Abstract
Purpose
To determine the role of dynamic cervical implant (DCI) replacement for single-level degenerative cervical disc disease in Chinese patients.
Methods
Thirty patients with single-level degenerative cervical disc disease were prospectively enrolled between April 2010 and August 2010 (12 women, 18 men; mean age 56.5 years). All patients underwent anterior cervical decompression, DCI replacement, clinical and radiological assessments preoperatively and at 1, 6, 12, and 24 months postoperatively, and Japanese Orthopaedic Association (JOA), Visual Analogue Scale (VAS), Neck Disability Index (NDI), and Short Form 36 (SF-36) scores. Lateral neutral radiographs provided the intervertebral space height. Lateral dynamic radiographs were taken to measure the range of motion (ROM) of the cervical spine and functional spinal unit (FSU) of the treated segment. We compared the amount of motion of the adjacent vertebral endplate and the intrinsic motion of the implant and calculated a correlation analysis.
Results
DCI showed good clinical and radiographic outcomes. At the final follow-up, JOA, VAS, NDI, and SF-36 average scores improved significantly. The intervertebral space height increased slightly after operation and was maintained during follow up. The ROM of the cervical spine and FSU decreased at early follow-up, but recovered to the preoperative level within 1–2 years. There was a high index of linear correlation between the motion of the adjacent vertebral endplate and the intrinsic motion of the implant.
Conclusions
DCI provided elastic dynamic stability for the targeted segment, and restored and sustained intervertebral space height and ROM of the cervical spine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anterior cervical decompression and fusion (ACDF) is the traditional method for treating degenerative cervical disc disease, providing satisfactory results in a high proportion of patients [1–3]. However, stabilization of the treated segment may result in loss of mobility as a functional spinal unit and acceleration of degeneration of the adjacent disc [4–6]. Reoperations may be required to treat complications of fusion, such as recurrent radicular symptoms, nonunion, graft collapse, or expulsion [7–9]. Therefore, it is ideal to achieve functional reconstruction of the treated segment to restore physiological motion after surgery [10]. Dynamic cervical implant (DCI) replacement (Fig. 1), as a cervical non-fusion technique, may offer a solution [11]. Currently, few reports of the clinical outcomes of DCI replacement in Asian patients appear in the English literature. Therefore, we performed a prospective study on DCI replacement for the treatment of single-level degenerative cervical disc disease in Asian patients.
Materials and methods
Patients
This was a prospective and non-randomized study. Between April 2010 and August 2010, 30 patients with single-level degenerative cervical disc disease in our department were enrolled, including 12 women (40 %) and 18 men (60 %) with a mean age of 56.5 years (range 36–78 years). The degenerative disc was located at C3–4 in 4 cases, C4–5 in 8, C5–6 in 12, and C6–7 in six. Simple degenerative disc herniation was found in 13 cases, which caused soft compression of the spinal cord or nerve root. Degenerative disc herniation combined with posterior osteophyte formation was found in 17 cases, which caused hard compression of the spinal cord or nerve root and segmental spinal canal stenosis. Eighteen patients had myelopathy and 14 patients had radiculopathy. Major symptoms of myelopathy included hand numbness, limb weakness, gait instability, and hyperreflexia. Major symptoms of radiculopathy included radicular pain in one arm, and sensory deficit or weakness of one upper extremity. All patients were evaluated using a visual analogue scale (VAS), Japanese Orthopaedic Association scale (JOA), the neck disability index (NDI), and the short form 36 (SF-36) before operation.
Inclusion criteria
Eligible patients were at least 18 years old with radiculopathy or myelopathy from single-level cervical disc herniation (C3–C7), and they had not responded to at least 4 weeks of non-operative treatment.
Exclusion criteria
Exclusion criteria included segmental instability, marked reduction or absence of intervertebral motion, facet joint arthrosis, marked spondylosis, cervical kyphosis, active infection, osteoporosis, diabetes mellitus, inflammatory spondyloarthropathies such as ankylosing spondylitis or rheumatoid arthritis, known allergy to titanium, and previous cervical spine surgery.
Operative technique
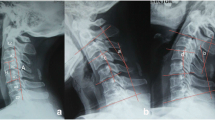
The surgical technique included the use of a conventional anterior cervical approach and discectomy. The patients were placed in the supine position with the neck approaching physiological lordosis. Discectomy was performed using a standard anterior cervical approach. After finishing the burring process of the vertebral body cartilage endplates at the treated disc space, the osteophyte located at the posterior edge of the vertebral body was completely resected. If the posterior longitudinal ligament was ruptured, it was excised and the sequestered disc completely removed to achieve better decompression of patients with myelopathy. Finally, a matching DCI was chosen and implanted under X-ray monitoring (Fig. 2). During the implant process, the location of the DCI was very important. The distance between the anterior/posterior edge of the DCI and the vertebral body endplates should be controlled to within 2–3 mm, and the lateral boundary of the DCI should not exceed the Luschka joint (Figs. 3, 4).
Postoperative management
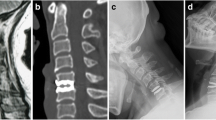
Drainage tubes were removed from the wound 24 h after operation, with all patients maintaining bed rest for that period. A cervical collar was used for the first week to help soft tissue healing and relieve pain. All patients were then permitted to begin physiological action of the cervical spine during daily life. Frontal and lateral, flexion–extension radiographs of the cervical spine were taken at 1, 6, 12, and 24 months after operation. Three-dimensional computed tomography (CT) views of the cervical spine were taken at 12 and 24 months after operation. VAS, JOA score, NDI, and the SF-36 assessments were completed at the same four follow-up points.
Radiographic measurements
The location of the DCI and the intervertebral height were observed on lateral neutral radiographs. The range of motion (ROM) of the cervical spine, the FSU of the treated segment, and the DCI itself were observed on lateral dynamic radiographs, postoperatively. The intervertebral height was measured by the distance from the midpoint of the upper endplate of the upper vertebral body to the midpoint of the lower endplate of the lower vertebral body. ROM was measured using the Cobb method (Figs. 5, 6). CT scans were evaluated for signs of prosthesis subsidence or extrusion, heterotopic ossification (HO), or spontaneous fusion. An independent radiologist and surgeon not participating in the operation analyzed the radiographs to determine the intervertebral height and the range of motion.
Results
Clinical outcomes
All patients underwent DCI replacement and there were no deaths, infections, or iatrogenic damage. A total of 30 DCIs were implanted for 30 patients with single-level cervical disc herniation. The postoperative follow-up ranged from 24 to 28 months (average 25.6 months).
The average intraoperative blood loss was 52.5 ± 7.8 mL (range 30–75 mL); the average operation time was 68.2 ± 5.4 min (range 55–85 min); and the average hospitalization time was 7.5 ± 1.8 days (range 5–11 days).
All patients showed significant improvement in neurological symptoms. The average JOA score before operation was 8.3 ± 1.2, with an average score of 11.8 ± 0.9 at 1 month, 15.2 ± 0.6 at 12 months, and 15.4 ± 0.7 at 24 months postoperatively. All differences were statistically significant (p < .05, paired t test) except between 12 and 24 months postoperatively (p > .05, paired t test). The average VAS score decreased from 7.8 ± 0.6 preoperatively to 4.2 ± 0.8 at 1 month, 2.1 ± 0.5 at 12 months, and 2.0 ± 0.3 at final follow-up. All differences were statistically significant (p < .05, paired t test) except between 12 and 24 months, postoperatively (p > .05, paired t test). The NDI was 42.5 ± 3.8 preoperatively, 35.8 ± 4.2 at 1 month, 26.4 ± 2.5 at 12 months, and 19.4 ± 3.1 at final follow-up. All differences were statistically significant between preoperative values and at 1, 6, 12, and 24 months, postoperatively (p < .05, paired t test). The mean SF-36 physical component summary score increased from 29.5 ± 3.3 to 46.7 ± 4.5 and the mean mental component summary scores increased from 35.8 ± 3.6 to 51.5 ± 2.8 at final follow-up. All differences were statistically significant (p < .05, paired t test) except between 6, 12, and 24 months, postoperatively (p > .05, paired t test).
Radiographic analysis
Elastic stabilization was achieved for all implanted DCIs; dynamic radiographs showed no instability at all treated levels; the distance between the edge of the vertebral body and the edge of the DCI showed no change during the 24 months follow-up; and no significant osteophyte development occurred on the posterior edge of the vertebral body (Figs. 7, 8). No resorption at the surface of the vertebral body or endplate collapse was observed in any patient. No patient had HO or spontaneous fusion at the implanted level and no prosthesis subsidence, rupture, or extrusion was found on 12- and 24-month CT images during the follow-up (Fig. 9).
The average intervertebral height was 35.4 ± 4.8 mm preoperatively; 38.8 ± 4.2 mm at 1 month postoperatively; 38.7 ± 4.1 mm at 12 months postoperatively; and 38.6 ± 3.2 mm at the final follow-up on the lateral radiographs. No differences were statistically significant (p > .05, paired t test).
ROM of the cervical spine was 68.2° ± 4.8° preoperatively; 59.5° ± 5.4° at 1 month postoperatively; 69.6 ± 5.3° at 12 months postoperatively; and 69.3° ± 3.8° at 24 months postoperatively. The ROM decreased at the 1 month follow-up (p < .05, paired t test), but recovered to the preoperative level at the final follow-up (p > .05, paired t test).
ROM of the FSU of the treated segment was 12.2° ± 2.5° preoperatively; 9.5° ± 2.3° 1 month postoperatively; 13.1° ± 2.1° at 12 months postoperatively; and 13.6° ± 1.7° at 24 months postoperatively. The ROM of the FSU decreased at the 1 month follow-up (p < .05, paired t test), but recovered to the preoperative level at the final follow-up (p > .05, paired t test).
The intrinsic motion (change of form with flexion–extension) of the implant was 13.0° ± 2.4° at 12 months postoperatively, and 13.3° ± 1.2° at 24 months postoperatively. We compared the amount of motion of the adjacent vertebral endplate and the intrinsic motion of the DCI and found no linear correlation (r = 0.983, p = .000) (Fig. 10).
Discussion
Many previous studies have shown that loss of motion at the fused level is compensated by increased motion at adjacent segments after ACDF, which induces a high rate of degenerative change adjacent to the fused segment [4, 9, 12]. Most researchers consider that the effects of ACDF in adjacent segment degeneration cannot be ignored and that functional reconstruction is the most desirable process after anterior cervical decompression, effectively avoiding the problem of fusion [13–15]. Cervical non-fusion techniques have increasingly been accepted and applied by spine surgeons. As a main component of cervical non-fusion techniques, artificial cervical disc replacement provides good ROM of the cervical spine [16–18], but high axial strength, no flexibility, and concussion buffering function can lead to HO and spontaneous fusion around the treated segment [19–22]. Therefore, researchers have been working to develop new cervical non-fusion techniques.
The first DCI was designed by Matgé in 2002 for treating cervical spondylosis. Paradigm spine introduced the second generation DCIs in 2005. A U-shaped appearance and axial elasticity are two the most significant characteristics (Fig. 1). A prospective study by Matgé et al. in 2009 showed that the clinical efficacy was satisfactory after DCI replacement in 102 cases of cervical spondylosis (120 segments) and during a 1-year follow-up, there was neither device migration nor subsidence [11]. Welke et al. showed that during flexion–extension, the DCI implant showed a tendency to stabilize the segment while allowing some degree of residual mobility and provided an alternative to cage-supported fusion, or total disc replacement in the cervical spine [23]. Anterior cervical decompression and non-fusion has been performed with DCI on Asian patients since the end of 2009 and since the beginning of 2010 in our institution, but few clinical reports of DCI replacement for Asian patients appear in the English literature. We focused our study on patients with single-level degenerative cervical disc disease to better exclude some uncertainties and improve the homogeneity of the research.
All of our patients had significant improvement in neurological symptoms after surgery. The JOA score (17 points) was 8.3 before operation and increased to 15.4 at the final follow-up. The NDI was 42.5 preoperatively and decreased to 19.4 at the final follow-up. The average VAS score decreased from 7.8 points preoperatively to 1.4 points at final follow-up. Based on these results, we believe that DCI provided elastic stabilization and restored the range of motion of the treated level; however, DCI replacement should not have much influence on the recovery of neurological function, because neurological improvement depends mainly on complete anterior decompression.
Range of motion is an important factor to evaluate the clinical outcomes of DCIs. We measured the motion of the DCI on lateral dynamic radiographs (Fig. 6). The average ROM of the cervical spine was 68.2° preoperatively, and 69.3° at final follow-up. Also, the average ROM at the treated level was 12.2° preoperatively, and 13.6° at the final follow-up, suggesting that DCI restored the physiological function of the cervical spine and the surgical segment. In dynamic stabilization, a secure, tight, and durable implant/bone interface is crucial because motion between the implant and bone may lead to long-term failure. To verify the stability of the implant/bone interface, we measured the intrinsic motion of the DCI and the amount of motion of the adjacent vertebral endplates. The comparison results showed a high index of linear correlation (Fig. 10) suggesting that the implant/bone interface was stable during flexion–extension of the cervical spine. Although our results showed that DCI replacement had good short-term clinical and radiographic outcomes, further follow-up is needed to determine the long-term efficacy.
HO and spontaneous fusion were found during follow-up in several studies of artificial cervical disc replacement [24–27], but until recently, there were few reports of how to prevent these consequences during cervical non-fusion surgery. Possible reasons for HO include muscle damage and residual bone dust left at the operative site [28]. We also consider that a hyperplastic posterior longitudinal ligament is a possible reason for HO. Resecting the ligament could avoid this issue and achieve better spinal cord decompression. Therefore, based on our clinical experience, improvement in surgical technique may play an effective role in preventing these complications. We recommend that after resecting the target cervical disc, posterior osteophytes of the vertebral body, and the hyperplastic posterior longitudinal ligament should be resected completely during anterior cervical decompression and the operative site should be flushed with normal saline. Equally important, a matching DCI prosthesis as large as possible should be implanted for the largest contact area between the DCI and the vertebral endplate. This can improve immediate elastic stability postoperatively and avoid bony contact between the adjacent vertebral endplates at the treated segment. None of our patients showed definite spontaneous fusion or HO of the treated segment during the 2 years of follow-up; therefore, we consider that resecting posterior osteophytes and the hyperplastic posterior longitudinal ligament, and implanting a matching DCI are two important methods for preventing spontaneous fusion and HO.
Despite favorable results, our study has some limitations, including the lack of a control group, limited sample size, and no measurement of adjacent level intradiscal pressure and facet joint force. Also, this study aimed to evaluate the short-term efficiency of DCI, so MRI was not used as an imaging evaluation measure to observe degenerative change of the adjacent segments. The anatomy of the cervical spine and the motion patterns of the functional spine units are complicated, and further research is needed to determine whether DCI can completely restore the physiological function of the treated segment. The follow-up time of our study was insufficient to confirm that DCI rupture would not occur during neck activity and that DCI replacement would not lead to HO and spontaneous fusion. Therefore, longer follow-up is needed to determine the long-term efficacy of DCI replacement.
We conclude that DCI replacement is a safe and efficient option for the treatment of patients with single-level degenerative cervical disc disease. It provides both elastic dynamic stability for the target segment, and restores and sustains intervertebral space height and ROM of the treated segment and the cervical spine. Longer follow-up is needed to determine the long-term effects.
References
Smith GW, Robinson RA (1958) The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Jt Surg Am 40-A(3):607–624
Cauthen JC, Kinard RE, Vogler JB, Jackson DE, DePaz OB et al (1998) Outcome analysis of noninstrumented anterior cervical discectomy and interbody fusion in 348 patients. Spine 23:188–192
Samartzis D, Shen FH, Lyon C, Phillips M, Goldberg EJ, An HS (2004) Does rigid instrumentation increase the fusion rate in onelevel anterior cervical discectomy and fusion? Spine J 4:636–643
Eck JC, Humphreys SC, Lim TH, Jeong ST, Kim JG, Hodges SD, An HS (2002) Biomechanical study on the effect of cervical spine fusion on adjacent level intradiscal pressure and segmental motion. Spine 27:2431–2434
Wigfield CC, Gill S, Nelson R, Langdon I, Metcalf N, Robertson J (2002) Influence of an artificial cervical joint compared with fusion on adjacent-level motion in the treatment of degenerative cervical disc disease. J Neurosurg 96(1 suppl):17–21
Sugawara T, Itoh Y, Hirano Y, Higashiyama N, Mizoi K (2009) Long-term outcome and adjacent disc degeneration after anterior cervical discectomy and fusion with titanium cylindrical cages. Acta Neurochir (Wien) 151(4):303–309
Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH (1999) Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Jt Surg Am 81:519–528
Goffin J, Geusens E, Vantomme N, Quintens E, Waerzeggers Y, Depreitere B et al (2004) Long-term follow-up after interbody fusion of the cervical spine. J Spinal Disord Tech 17:79–85
Kulkarmi V, Rajshekhar V, Raghuram L (2004) Accelerated spondylotic changes adjacent to the fused segment following central cervical corpectomy magnetic resonance imaging study evidence. J Neurosurg 100(1 Suppl Spine):2–6
Dmitriev AE, Cunningham BW, Hu N, Sell G, Vigna F, McAfee PC (2005) Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine 30(10):1165–1172
Matgé G, Eif M, Herdmann J (2009) Dynamic cervical implant (DCITM): clinical results from an international multicenter prospective study. Paradig Spine 1:1–3
DiAngelo DJ, Roberston JT, Metcalf NH, Mcvay BJ, Davis RC (2003) Biomechanical testing of an artificial cervical joint and an anterior cervical plate. J Spinal Disord Tech 16:314–323
Robertson JT, Papadopoulos SM, Traynelis VC (2005) Assessment of adjacent segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine 3:417–423
Pracyk JB, Traynelis VC (2005) Treatment of the painful motion segment. Spine 30:S23–S32
Phillips FM, Garfin SR (2005) Cervical disc replacement. Spine 30:S27–S33
Robertson J, Metcalf N (2004) Long-term outcome after implantation of the Prestige I disc in an end-stage indication: 4-year results form a pilot study. Neurosurg Focus 17(3):E10
Lafuente J, Casey AT, Petzold A, Brew S (2005) The Bryan cervical disc prosthesis as an alternative to arthrodesis in the treatment of cervical spondylosis: 46 consecutive cases. J Bone Jt Surg Br 87:508–512
Mummaneni PV, Burkus JK, Haid RW, Traynelis VC, Zdeblick TA (2007) Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine 6:198–209
Parkinson JF, Sekhon LH (2005) Cervical arthroplasty complicated by delayed spontaneous fusion. Case report. J Neurosurg Spine 2(3):377–380
Leung C, Casey AT, Goffin J, Kehr P, Liebig K, Lind B et al (2005) Clinical significance of heterotopic ossification in cervical disc replacement: a prospective multicenter clinical trial. Neurosurgery 57:759–763
Mehren C, Suchomel P, Grochulla F, Barsa P, Sourkova P, Hradil J et al (2006) Heterotopic ossification in total cervical artificial disc replacement. Spine 31(24):2802–2806
Heidecke V, Burkert W, Brucke M, Rainov NG (2008) Intervertebral disc replacement for cervical degenerative disease-clinical results and functional outcome two years in patients implanted with the bryan cervical disc prosthesis. Acta Neurochir (Wein) 150:453–459
Welke B, Hurschler C, Packheiser A, Richter B, Daentzer D (2010) Biomechanical concept of a novel dynamic cervical implant: first biomechanical comparison among fusion, TDR, and dynamic stabilization. Eur spine 19:1963–2073
Suchomel P, Jurák L, Benes V 3rd, Brabec R, Bradác O, Elgawhary S (2010) Clinical results and development of heterotopic ossification in total cervical disc replacement during a 4-year follow-up. Eur Spine J 19(2):307–315
Chen J, Wang X, Bai W, Shen X, Yuan W (2012) Prevalence of heterotopic ossification after cervical total disc arthroplasty: a meta-analysis. Eur Spine J 21(4):674–680
Guérin P, Obeid I, Bourghli A, Meyrat R, Luc S, Gille O et al (2012) Heterotopic ossification after cervical disc replacement: clinical significance and radiographic analysis. Prospect study. Acta Orthop Belg 78(1):80–86
Yi S, Shin DA, Kim KN, Choi G, Shin HC, Kim KS (2013) The predisposing factors for the heterotopic ossification after cervical artificial disc replacement. Spinal J 13(9):1048–1054
Ahrengart L, Sahlin K, Lindgren U (1987) Myositis ossification after total hip replacement and perioperative muscle ischemia. J Arthroplast 2:65–69
Conflict of interest
None of the authors has any potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, L., Song, Ym., Liu, Lm. et al. Clinical and radiographic outcomes of dynamic cervical implant replacement for treatment of single-level degenerative cervical disc disease: a 24-month follow-up. Eur Spine J 23, 1680–1687 (2014). https://doi.org/10.1007/s00586-014-3180-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3180-7