Abstract
Objective
To identify the independent risk factors, based on available evidence in the literature, for patients developing surgical site infections (SSI) after spinal surgery.
Methods
Non-interventional studies evaluating the independent risk factors for patients developing SSI following spinal surgery were searched in Medline, Embase, Sciencedirect and OVID. The quality of the included studies was assessed by a modified quality assessment tool that had been previously designed for observational studies. The effects of studies were combined with the study quality score using a best-evidence synthesis model.
Results
Thirty-six observational studies involving 2,439 patients with SSI after spinal surgery were identified. The included studies covered a wide range of indications and surgical procedures. These articles were published between 1998 and 2012. According to the quality assessment criteria for included studies, 15 studies were deemed to be high-quality studies, 5 were moderate-quality studies, and 16 were low-quality studies. A total of 46 independent factors were evaluated for risk of SSI. There was strong evidence for six factors, including obesity/BMI, longer operation times, diabetes, smoking, history of previous SSI and type of surgical procedure. We also identified 8 moderate-evidence, 31 limited-evidence and 1 conflicting-evidence factors.
Conclusion
Although there is no conclusive evidence for why postoperative SSI occurs, these data provide evidence to guide clinicians in admitting patients who will have spinal operations and to choose an optimal prophylactic strategy. Further research is still required to evaluate the effects of these above risk factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical site infections (SSI) after adult spinal surgery are one of the most common causes of morbidity and mortality and have been reported to occur in 0.7–12.0 % of patients [39]. Spinal surgeries report a higher rate of infection compared with other orthopedic surgeries such as total hip arthroplasty [38]. According to the Center for Disease Control’s National Nosocomial Infections Surveillance (NNIS) System, which reflects the rate of hospital-acquired infections in the United States, SSI is the third most commonly reported type of nosocomial infection, accounting for 14–16 % of all nosocomial infections [16]. Moreover, 77 % of acute mortalities in patients with SSI were found to be related to the infections [32]. It has also been reported that SSI can increase medical, social and economical costs for the patients by up to four times, as well as increase costs for hospitals and the health insurance companies. Recognition of a patient’s risk factors for SSI may allow for interventions that prevent severe infections. Thus, several studies were performed to identify the specific risk factors for SSI. Identifying risk factors may aid in preventing infections and the associated patient suffering.
The incidence of SSI after spinal surgery is influenced both by patient characteristics and pre- or intra-operative factors. A variety of risk factors for SSI following spinal surgery have been identified including diabetes, obesity, longer operation times, smoking, history of previous SSI, type of surgical approach, larger blood loss, and use of spinal instrumentation. Furthermore, some of the above factors are impossible to modify and their effects can also be compounded by interactions, so a zero rate of SSI risk cannot be expected. Therefore, orthopedic surgeons aims to minimize infection rates to an acceptably low level.
Recently, Schuster et al. [51] published a systematic review on the risk factors for SSI after spinal surgery with the levels of evidence from the included studies. Pull et al. [45] also published a systematic review with appraised validity of identified risk factors for SSI. However, neither of these systematic reviews performed an evidence synthesis according to the study characteristics and the study quality score. We performed this systematic review of the literature to identify the independent risk factors for SSI in patients after spinal surgery and to grade the evidence according to the quality of included studies. This synthesis will provide an evidence base from which orthopedic surgeons can assess the probability of SSI for each patient. The ultimate goal is to decrease the risk for SSI and thereby decrease patient morbidity, mortality and healthcare costs based upon any identified independent risk factors.
Materials and methods
Search strategy
All methods used in this review followed the CRD, PRISMA [37], and MOOSE [53] guidelines. The primary sources of the reviewed studies were Medline, Embase, Sciencedirect, and OVID. The search included literature published exclusively in English up to June 2012 with no lower date limit on the search results. Searches included the terms “surgery”, “spinal”, “infection”, “surgical”, “spine”, “prophylaxis”, and “prevention”. The electronic search was reinforced with manual searches for reference lists of all retrieved articles. In addition, all relevant conference databases that provided grey literature were also searched.
Eligibility of relevant studies
To begin, SSI was defined by the ICD-9-CM codes, National Nosocomial Infection Surveillance System (NNIS) definitions [40], Centers for Disease Control and Prevention (CDC) definitions [32], NHSN criteria [23] or other criteria such as definite pus discharge from the wound, local redness and heating, or identification of germs in exudates. We included studies in that (1) were observational in design, (2) consisted of a clearly defined group of adult patients, (3) investigated SSI (but were not limited to a specific pathogen) and (4) reported data on the independent risk factors for SSI but not on specific management strategies. However, studies were excluded if they were pediatric studies (age <18 years old), for the treatment of osteomyelitis, other than spinal surgery, or for evaluating antibiotics. Articles published in languages other than English were also excluded. Other excluding criteria included editorials, reviews and animal studies.
Study selection
Two reviewers (D. X. and XL. M.) independently analyzed each of the titles and abstracts based on the eligibility criteria. Articles that could not be excluded from our study based on the title and abstract were retrieved for independent full-text review by the same two reviewers. Any disagreements were resolved by consensus.
Data abstraction
The two independent reviewers (D. X. and JX. M.) extracted the following data from the qualifying articles. A form for extracting data, which had been previously piloted, was used to record information about population, study design, sample size, diagnosis, surgical procedure, and independent risk factors for SSI. The corresponding author of each study was contacted to obtain any missing information that was required. In the case of discrepancies, the third reviewer (Y. C.) was involved.
Quality assessment
The quality of the included studies was independently assessed by two authors (D. X. and Y. C.). The methodological quality of studies was evaluated using a previously designed criteria list (Table 1), which was adapted from Lievense et al. [31]. These systems were designed for methodological quality assessment of observational studies and had been used in previous observational systematic review articles [7, 22, 31]. Some of the original 15 criteria were not included in this review because they were not relevant to our topic or not specific to the study design of the included studies. Therefore, our methodological assessment tool was based on 12 criteria that were specific to case-series studies, which were scored as positive (1), negative (0), or unsure (?). Included studied were classified into three different quality levels (high, moderate, low quality) according to their methodological quality score, which is presented in Table 2. A consensus method was used to resolve differences in quality rating, with a third reviewer (XL. M.) available for consultation if needed.
Evidence synthesis
Given the heterogeneity of the included studies and independent risk factors, we were unable to perform a meta-analysis directly. In addition to assessing the quality of the studies, we graded the body of evidence. Therefore, we summarized the results using the model of “best-evidence synthesis” (Table 2) by which the potential risk factors were classified. The risk factors of included studies with different methodological qualities were summarized according to the quantity and quality of relevant studies. This is a less-common approach, but is increasingly recognized as pertinent because it provides a conclusion that incorporates both the quality of studies and their outcomes [6, 19, 31, 55]. Using the key elements for grading systems suggested by the US Agency for Healthcare Research and Quality [59], we rated the evidence by synthesis according to five levels: no, conflicting, limited, moderate or strong evidence. “Strong evidence” means that further study is very unlikely to change our confidence in the estimate of effect. “Moderate evidence” means that further research is likely to have an impact on our confidence in the estimate of the effect and may change the results. “Low evidence” means that further research is very likely to change the results. “Conflicting evidence” means that any estimate of effect is very uncertain. “No evidence” means that no statistically analyzed or discussed factors are presented.
Results
Study identification
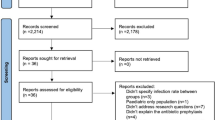
From our research, we identified 412 potential citations after duplications were excluded. After screening the titles and abstracts of these citations, we identified 177 publications for full-text reading. Based on the full-text screening, 36 studies were included ultimately in the current review. The most frequent reason for exclusion was the inclusion of pediatric patients (Fig. 1).
Characteristics of studies included in the analysis
Characteristics of the studies are presented in Table 3. A total of 36 studies were included involving 2,439 patients with SSI after spinal surgery. The number of subjects in each study ranged from 45 to 24,774. The number of patients with SSI ranged from 6 [30] to 752 [56]. All of the investigations evaluated the risk factors for SSI after spinal surgery. These articles were published between 1998 and 2012. Included studies consisted of 14 (38.9 %) case–control studies (602 patients) and 22 (61.1 %) case-series studies (1,837 patients). There were 23 studies conducted in the USA, 3 in Japan, 2 in Taiwan, 2 in Netherlands, 2 in Korea, and 1 each in Lebanon, Italy, China and Austria. Twenty-six studies (72.2 %) reported the level of the spine in question: the cervical spine was operated on in 15 studies, the thoracic spine in 19 studies, the lumbar spine in 25 studies, and the sacral spine in 12 studies. Although 13 of the 36 studies did not provide data on the average age of patients, the lowest and highest mean ages reported by the remaining studies were 44 and 72.1 years. The most frequently performed surgical procedures were spinal fusion and spinal laminectomy/decompression. A variety of definitions were used to define SSI after spinal surgery. Although most of the studies used the criteria established by the Center for Disease Control and Prevention (CDC), other studies reporting SSI included findings from physical examinations, anamnesis, radiographic investigations, blood tests, MRI findings, and applied treatments. Although statistical methods were used in all 30 studies, multivariate regression analysis was performed in only 20 of 30 studies.
Quality of included studies
According to the methodological quality criteria for observational studies, 15 studies (41.7 %) were deemed to be high-quality studies, 5 (13.9 %) as moderate-quality studies and 16 (44.4 %) as low-quality studies, as presented in Table 3.
Evidence level of independent risk factors
There were 46 potential independent risk factors that were identified by more than one study and were included in the final analysis. As shown in Table 4, six were identified as strong-evidence factors, including obesity/BMI, longer operation times, diabetes, smoking, history of previous SSI and type of surgical procedure. There were eight moderate-evidence independent risk factors identified including level of spinal surgery, number of spinal levels operated on, surgery involving the sacrum or pelvis, larger intraoperative blood loss, surgery with spinal instrumentation, earlier surgery, blood transfusions and ASA (American Anesthetists Society score) classification. Thirty-one limited-evidence independent risk factors were identified, presented in Table 4. Only the number of resident surgeons participating in the operative procedure was identified as a conflicting-evidence factor.
Discussion
Surgical site infections following spinal surgery are frequently seen and have been previously linked to the length of surgical procedure, morbidity, and mortality. Therefore, it is important to identify variables that increase the risk of SSI so that strategies can be developed to minimize patient risk. The purpose of this systematic review was to identify independent risk factors for adult patients who develop SSI after spinal surgery and to synthesize these risk factors according to the level of available evidence, based on the methodological quality of the included studies.
Although multiple risk factors for SSI have previously been identified, there had been no synthesis of the evidence according to different level of study quality. In agreement with other review articles, Pull et al. [45]. reviewed 24 studies and reported 73 different types of factors related to SSI. Schuster et al. [51] reviewed 32 studies to evaluate the influence of perioperative risk factors and therapeutic interventions on SSI and rated level of evidence according to a certain methodological criteria. However, the authors evaluated the risk factors without an evidence-based synthesis. While the above reviews partly agreed with our results, the present systematic analysis introduced a quality assessment of the included studies and performed an evidence synthesis to evaluate the independent risk factors for SSI after spinal surgery, according to the differing quality levels of the studies.
Because of study heterogeneity, it was impossible to pool data between studies and conduct a meta-analysis to determine the estimates the effects of each risk factor. However, the evidence synthesis method is also a useful method for arriving at clinical recommendations. In the present systematic review, we only included observational studies, which might result in an incomplete detection of preoperative exposures and a higher bias of risk. In addition, heterogeneity of the included studies was induced, to a certain degree, by surgical approach, study population, preoperative state, primary disease, surgical techniques of surgeons, and different indications for surgery. Accordingly, while the results of this systematic review should be considered valid, these methodological quality considerations should be taken into account when interpreting the findings.
By conducting this systematic review of the current evidence base of 36 included studies, we have identified 6 strong-evidence independent risk factors, including obesity/BMI, longer operation times, diabetes, smoking, history of previous SSI and type of surgery procedure. Diabetes was associated with increased risk for SSI, with some studies reporting an odds ratio as high as 4.2 [18]. Diabetic patients are known to be susceptible to postoperative SSI due to their immunocompromised state, reduced wound healing potential and poor microvascularization. In addition, diabetes impairs wound healing because microangiopathic changes lead to local tissue ischemia [20] and lower tissue concentrations of antibiotics. Thus, tight regulation of serum glucose levels may decrease the incidence of SSI following spinal surgery [15]. Moreover, several studies reported that elevated preoperative or postoperative serum glucose level, which was regarded as a limited-evidence risk factor in the present study, was independently associated with an increased risk of SSI [30, 40]. Koutsoumbelis et al. [26] reported that postoperative serum glucose levels were significantly higher in the infected group, but they were not a risk factor according to a multivariate logistic regression analysis. Therefore, while preoperative and postoperative regulation of serum glucose levels had weaker evidence for being a risk factor than diabetes, it may influence us to pay more attention to the regulation of serum glucose levels pre- and post-operatively. Obesity was found to be an independent risk factor for SSI. The obese patients have thick subcutaneous adipose layers that form dead space after closure of the surgical wound. Thus, the necrosis of local fat can result in a localized wound infection. The use of subcutaneous drains to prevent dead space or recommending weight loss preoperatively may be effective strategies for preventing SSI. Although the different cutoffs (BMI >30 or BMI >35) or different definitions for obesity were utilized in different studies, the influence of obesity on SSI showed a similar trend. Therefore, weight loss may lower risk of SSI after spinal surgery. Our results confirmed the finding that patients who smoked had an increased risk for SSI. Smoking impairs wound healing because microvascular traction leads to tissue ischemia. Thomsen et al. [54] reported that patients may benefit from intensive preoperative smoking cessation interventions, which must have been initiated at least 4 weeks before surgery and have included nicotine replacement therapy. Therefore, patients have to stop smoking before surgery. Longer operative times were found to have a higher risk for SSI. The reason may be that longer surgical procedures result in increased duration of tissue traction and resulting ischemia and necrosis. Longer surgical procedures can also increase the risk of wound contamination. Although longer operative times were dictated mostly by the surgical complexity of the procedure, they can be mitigated by factors such as the proficiency of the surgeon and the operation methods [32]. The proper methods, which can minimize tissue necrosis and decrease infection rates, include releasing the tension on self-retractors often [49] and frequent irrigation of the surgical wound with saline during surgical procedures [9]. Moreover, full and long-term training for surgeons must be required to improve surgical proficiency. The history of previous SSI also significantly increased the risk of SSI. Although patients may not have any symptoms or signs of active infection, bacteria can remain encapsulated in the scar tissue. Bacteria may lie dormant until being released by a new surgery, which will lead to wound contamination [42]. If surgeons can determine the organism that caused the prior SSI before a revision surgery, an appropriate prophylactic antibiotic regimen may help to minimize the risk of SSI. A posterior surgical approach was independently associated with an increased risk of SSI. Levi et al. [29] found that all deep wound infections in patients for whom surgery involved spinal instrumentation occurred after a posterior approach. Wimmer et al. [61] reported that most of their patients with SSI had previously undergone surgery that involved posterior fusion and placement of instrumentation. Therefore, interventions to reduce the risk of SSI must focus on methods to reduce contamination of the posterior wound with urinary, fecal or skin flora. More attention must be paid to antisepsis of the posterior wounds, both in the hospital and by caregivers after discharge.
Beyond the six discussed risk factors for which there is strong evidence, we identified eight moderate-evidence factors. Friedman et al. [18] reported that laminectomy at a level other than cervical was one of independent risk factors. This may be attributed to the fact that laminectomy at a level other than cervical was always performed via a posterior approach, which has been identified as a strong-evidence risk factor. Mirza et al. [36] demonstrated that the number of levels operated on and blood transfusion were associated with longer surgical procedures, which was regarded as one of the strong-evidence risk factors. Therefore, an increased number of operated levels and blood transfusion may increase the risk of SSI. Although spinal instrumentation was regarded as foreign body that may increase the risk of infection, instrumentation could stabilize the motion segment, which could theoretically decrease inflammation due to instability and promote bone healing [41]. Using instrumentation had an effect on risk of SSI due to increased dead space by foreign bodies and longer operative times [27]. Surgery involving the sacrum or pelvis was a moderate risk factor because it may be associated with larger blood loss and longer operative times. As one of the moderate-evidence factors, larger blood loss may be directly associated with blood transfusion, which results in immune suppression in the recipient [46] and an increased risk of infection for all types of surgery [4]. Previous surgery may result in bowel and bladder dysfunction or complex tissue reconstruction, which to some extent may increase the risk of SSI after spinal surgery. We also identified 31 limited-evidence factors, and only the number of resident surgeons participating in the operative procedure was identified as a conflicting-evidence factor. A better understanding of these independent risk factors could help to identify patients at different degrees of risk for SSI and help to develop interventions. Future studies are required to evaluate the effects of these risk factors.
The limitations of this systematic review primarily include the following: (1) systematic reviews of observational studies remain contentious in research [6, 18, 19, 55]. Although the criteria of methodological quality assessment and evidence synthesis have been adopted by several systematic reviews recently [6, 19, 55], the choice of this method is still under dispute. (2) Observational studies were sensitive to selection, detection, performance and publication bias and confounds. Publication bias occurs for the following reason: significant conclusions are easier to publish. Moreover, most authors prefer significant results by multivariate analysis. (3) Heterogeneity of included studies was induced by surgical approach, patient selection, preoperative state, surgical techniques of surgeons, analysis of putative risk factors, different indication for surgery, and definitions of SSI outcomes. (4) The classification of the evidence is weakened by heterogeneity and the varying methodological quality of the included studies. The evidence synthesis might overestimate the predicted effects and should be interpreted with the above limitations in mind.
Conclusion
This systematic review provided an overview of the current knowledge concerning independent risk factors for patients with SSI after spinal surgery. It included 36 studies involving 2,439 patients with SSI after spinal surgery. Although the available observations form a heterogeneous group, and there is no conclusive evidence, we have identified six strong-evidence risk factors including obesity/BMI, longer operation times, diabetes, smoking, history of previous SSI and type of surgical procedure. We also identified 8 moderate-evidence, 31 limited-evidence and one conflicting-evidence risk factor. We believe disclosure of these data will provide evidence to guide clinicians who treat patients with spinal surgery. However, high-quality studies are still required to clarify or to evaluate the effects of these risk factors. They should be based on a prospective design and with a large sample size. Multivariate analysis should be performed to adjust for, at a minimum, age, gender, primary diagnosis and comorbidities. In addition, details of interventions before surgery and preoperative exposures should be specified and randomized. Meanwhile, the results of the analysis should be fully presented, including negative factors.
References
Abdul-Jabbar A, Takemoto S, Weber MH, Hu SS, Mummaneni PV, Deviren V, Ames CP, Chou D, Weinstein PR, Burch S, Berven SH (2011) Surgical site infection (SSI) in spinal surgery: description of surgical and patient-based risk factors for postoperative infection using administrative claims data. Spine (Phila Pa 1976) 37:1340–1345. doi:10.1097/BRS.0b013e318246a53a
Ahn DK, Park HS, Choi DJ, Kim TW, Chun TH, Yang JH, Kim DG (2012) The difference of surgical site infection according to the methods of lumbar fusion surgery. J Spinal Disord Tech. doi:10.1097/BSD.0b013e31825c6f7b
Apisarnthanarak A, Jones M, Waterman BM, Carroll CM, Bernardi R, Fraser VJ (2003) Risk factors for spinal surgical-site infections in a community hospital: a case–control study. Infect Control Hosp Epidemiol 24:31–36. doi:10.1086/502112
Banbury MK, Brizzio ME, Rajeswaran J, Lytle BW, Blackstone EH (2006) Transfusion increases the risk of postoperative infection after cardiovascular surgery. J Am Coll Surg 202:131–138. doi:10.1016/j.jamcollsurg.2005.08.028
Banco SP, Vaccaro AR, Blam O, Eck JC, Cotler JM, Hilibrand AS, Albert TJ, Murphey S (2002) Spine infections: variations in incidence during the academic year. Spine (Phila Pa 1976) 27:962–965
Battle CE, Hutchings H, Evans PA (2012) Risk factors that predict mortality in patients with blunt chest wall trauma: a systematic review and meta-analysis. Injury 43:8–17. doi:10.1016/j.injury.2011.01.004
Borghouts JA, Koes BW, Bouter LM (1998) The clinical course and prognostic factors of non-specific neck pain: a systematic review. Pain 77:1–13
Boston KM, Baraniuk S, O’Heron S, Murray KO (2009) Risk factors for spinal surgical site infection, Houston, Texas. Infect Control Hosp Epidemiol 30:884–889. doi:10.1086/605323
Brown EM, Pople IK, de Louvois J, Hedges A, Bayston R, Eisenstein SM, Lees P (2004) Spine update: prevention of postoperative infection in patients undergoing spinal surgery. Spine (Phila Pa 1976) 29:938–945
Chen KW, Yang HL, Lu J, Wang GL, Ji YM, Bao ZH, Wu GZ, Gu Y, Sun ZY, Zhu RF (2011) Risk factors for postoperative wound infections of sacral chordoma after surgical excision. J Spinal Disord Tech 24:230–234. doi:10.1097/BSD.0b013e3181ea478a
Chen S, Anderson MV, Cheng WK, Wongworawat MD (2009) Diabetes associated with increased surgical site infections in spinal arthrodesis. Clin Orthop Relat Res 467:1670–1673. doi:10.1007/s11999-009-0740-y
Chikawa T, Sakai T, Bhatia NN, Sairyo K, Utunomiya R, Nakamura M, Nakano S, Shimakawa T, Minato A (2011) Retrospective study of deep surgical site infections following spinal surgery and the effectiveness of continuous irrigation. Br J Neurosurg 25:621–624. doi:10.3109/02688697.2010.546902
Cizik AM, Lee MJ, Martin BI, Bransford RJ, Bellabarba C, Chapman JR, Mirza SK (2012) Using the spine surgical invasiveness index to identify risk of surgical site infection: a multivariate analysis. J Bone Joint Surg Am 94:335–342. doi:10.2106/JBJS.J.01084
Demura S, Kawahara N, Murakami H, Nambu K, Kato S, Yoshioka K, Okayama T, Tomita K (2009) Surgical site infection in spinal metastasis: risk factors and countermeasures. Spine (Phila Pa 1976) 34:635–639. doi:10.1097/BRS.0b013e31819712ca
Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ (2007) Clostridium difficile-associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis 45:1543–1549. doi:10.1086/523582
Emori TG, Gaynes RP (1993) An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev 6:428–442
Fang A, Hu SS, Endres N, Bradford DS (2005) Risk factors for infection after spinal surgery. Spine (Phila Pa 1976) 30:1460–1465
Friedman ND, Sexton DJ, Connelly SM, Kaye KS (2007) Risk factors for surgical site infection complicating laminectomy. Infect Control Hosp Epidemiol 28:1060–1065. doi:10.1086/519864
Gomes B, Higginson IJ (2006) Factors influencing death at home in terminally ill patients with cancer: systematic review. BMJ 332:515–521. doi:10.1136/bmj.38740.614954.55
Goodson WR, Hung TK (1977) Studies of wound healing in experimental diabetes mellitus. J Surg Res 22:221–227
Gruskay J, Kepler C, Smith J, Radcliff K, Vaccaro A (2011) Is surgical case order associated with increased infection rate after spine surgery?. Spine (Phila Pa 1976). doi:10.1097/BRS.0b013e3182407859
Hoogendoorn WE, van Poppel MN, Bongers PM, Koes BW, Bouter LM (1999) Physical load during work and leisure time as risk factors for back pain. Scand J Work Environ Health 25:387–403
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. doi:10.1016/j.ajic.2008.03.002
Kanafani ZA, Dakdouki GK, El-Dbouni O, Bawwab T, Kanj SS (2006) Surgical site infections following spinal surgery at a tertiary care center in Lebanon: incidence, microbiology, and risk factors. Scand J Infect Dis 38:589–592. doi:10.1080/00365540600606440
Klekamp J, Spengler DM, McNamara MJ, Haas DW (1999) Risk factors associated with methicillin-resistant staphylococcal wound infection after spinal surgery. J Spinal Disord 12:187–191
Koutsoumbelis S, Hughes AP, Girardi FP Jr, Cammisa FP, Finerty EA, Nguyen JT, Gausden E, Sama AA (2011) Risk factors for postoperative infection following posterior lumbar instrumented arthrodesis. J Bone Joint Surg Am 93:1627–1633. doi:10.2106/JBJS.J.00039
Kuo CH, Wang ST, Yu WK, Chang MC, Liu CL, Chen TH (2004) Postoperative spinal deep wound infection: a six-year review of 3,230 selective procedures. J Chin Med Assoc 67:398–402
Lee SE, Kim KT, Park YS, Kim YB (2010) Association between asymptomatic urinary tract infection and postoperative spine infection in elderly women: a retrospective analysis study. J Korean Neurosurg Soc 47:265–270. doi:10.3340/jkns.2010.47.4.265
Levi AD, Dickman CA, Sonntag VK (1997) Management of postoperative infections after spinal instrumentation. J Neurosurg 86:975–980. doi:10.3171/jns.1997.86.6.0975
Liao JC, Chen WJ, Chen LH, Niu CC (2006) Postoperative wound infection rates after posterior instrumented spinal surgery in diabetic patients. Chang Gung Med J 29:480–485
Lievense AM, Bierma-Zeinstra SM, Verhagen AP, van Baar ME, Verhaar JA, Koes BW (2002) Influence of obesity on the development of osteoarthritis of the hip: a systematic review. Rheumatology (Oxford) 41:1155–1162
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 20:250–278, 279–280. doi:10.1086/501620
Maragakis LL, Cosgrove SE, Martinez EA, Tucker MG, Cohen DB, Perl TM (2009) Intraoperative fraction of inspired oxygen is a modifiable risk factor for surgical site infection after spinal surgery. Anesthesiology 110:556–562. doi:10.1097/ALN.0b013e3181974be7
Mastronardi L, Tatta C (2004) Intraoperative antibiotic prophylaxis in clean spinal surgery: a retrospective analysis in a consecutive series of 973 cases. Surg Neurol 61(129–135):135
Mehta AI, Babu R, Karikari IO, Hughes BD, Agarwal VJ, Owens TR, Friedman AH, Bagley CA, Gottfried ON (2011) The distribution of body mass as a significant risk factor for lumbar spinal fusion postoperative infections. Spine (Phila Pa 1976). doi:10.1097/BRS.0b013e318241b186
Mirza SK, Deyo RA, Heagerty PJ, Konodi MA, Lee LA, Turner JA, Goodkin R (2008) Development of an index to characterize the “invasiveness” of spine surgery: validation by comparison to blood loss and operative time. Spine (Phila Pa 1976) 33:2651–2661. doi:10.1097/BRS.0b013e31818dad07 (discussion 2662)
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Muilwijk J, van den Hof S, Wille JC (2007) Associations between surgical site infection risk and hospital operation volume and surgeon operation volume among hospitals in the Dutch nosocomial infection surveillance network. Infect Control Hosp Epidemiol 28:557–563. doi:10.1086/513613
Olsen MA, Mayfield J, Lauryssen C, Polish LB, Jones M, Vest J, Fraser VJ (2003) Risk factors for surgical site infection in spinal surgery. J Neurosurg 98:149–155
Olsen MA, Nepple JJ, Riew KD, Lenke LG, Bridwell KH, Mayfield J, Fraser VJ (2008) Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am 90:62–69. doi:10.2106/JBJS.F.01515
Picada R, Winter RB, Lonstein JE, Denis F, Pinto MR, Smith MD, Perra JH (2000) Postoperative deep wound infection in adults after posterior lumbosacral spine fusion with instrumentation: incidence and management. J Spinal Disord 13:42–45
Pull Ter Gunne, Cohen DB (2009) Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976) 34:1422–1428. doi:10.1097/BRS.0b013e3181a03013
Ter Gunne Pull, van Laarhoven CJ, Cohen DB (2010) Surgical site infection after osteotomy of the adult spine: does type of osteotomy matter? Spine J 10:410–416. doi:10.1016/j.spinee.2009.11.017
Ter Gunne Pull, van Laarhoven CJ, Cohen DB (2010) Incidence of surgical site infection following adult spinal deformity surgery: an analysis of patient risk. Eur Spine J 19:982–988. doi:10.1007/s00586-009-1269-1
Pull TGD, Hosman DA, Cohen DD, Schuetz PM, Habil D, van Laarhoven PC, van Middendorp DJ (2012) A methodological systematic review on surgical site infections following spinal surgery. Part 1: risk factors. Spine (Phila Pa 1976). doi:10.1097/BRS.0b013e31825bfca8
Quintiliani L, Pescini A, Di Girolamo M, Iudicone P, Martini F, Guglielmetti M, Buzzonetti A, Fascioli S (1997) Relationship of blood transfusion, post-operative infections and immunoreactivity in patients undergoing surgery for gastrointestinal cancer. Haematologica 82:318–323
Rao SB, Vasquez G, Harrop J, Maltenfort M, Stein N, Kaliyadan G, Klibert F, Epstein R, Sharan A, Vaccaro A, Flomenberg P (2011) Risk factors for surgical site infections following spinal fusion procedures: a case–control study. Clin Infect Dis 53:686–692. doi:10.1093/cid/cir506
Rechtine GR, Bono PL, Cahill D, Bolesta MJ, Chrin AM (2001) Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma 15:566–569
Sasso RC, Garrido BJ (2008) Postoperative spinal wound infections. J Am Acad Orthop Surg 16:330–337
Schimmel JJ, Horsting PP, de Kleuver M, Wonders G, van Limbeek J (2010) Risk factors for deep surgical site infections after spinal fusion. Eur Spine J 19:1711–1719. doi:10.1007/s00586-010-1421-y
Schuster JM, Rechtine G, Norvell DC, Dettori JR (2010) The influence of perioperative risk factors and therapeutic interventions on infection rates after spine surgery: a systematic review. Spine (Phila Pa 1976) 35:S125–S137. doi:10.1097/BRS.0b013e3181d8342c
Schwarzkopf R, Chung C, Park JJ, Walsh M, Spivak JM, Steiger D (2010) Effects of perioperative blood product use on surgical site infection following thoracic and lumbar spinal surgery. Spine (Phila Pa 1976) 35:340–346. doi:10.1097/BRS.0b013e3181b86eda
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Thomsen T, Tonnesen H, Moller AM (2009) Effect of preoperative smoking cessation interventions on postoperative complications and smoking cessation. Br J Surg 96:451–461. doi:10.1002/bjs.6591
Urquhart DM, Hanna FS, Brennan SL, Wluka AE, Leder K, Cameron PA, Graves SE, Cicuttini FM (2010) Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty 25:1216–1222. doi:10.1016/j.arth.2009.08.011
Veeravagu A, Patil CG, Lad SP, Boakye M (2009) Risk factors for postoperative spinal wound infections after spinal decompression and fusion surgeries. Spine (Phila Pa 1976) 34:1869–1872. doi:10.1097/BRS.0b013e3181adc989
Watanabe M, Sakai D, Matsuyama D, Yamamoto Y, Sato M, Mochida J (2010) Risk factors for surgical site infection following spine surgery: efficacy of intraoperative saline irrigation. J Neurosurg Spine 12:540–546. doi:10.3171/2009.11.SPINE09308
Weinstein MA, McCabe JP Jr, Cammisa FP (2000) Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord 13:422–426
West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, Lux L (2002) Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ) (47):1–11
Willems KF, Slot GH, Anderson PG, Pavlov PW, de Kleuver M (2005) Spinal osteotomy in patients with ankylosing spondylitis: complications during first postoperative year. Spine (Phila Pa 1976) 30:101–107
Wimmer C, Gluch H, Franzreb M, Ogon M (1998) Predisposing factors for infection in spine surgery: a survey of 850 spinal procedures. J Spinal Disord 11:124–128
Acknowledgments
The authors thank Professor Edmund Y.S. Chao and Jun Lu for their advice on researching the unpublished literature. This study was supported by grants from the National Natural Science Foundation of China (No. 81102607), the Key Technologies & Program of Tianjin (No. 11ZCGYSY01800) and the Scientific and Technological Project of Tianjin Public Health Bureau (No. 11KG137).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
D. Xing and J.-X. Ma contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xing, D., Ma, JX., Ma, XL. et al. A methodological, systematic review of evidence-based independent risk factors for surgical site infections after spinal surgery. Eur Spine J 22, 605–615 (2013). https://doi.org/10.1007/s00586-012-2514-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-012-2514-6