningful. Okada et al. [13] measured the areas of the spinal cord, dural sac and spinal canal on T1-weighted axial images, and proposed a ratio that resulted from the area of the cord divided by the spinal canal to prevent individual variation. Based on the these previous research results, we established the concept “occupation ratio”, which was defined as the ratio between the area of the cord and the dual sac instead of the spinal canal, so that some soft tissues such as posterior longitudinal ligament or ligamentum flavum would not distract the assessment of real reserving space for the spinal cord. Okada reported its maximal value at C4 and C5; whereas the minimal at C7 [13]. Our results showed the maximal value at C5 and the minimal at C7 in both stenosis and non-stenosis groups, which suggested that the cerebral fluid in each transverse area at C5 was less than that at C7. Claire et al. [14] found that the spinal cord deformation was significantly reduced in the presence of CSF compared with the absence of CSF when applied upon transverse impact. These facts suggested that CSF may involve in the prevalence of CSM in DCS. We found the occupation ratio was larger in stenosis group but with no significance, which suggested that the transverse area of the CSF might be smaller than that in non-stenosis group. Therefore, the decrease of the sagittal diameter probably represented a decrease of the whole area of the dural sac and the reserving space for the cord. However, the incidence of such a difference was not as usual as the ones in MRI Pavlov ratio, which presumably indicated that the development of the spinal cord may adapt passively to the surrounding structures. A possible phenomenon may occur as “small spinal canal with small spinal cord”. Meanwhile, the RR was better in non-space-reserving subgroup than that in space-reserving subgroup, proposing that the more compressed the spinal cord was, the better it recovered after sufficient decompression.
The surgical treatment for CSM can be roughly categorized as anterior or posterior approach. The effective way of anterior decompression and fusion was intended for those who have herniated discs or osteophytes compressing spinal cord. On the other hand, the posterior laminoplasty was thought to be indicated for those who have both anterior and posterior compression as multiple segmental disc herniation, or for those who have degenerative or developmental canal stenosis so as to enlarge the spinal canal and make the spinal cord shift backwards [15–17]. Researches on the comparisons between the two surgical approaches have continued during the past four decades [18–23]. Based on our study about CSM patients with DCS, a decrease of the sagittal diameter of the spinal canal was not thought to be the only determinant for the surgical approach. Both anterior and posterior approaches can achieve satisfying curative results in non-space-reserving subgroup in our cohort, while anterior approach brought better outcomes in space-reserving subgroup, with respect to its RR. The possible reasons are as follows: first of all, the direct anterior decompression of the spinal cord and anterior spinal artery, which removes both the osteophytes and herniating discs, may ameliorate the blood supply of the nerve conduction bundle; [24, 25] second, a better CSF circulation might improve the inner environment surrounding the reserving space; [14] third, the anterior local bone graft fusion provides solid stability to prevent a micro-trauma on the spinal cord; fourth, the backwards shift of the spinal cord after laminoplasty may be an unpredictable factor for individual patient despite that such a mechanism was proved to be effective for clinical recoveries after posterior approach; finally, the enlargement of the whole space available for the spinal cord achieved from posterior single-door laminoplasty may not be able to relieve the neurogenic symptoms for patients who already have enough reserving space. On the contrary, for those without reserving space, the spinal cord that is compressed both ventrally and dorsally could be relieved by both subtotal vertebral corpectomy and laminoplasty to achieve a satisfactory clinical outcome. The mJOA RR was similar between the anterior and posterior approach groups, which suggested the local CSF environment was a critical factor for the spinal cord recovery after surgery.
Limitation
Due to its retrospective nature and short-time follow-up, the results of this study might be limited. A more convincing clinical conclusion would be drawn if a larger asymptomatic cohort is included.
Conclusion
DCS is associated with a smaller sagittal diameter of the dural sac, so-called as “small spinal canal with small spinal cord”; however, it does not lead to a significant decrease of intra-dural space available for the cord. For patients with normal intra-dural space, the recovery after anterior decompression surgery was better than posterior approach; while for patients with insufficient intra-dural space, the recovery after the two surgical decompression approaches was similar.
References
Pavlov H, Torg JS, Robbie B, Jahre C (1987) Cervical spinal stenosis: determination with vertebral body ratio method. Radiology 164:771–775
Prasad SS, O’Malley M, Caplan M, Shackleford IM, Pydisetty RK (2003) MRI measurements of the cervical spine and their correlation to Pavlov’s ratio. Spine 28(12):1263–1268
Park HJ, Kim SS, Chung EC, Lee SY, Park NH, Rho MH (2012) Clinical correlation of a new practical MRI method for assessing cervical spinal canal compression. Am J Roentgenol 199(2):W197–W201
Rajshekhar V, Arunkumar MJ, Kumar SS (2003) Changes in cervical spine curvature after uninstrumented one and two-level corpectomy in patients with spondylotic myelopathy. Neurosurgery 52:804–805
Ohara A, Miyamoto K, Naganwa T, Matsumoto K, Shimizu K (2006) Reliabilities of and correlations among five standard methods of assessing the sagittal alignment of the cervical spine. Spine 31(22):2585–2591
Harabayashi K, Mikayawa J, Satomi K, Maruyama T, Wakano K (1981) Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine 6:354–364
Wolf BS, Khilnani M, Malis L (1956) The sagittal diameter of the bony cervical spinal canal and its significance in cervical spondylosis. J Mt Sinai Hosp 23:283–292
Payne EE, Spillane JD (1957) The cervical spine. An anatomicopathological study of 70 specimens (using a special technique) with particular reference to the problem of cervical spondylosis. Brain 80:571–596
Herzog RJ, Weins JJ, Dillingham MF, Sontag MJ (1991) Normal cervical spine morphometry and cervical spine stenosis in asymptomatic professional football players. Spine 16:178–186
Blackley HR, Plank LD, Robertson PA (1999) Determining the sagittal dimensions of the cervical spine. J Bone Joint Surg Br 81:110–112
Hulcelle P, Dooms G, Demeure R, Mathurin P, Cornelis G (1990) Cervical myelopathy: MRI evaluation of cord compression. J Belg Radiol 73(1):15–19
Zhang L, Zeitoun D, Rangel A et al (2011) Preoperative evaluation of the cervical spondylotic myelopathy with flexion-extension magnetic resonance imaging: about a prospective study of fifty patients. Spine 36:E1134–E1139
Okada Y, Ikata T, Katoh S, Yamada H (1994) Morphologic analysis of the cervical spinal cord, dural sac, and spinal canal by magnetic resonance imaging in normal adults and patients with cervical spondylotic myelopathy. Spine 19:2331–2335
Jones CF, Kroeker SG, Cripton PA, Hall RM (2008) The effect of cerebrospinal fluid on the biomechanics of spinal cord: an ex vivo bovine model using bovine and physical surrogate spinal cord. Spine 33:E580–E588
Hirabayashi K, Toyama Y, Chiba K (1999) Expansive laminoplasty for myelopathy in ossification of the longitudinal ligament. Clin Orthop Relat Res 359:35–48
Tsuzuki N, Abe R, Saiki K, Zhongshi L (1996) Extradural tethering effect as one mechanism of radiculopathy complication posterior decompression of the cervical spinal cord. Spine 21:203–211
Uematsu Y, Tokuhashi Y, Matsuzaki H (1998) Radiculopathy after laminoplasty of the cervical spine. Spine 23:2057–2062
Douglas R, Thomas A (1999) Cervical spondylotic myelopathy approaches to surgical treatment. Clin Orthop Relat Res 359:158–166
Yonenobu K, Fuji T, Ono K, Okada Y, Yamanoto T, Harada N (1985) Choice of surgical treatment for multisegmental cervical spondylotic myelopathy. Spine 10(8):710–716
Kimura I, Shingu H, Nasu Y (1995) Long-term follow-up of cervical spondylotic myelopathy treated by canal-expansive laminoplasty. J Bone Joint Surg 77B:956–961
Goto S, Motizuki M, Watanabe T, Hiramatu K, Tanno T, Kitahara H et al (1993) Long-term follow-up of anterior surgery for cervical spondylotic myelopathy: with special reference to the MRI findings in 52 cases followed up over 12 years. Clin Orthop Relat Res 291:142–153
Yamasaki S, Kokubun M, Sato T, Ishii Y (1997) Anterior decompression and fusion for cervical myelopathy due to disc herniation: long-term evaluation of results. Spine Spinal Cord 10:797–801 (in Japanese)
Kawakami M, Tamaki T, Iwasaki H, Yoshida M, Ando M, Yamada H (2000) A comparative study of surgical approaches for cervical compressive myelopathy. Clin Orthop Relat Res 381:129–136
Breig A, el-Nadi A (1966) Biomechanics of the cervical spinal cord. Relief of contact pressure on and overstretching of the spinal cord. Acta Radiol Diagn (Stockb) 4:602–624
Breig A, Turnbull I, Hassler O (1966) Effects of mechanical stresses on the spinal cord in cervical spondylosis. A study on fresh cadaver material. J Neurosurg 25:45–56
Acknowledgments
The authors really appreciate the generous help from Mr. Wanheng Hu in correcting the English language of this article.
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Miao Yu and Yanchao Tang contributed equally to this work and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Yu, M., Tang, Y., Liu, Z. et al. The morphological and clinical significance of developmental cervical stenosis. Eur Spine J 24, 1583–1589 (2015). https://doi.org/10.1007/s00586-015-3896-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-3896-z