Abstract
Purpose
Some patients will experience post-operative back pain following lumbar discectomy, and the potential sources for that pain are poorly understood. One potential source is the vertebral endplates. The goal of this study was to document the changes that occur in lumbar endplates following discectomies, and to assess associations between endplate changes and clinical outcomes.
Methods
Changes in lumbar endplates and discs were assessed from X-rays, CT and MRI exams by comparing preoperative imaging with imaging obtained at yearly intervals up to 5 years. 260 endplates in 137 patients with single-level herniation and discectomy were analyzed. The geometry of osseous defects in the endplates was measured from the CT exams, and marrow and disc changes adjacent to endplates were assessed from the MRI exams. Clinical outcome assessments were collected at each time point. Descriptive statistics were used to describe endplate defect sizes, and logistic regression and analysis of variance were used to identify potential associations between endplate and vertebral body changes and clinical outcomes.
Results
Approximately 14 % of the endplates had osseous defects prior to surgery. After surgery, 24 % of inferior and 43 % of superior endplates had defects. Change occurred within the first year and remained relatively constant over the next few years. Disc signal intensity worsened and disc height decreased following surgery. New Modic changes were also observed. None of these changes were associated with having achieved a clinically significant improvement in outcome scores. The follow-up rates were low at the later time points and significant associations cannot be ruled out.
Conclusions
This study documents lesion characteristics in detail and supports that osseous defects in the endplates at the level of a lumbar discectomy may be a relatively common finding following surgery, along with disc height loss, loss of disc signal intensity, and Modic changes. The clinical significance of these imaging findings could not be conclusively determined in this study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nearly half a million lumbar discectomies are performed in the United States each year [1] and although the clinical outcomes are generally good, there are complications associated with the procedure including re-herniations, accelerated disc degeneration, and post-operative low back pain. The latter—post-operative low back pain—is relatively common but remains poorly understood. There are several possible sources of this back pain. One such source is the vertebral endplates which are known to be extensively innervated [2, 3].
There is evidence of an association between lesion size and back pain [4]. Endplates may be injured as a result of the original disc herniation [5–10] and there may also be changes that occur in the endplate that are directly associated with the surgical procedure itself [11]. Fragments of endplates can be found in extruded disc material [6, 12]. Thus, it might prove useful to fully delineate the endplate changes that occur following discectomy to better understand this as a potential source of pain. Some of the post-discectomy changes that can occur in the marrow adjacent to endplates have been described [13–15], but the specific osseous changes that can occur involving the subchondral plate of the endplate have not been delineated in detail.
Accordingly, the aim of this study was to provide detailed information regarding the endplate changes that can occur following lumbar discectomy. The preoperative endplate condition was used as the baseline internal control for pre-existing defects. Knowledge regarding subsequent endplate changes can help us to better understand this possible source of post-discectomy symptoms and may help identify possible intervention strategies aimed at reducing their occurrence.
Methods
Pre- and post-discectomy X-rays, CT, and MRI exams were obtained for patients who had a single-level lumbar discectomy procedure performed at one of five clinical sites in Europe. A total of 137 patients were enrolled in the study. All patients signed informed consents as approved by the respective ethics committees.
The CT exams were carefully assessed for apparent endplate defects. Axial, sagittal, and coronal plane slices were reviewed. Multi-planar reconstructions were also used to obtain sections parallel and perpendicular to the endplate. Endplate defects were defined as any localized morphologic feature of the osseous anatomy that could not be explained by the overall shape of the endplate, recognizing that endplates can have a variety of morphologies [16]. Defects present pre-operatively were identified and measured. Defects present post-operatively were also identified and measured, with particular attention to any changes that occurred between pre- and post-operative CT exams. The superior and inferior endplates of the intervertebral disc were assessed separately.
The total number of identifiable lesions was recorded. Quantitative measurements of lesion size were made for the two largest lesions in each endplate and these were labeled as the primary and secondary lesions. Defect size in the plane of the endplate was measured from sagittal and coronal plane slices and the depth of the defect was also measured perpendicular to the plane of the endplate in the sagittal and coronal planes. Each lesion was also approximated as an ellipse in the plane of the endplate using the axial, sagittal and coronal plane slices. The major and minor axis of each lesion were identified and measured. The depth was measured perpendicular to the plane of the endplate. Multi-planar reconstructions were used to assure that the measurement landmarks were consistent between sagittal, axial, and coronal views. In addition, the location of each defect was classified by the position on a 5 × 5 grid virtually overlaid on to each endplate (Fig. 1). The grid assigned to each defect was the grid that contained the largest proportion of the defect.
Illustration of the 5 × 5 grid that was used to define the location of the endplate defects in the axial plane, and the proportion of defects located in each grid box. Data for the superior and inferior endplates are provided, both at PreOp (113 disc spaces examined) and at 1 year PostOp (11 disc spaces examined). The lesion location was the grid box that contained the largest proportion of the lesion. Pre-operatively, most of lesions were toward the center of the endplate, with a higher proportion of lesions located along the posterior border of the endplates following discectomy
In addition, MRI exams were used to assess disc signal intensity (T2-weighted images) using the Pfirrmann et al. [17] grading system (Table 1). Modic changes in the vertebral bodies adjacent to the treated disc were also assessed (Table 2) [18]. Disc height and spondylolisthesis were measured from neutral lateral X-rays. These measurements and the changes between time points were measured using previously validated computer-assisted software [19] (QMA®, Medical Metrics, Inc). All assessments (CT, MRI, X-ray) were done by radiologists at an independent core lab (Medical Metrics, Inc, Houston, TX, USA), with radiologists blinded to clinical outcomes.
In addition to the imaging assessments, the Oswestry Disability Index (ODI) and the Visual Analog Scale (VAS) for back pain were collected pre-operatively and at 1 and 2 years PostOp. The change in these scores between PreOp and PostOp was calculated for each patient. The patient was classified as having a good outcome if the ODI dropped by 12.8 points (with ODI scored on a scale where 100 = worst possible disability) or the VAS back pain score dropped by 12 points (scored on a scale where 100 = worst possible pain). These thresholds were based on a study of the Minimum Clinically Important Difference (MCID) in lumbar spine surgery patients [20].
Data were analyzed using descriptive statistics, analysis of variance, and by logistic regression analyses (Stata Ver 11, College Station, TX, USA) to determine if each measure of the number and size of the defects was associated with whether the patient received a good outcome at 1 year PostOp (a 12.8-point improvement in the ODI or 12-point improvement in the VAS back pain score). Ordered logistic regression was used to analyze the number of lesions per endplate as an ordinal variable. Logistic regression does not assume or require normality, linearity, and homogeneity of variance for the independent variables, and can assess for the significance of relationships between the dependent variable and metric or dichotomous independent variables.
Results
Some of the endplates could not be reliably assessed because multi-planar reconstructions were not possible due to variable slice thickness or gantry tilt. Reliable CT-based assessments could be made on endplates of 113 disc spaces at PreOp, 111 disc spaces at 1 year, 68 disc spaces at 2 and 3 years, and 38 disc spaces at 4 years. Using MRI, disc signal intensities and Modic changes could be assessed in 52 patients at PreOp, 86 patients at 1 year, 44 at 2 years, 26 at 3 years, 16 at 4 years, and 7 at 5 years. The change in disc height relative to PreOp could be calculated for 130 patients at 1 year and 78, 66, 34, and 10 patients at 2, 3, 4, and 5 years. Clinical outcome scores were available for 136 patients at PreOp, 94 patients at 1 year, and 38 patients at 2 years. The statistical tests involving outcomes data should be interpreted with an appreciation of the low follow-up. It is possible that patients who did not return for follow-up may differ from those that did.
Endplate bony defects
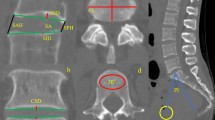
The defects tended to be located near the center of the endplate at PreOp (Fig. 1). Following discectomy, however, new endplate defects were also commonly observed in the posterior regions of the endplate. The numbers of defects in each zone were too small for meaningful statistics comparing distributions within zones, so the observations should be viewed as trends. A range of osseous endplate defect morphologies was observed. In general, these defects were similar to the morphologies described in the literature on Schmorl’s nodes [21–25]. Examples of endplate lesions observed pre- and post-operatively are shown in Fig. 2. There were no observations that would suggest that the observed lesions were morphologically different at PostOp compared to PreOp or that the defects were morphologically different than what has been described as a typical Schmorl’s node [21–25].
Examples of endplate defects that were observed following discectomy. In all of these three patients, there was no defect in the endplate pre-operatively. The top two images are axial and sagittal plane slices through a defect in one patient at 3 years PostOp. The bottom figures shows a three-dimensional reconstructions of vertebral endplates from an additional patient, created from a thin-slice CT scan taken 3 years after discectomy
The proportion of superior and inferior endplates that were free of endplate lesions at each time point decreased in the first year after surgery but did not appear to change in the subsequent years (Fig. 3). This early change was more pronounced in the superior endplate of the treated disc space, where there was almost 50 % fewer superior endplates that were free of defects following surgery. Based on ordered logistic regression, the change from PreOp to 1 year in number of endplate lesions was nearly significant at the inferior endplates (P = 0.079) and was highly significant at the superior endplates (P < 0.0001).
The percent of endplates with no defects decreased following discectomy, but did not appear to change with time following surgery. Data based on assessing 113 disc spaces at PreOp, 111 disc spaces at 1 year, 68 disc spaces at 2 and 3 years, and 38 disc spaces at 4 years. Note that there is relatively low follow-up at later time points
The average size of the primary and secondary lesions is summarized in Table 3. With data for superior and inferior endplates pooled, there was no difference in the size of the lesions measured from PostOp versus PreOp CT scans (P = 0.51). Analysis of variance tests indicated no significant difference in the size of the lesions in the superior versus inferior endplates at PreOp (P = 0.36); however, the defects tended to be larger in the inferior versus superior endplates at 1 year PostOp (6.3 ± 2.9 mm versus 4.9 ± 1.6 mm, P = 0.047).
Clinical outcomes
Pre-operatively, the ODI score averaged 49.9 ± 17.8 for the 136 patients who completed the questionnaire. At 1 year, ODI averaged 19.3 ± 16.6 for the 94 patients who completed the questionnaire. The average change in ODI was −30.4 ± 21.6 at the 1-year follow-up and −32.6 ± 23.0 at the 2-year follow-up. At the 1- and 2-year follow-up, 84.0 and 81.6 % of patients had achieved at least a 12.8-point improvement in the ODI score, 54.3 and 65.8 % had achieved at least a 12-point improvement in the VAS back pain score, and 88.3 and 86.8 % had achieved a MCID in either the ODI or the VAS back scores.
Table 4 provides the statistical significance value for logistic regression tests of whether each specific measurement of lesion number or size was independently associated with whether a patient received at least a 12.8 point improvement in the ODI score 1 year PostOp. There were no variables that were found to be close to significant. Similar tests were run using an improvement in VAS back of ≥12 points as the measure of whether a patient achieved a good clinical outcome. No measure of the number or size of endplate defects was associated with this definition of a good outcome (P > 0.50 for all tests).
MRI assessment of degeneration
The MRI exams document evidence of progressive disc degeneration following discectomy. The intervertebral disc signal intensity data show a trend toward loss of disc hydration over time (Fig. 4). Based on analysis of variance tests, however, there was no statistically significant evidence that disc signal intensity was associated with the change in clinical outcomes following discectomy (P > 0.11 for all outcome scores). Similarly, logistic regression analysis indicated no association between disc signal intensity and whether a patient had at least a 12.8 improvement in the ODI score 1 year PostOp.
Disc height measurements also support a progressive degeneration of the intervertebral disc following discectomy. Disc height significantly decreased with time following discectomy, based on the change in disc height relative to Preop, that was calculated on a per-patient basis and then averaged (Fig. 5, P = 0.014, oneway analysis of variance). Using >1 mm as the threshold to define a significant loss in disc height relative to PreOp, the data suggest that approximately 1/2 of the patients have this loss within the first year, with an additional 25 % of patients developing this loss over subsequent years (Fig. 6). There was no evidence of a significant change in spondylolisthesis, on average, in the first 5 years following discectomy (P = 0.19). Two patients developed a change in spondylolisthesis >10 % of the endplate during the course of the study.
The assessments of Modic changes adjacent to the endplates at the treated level support several observations (Fig. 7), including: (1) most endplates having no Modic changes at PreOp; (2) 30–40 % developing type I changes during the first 3 years, with this proportion declining during the 4th and 5th years; (3) a progressive increase to where nearly 80 % of patients have type II changes by 5 years PostOp. There was no evidence for a significant relationship between the Modic change and clinical outcome scores. Interestingly, there was a significant association between loss of PreOp disc height and the Modic grade 1 year following discectomy (P = 0.02, Fig. 8). Having multiple measureable defects in the endplates was a significant (P < 0.0001) risk factor for having Type I Modic changes.
Discussion
New endplate defects were common following limited lumbar discectomy involving removal of free and loose fragments outside and within the disc space with no direct intentional damage to the endplates. It is not known how many of these patients would have had new lesions if they had not had a discectomy. However, the natural history data for Schmorl’s nodes [26, 27] and our understanding of the rate of endplate marrow changes [28] would suggest that endplate changes generally progress slowly over time. A study by Wu et al. was the only previous study found reporting significant endplate changes over the course of a few years and they found this in only a small proportion of patients studied [27]. Barth et al. [14] reported that endplate degeneration was observed in 47 % of microdiscectomy patients and 14 % of microscopic sequestrectomy patients 2 years after surgery. These proportions span the proportion found in the current study. The results of the Barth et al. suggest an influence of surgical technique on endplate changes.
Despite the apparent spike in the proportion of endplates with lesions, there was no evidence that the presence of defect or any measurement of defect size was associated with clinical outcomes. This observation is limited by the low follow-up at later time points. A wide range of defect size measurements were explored to assure that a critical size measurement was not missed. Size of defect does not appear to determine patient satisfaction. Jensen et al. found that preoperative Modic lesions can significantly affect clinical outcomes [29], suggesting the possibility that the marrow changes may be clinically more important than osseous defects in the subchondral plate.
Strengths of this study include a thorough quantitative assessment of osseous defects. To assure that the true dimensions were measured as accurately as possible, the lesion dimensions were made from sagittal plane and coronal plane slices from the CT exams, with axial-plane slices used when they provided the best visualization of defect size. (This also resulted in lesser quality CT studies being excluded). Consistency of some results with previously published studies was also noted. The proportions of patients with Modic changes at the different time points are similar to those reported by Rahme et al. [30]. At PreOp, Rahme et al. reported a higher proportion of patients with any type of Modic change (46 versus 20 %), while at their median follow-up period of 41 months, they reported type II changes in 63 % of patients whereas we found Type II changes in 50 % of patients at 3 years and 66 % at 4 years. Rahme et al. reported that all of their discectomy patients had some evidence of disc height loss at a median follow-up of 41 months, consistent with our observation of 80 % having >1 mm height loss by 5 years. Rahme et al. also found no significant correlation between Modic changes and symptoms. Ohtori et al. also reported no association between Modic changes and clinical outcomes following discectomy surgery [31].
This study has several limitations. While all patients had limited discectomies, the discectomies were performed at five different sites and there may have been differences in techniques used to perform the discectomy. The follow-up rate was less than ideal at later time points, but good early on and the PreOp CT and MRI images afforded a solid internal control. That said, there was no non-operative control group so it is not known how many would have had significant changes without surgery. It is also not known whether defects may have been a consequence of a specific surgical technique. This was a physician-sponsored study and dedicated resources were not available for encouraging patients to return for follow-up. It is not known why some patients agreed to return for follow-up while others did not, or how inclusion of only those patients who volunteered for follow-up may have biased the results. In addition, it would have been of value to also have assessments of the adjacent levels to better understand the natural history of endplate changes. Resource limitations did not allow for those assessments. The sites did not provide details on patient sex and age, since it was not expected that the study would have the statistical power to analyze for sex- or age-related differences. It is assumed that the patients represent the typical population of patients undergoing discectomy surgery. Potential interactions between variables were not addressed in the current study, and it is possible that pre-existing Schmorl’s nodes or PreOp degenerative changes might have affected the occurrence of new defects or endplate changes. A much larger sample size would be required to assess such interactions. And finally, due to sample size, the statistical power does not allow us to state definitively that there is truly no association between the presence or size of a defect or endplate change and PostOp symptoms. With a much larger sample size it is possible that a sub-group of patients could be identified where the endplate lesion is associated with symptoms. Nevertheless, this study provides new observations on the characteristics of lesions that may form following discectomy. The observations need to be interpreted with respect to the limitations of the study.
Conclusion
Using the preoperative condition of each endplate as its own control, the proportion of endplates with osseous defects increased in the first year following surgery with no changes detected over the next 3 years, based on the lesions detected from CT scans obtained pre-operatively and following lumbar discectomy. The change is much more pronounced in the superior endplate of the treated disc space. New post-discectomy defects tended to be slightly larger but otherwise morphologically similar to lesions observed at PreOp. Consistent with prior studies, the new endplate changes were not significantly correlated with clinical outcomes. However, due to the unknown outcome of patients lost to follow-up, the association between new endplate changes and clinical outcomes remains uncertain.
References
Sherman J, Cauthen J, Schoenberg D, Burns M, Reaven NL, Griffith SL (2010) Economic impact of improving outcomes of lumbar discectomy. Spine J 10(2):108–116
Antonacci MD, Mody DR, Heggeness MH (1999) Innervation of the human vertebral body: a histologic study. J Spinal Disord 11(6):526
Brown MF, Hukkanen MV, McCarthy ID, Redfern DR, Batten JJ, Crock HV, Hughes SP, Polak JM (1997) Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Jt Surg Br 79(1):147–153
Wang Y, Videman T, Battié MC (2012) Lumbar vertebral endplate lesions: prevalence, classification, and association with age. Spine 37(17):1432–1439
Bonneville JF, Runge M, Cattin F, Potelon P, Tang YS (1989) Extraforaminal lumbar disc herniations: CT demonstration of Sharpey’s fibers avulsion. Neuroradiology 31(1):71–74
Schmid G, Witteler A, Willburger R, Kuhnen C, Jergas M, Koester O (2004) Lumbar Disk Herniation: correlation of Histologic Findings with Marrow Signal Intensity Changes in Vertebral Endplates at MR Imaging 1. Radiology 231(2):352
Veres SP, Robertson PA, Broom ND (2008) ISSLS prize winner: microstructure and mechanical disruption of the lumbar disc annulus: part II: how the annulus fails under hydrostatic pressure. Spine 33(25):2711
Veres SP, Robertson PA, Broom ND (2010) ISSLS Prize Winner: how loading rate influences disc failure mechanics: a microstructural assessment of internal disruption. Spine 35(21):1897
Veres SP, Robertson PA, Broom ND (2010) The influence of torsion on disc herniation when combined with flexion. Eur Spine J 19(9):1468–1478
Veres SP, Robertson PA, Broom ND (2009) The morphology of acute disc herniation: a clinically relevant model defining the role of flexion. Spine 34(21):2288
Malinin T, Brown M (2007) Changes in vertebral bodies adjacent to acutely narrowed intervertebral discs: observations in baboons. Spine 32(21):E603
Rätsep T, Minajeva A, Asser T (2013) Relationship between neovascularization and degenerative changes in herniated lumbar intervertebral discs. Eur Spine J 22:2474–2480
Babar S, Saifuddin A (2002) MRI of the post-discectomy lumbar spine. Clin Radiol 57(11):969–981
Barth M, Diepers M, Weiss C, ThomÈ C (2008) Two-year outcome after lumbar microdiscectomy versus microscopic sequestrectomy: part 2: radiographic evaluation and correlation with clinical outcome. Spine 33(3):273
Grand C, Bank W, Baleriaux D, Matos C, Levivier M, Brotchi J (1993) Gadolinium enhancement of vertebral endplates following lumbar disc surgery. Neuroradiology 35(7):503–505
Oetgen M, Yue J, Jaramillo-de la Torre J, Bertagnoli R (2008) Does Vertebral Endplate Morphology Influence Outcomes in Lumbar Total Disc Arthroplasty?: Part II: Clinical and Radiographic Results as Evaluated Utilizing the Vertebral Endplate Yue-Bertagnoli (VEYBR) Classification. SAS J 2(2):101–106
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26(17):1873–1878
Modic M, Masaryk T, Ross J, Carter J (1988) Imaging of degenerative disk disease. Radiology 168(1):177
Zhao KD, Yang C, Zhao C, Stans AA, An KN (2005) Assessment of non-invasive intervertebral motion measurements in the lumbar spine. J Biomech 38(9):1943–1946
Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY (2008) Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J 8(6):968–974
Hilton RC, Ball J, Benn RT (1976) Vertebral end-plate lesions (Schmorl’s nodes) in the dorsolumbar spine. Ann Rheum Dis 35(2):127–132
Peng B, Wu W, Hou S, Shang W, Wang X, Yang Y (2003) The pathogenesis of Schmorl’s nodes. J Bone Jt Surg Br 85(6):879
Pfirrmann CW, Resnick D (2001) Schmorl nodes of the thoracic and lumbar spine: radiographic-pathologic study of prevalence, characterization, and correlation with degenerative changes of 1,650 spinal levels in 100 cadavers. Radiology 219(2):368–374
Saluja G, Fitzpatrick K, Bruce M, Cross J (1986) Schmorl’s nodes (intravertebral herniations of intervertebral disc tissue) in two historic British populations. J Anat 145:87
Silberstein M, Opeskin K, Fahey V (1999) Spinal Schmorl’s nodes: sagittal sectional imaging and pathological examination. Australas Radiol 43(1):27–30
Girard C, Schweitzer M, Morrison W, Parellada J, Carrino J (2004) Thoracic spine disc-related abnormalities: longitudinal MR imaging assessment. Skelet Radiol 33(4):216–222
Wu H, Morrison W, Schweitzer M (2006) Edematous Schmorl’s nodes on thoracolumbar MR imaging: characteristic patterns and changes over time. Skelet Radiol 35(4):212–219
Kuisma M, Karppinen J, Niinimäki J, Kurunlahti M, Haapea M, Vanharanta H, Tervonen O (2006) A 3-year follow-up of lumbar spine endplate (Modic) changes. Spine 31(15):1714
Jensen RK, Leboeuf-Yde C, Wedderkopp N, Sorensen JS, Jensen TS, Manniche C (2012) Is the development of Modic changes associated with clinical symptoms? A 14-month cohort study with MRI. Eur Spine J 21(11):2271–2279
Rahme R, Moussa R, Bou-Nassif R, Maarrawi J, Rizk T, Nohra G, Samaha E, Okais N (2010) What happens to Modic changes following lumbar discectomy? Analysis of a cohort of 41 patients with a 3- to 5-year follow-up period. J Neurosurg Spine 13(5):562–567. doi:10.3171/2010.5.SPINE09818
Ohtori S, Yamashita M, Yamauchi K, Inoue G, Koshi T, Suzuki M, Orita S, Eguchi Y, Ochiai N, Kishida S (2010) Low back pain after lumbar discectomy in patients showing endplate Modic type 1 change. Spine 35(13):E596
Conflict of interest
Patient follow-up and data analysis were supported by Intrinsic Therapeutics, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weiner, B.K., Vilendecic, M., Ledic, D. et al. Endplate changes following discectomy: natural history and associations between imaging and clinical data. Eur Spine J 24, 2449–2457 (2015). https://doi.org/10.1007/s00586-014-3734-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3734-8