Abstract
Purpose
The hypothesis for this study was that the simulated wear behavior of a hydroxyapatite coated, self-mating PEEK cervical disc arthroplasty device would be dependent on the simulated testing environment.
Methods
Five groups of devices were evaluated under suggested ASTM and ISO load and motion profiles. The groups utilized different testing frequencies and protein content of simulator fluid, in addition to assessing the potential for third body wear. The average wear rates were determined using linear regression analysis with a generalized estimating equation. Significant differences between groups were determined using the Wald’s test.
Results
The simulated wear behavior was shown to be highly dependent on the testing environment, where protein content more than decreasing the cyclic loading frequency resulted in increased wear, but was not dependent on the suggested load and motion profiles. It was demonstrated that a self-mating PEEK cervical disc arthroplasty device has wear rates that are similar to existing material combinations for cervical disc arthroplasty.
Conclusions
This study showed that at a time when data from retrieval analyses is deficient, it is important to test the wear resistance of cervical disc arthroplasty devices under various conditions. Long-term clinical results and ongoing implant retrievals are required for validation between clinical performance and simulator inputs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical total disc replacement (TDR) is a well-established alternative treatment option for anterior cervical decompression and fusion (ACDF). The goal of TDR is to relieve the symptoms associated with degenerative disc disease (radiculopathy and/or myelopathy) and preserve motion at the index level, while simultaneously providing biomechanical stability and overall global neck mobility and alignment. The benefits of TDR are the maintenance of motion at the index level leading to potential reduction of adjacent level degenerative changes brought upon by the ACDF process, while still leaving the fusion option available. The history of TDR goes back more than 40 years starting with the use of stainless steel (SS) balls [1, 2] to more recent implants that consist of a diverse selection of material combinations, such as polyethylene on metal, metal on metal and elastomeric constructs. Subsequently, the clinical outcome of many of these modern implants have shown promise in providing equivalent or superior clinical performance to ACDF in randomized, controlled studies [3–6]. However, currently ACDF still remains the gold standard of care.
A central element in the design of TDR devices is an articulating or elastomeric core component as the premise for their motion-preserving characteristics. Therefore, as compared to ACDF, TDR devices face additional issues, such as wear and biodurability. Given that these devices are indicated for skeletally mature patients (≥18 years of age) [3–5], the durability of the device should be evaluated as part of the preclinical evaluation, since these prostheses are expected to last for the lifetime of the recipient. To assess the durability of motion-preserving devices, in vitro wear simulations are performed as a component of preclinical safety and effectiveness evaluations to satisfy medical device directives (MDD) and investigational device exemption (IDE) submissions. The American Society for Testing and Materials (ASTM) and the International Organization for Standardization (ISO) have developed a guide [7] and standard [8], respectively, detailing the testing methodology for evaluating the wear properties of cervical disc replacements. However, knowledge about the clinical wear behavior of these devices remains limited. Unlike total joint replacements, to date there is no substantial clinical retrieval history, and although the loading profiles and kinematics for these devices may have been established, they have not been clinically validated. The limited explant analyses that have been performed to date are from patients with a short clinical follow-up and have undergone revision due to recurring symptoms that likely precluded them from utilizing normal ranges of motion (ROM) [9–13]. In addition, the environment that a cervical arthroplasty device resides in is not truly known postoperatively, as the cervical intervertebral disc is not a synovial joint, but rather a cartilaginous joint, and similar to lumbar disc arthroplasty, much of what is speculated comes from the history of total joint arthroplasty. It has also been well established that the wear behavior of arthroplasty devices can be critically influenced by the load and kinematic parameters, test frequency, lubricant, material selection and device design. To this end, without long-term clinical retrievals and the subsequent analyses, the assessment of the various potential influences on a respective devices’ wear properties rather than relying on one set of parameters is essential, especially in evaluating candidate materials for cervical arthroplasty. Therefore, how deviation from a single set of testing parameters translates to differences in the amount of wear is clinically important.
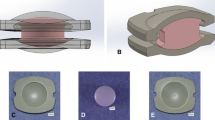
The objective of the current study was to assess the in vitro wear properties of PEEK-OPTIMA LT1 (PEEK) under various testing parameters to determine the dependence of wear on simulated conditions for cervical arthroplasty applications. NuNec (Pioneer Surgical, Marquette, MI, USA) is a machined, self-mating PEEK TDR with a ball- and socket-type articulation (Fig. 1). The device has tantalum (Ta) markers for visualization within the disc space, titanium (Ti) cam blades for primary fixation and a hydroxyapatite (HA) coating on its outer endplates for supplemental fixation. CE mark was obtained in 2008 and it has since undergone wide clinical use. The hypothesis for this study was that the simulated wear behavior of this self-mating PEEK TDR device would be highly dependent on the simulator testing environment, such as loading and kinematic conditions, test frequency, protein content of simulated body fluid and third body wear.
Methods
All implants used in this study were applicable to a clinical setting. The device has the same bearing surface area and thus the same contact area for all footprints and heights. The smallest height and footprint was tested, with standard sterilization of 3.0 MRad of gamma radiation. Five separate groups of six implants each were utilized in this wear study. Table 1 summarizes the test methodology. Groups 1a–3 consisted of implants that had not gone through the HA coating process, while Groups 4 and 5 were HA coated devices. All implants were pre-soaked in test fluid for at least 6 weeks. All groups were then tested on a six-station spine wear simulator (MTS, Eden Prairie, MN, USA) with a soak control as reference. Cleaning, drying and weighing of all implants were performed similar to ASTM F2025-6 [14].
For Groups 1a, b, the testing fluid consisted of newborn calf serum diluted with phosphate buffered saline (PBS) to a final protein content of 20 g/L. Ethylene-diamine tetraacetic acid (EDTA) was added to the serum at a concentration of 20 mM to bind the Ca++ present in the serum. EDTA is a known preservative, and together with the low protein content and PBS, the addition of sodium azide or other anti-bacterial agent was not used. The pH of the lubricant was monitored. Group 1a was tested under ASTM F2423-05 [7] recommended load and motion profiles (Fig. 2) for 10 million cycles (Mc). After 10 Mc, these same implants were then tested under ISO 18192-1 [8] recommended load and motion profiles for another 10 Mc (Group 1b) (Fig. 2). The test was stopped at 0.5, 1.0 Mc and every Mc interval thereafter to clean and gravimetrically weigh the samples. The ASTM method was chosen first as it was thought that the recommendation of a static compressive load would deprive the articulating surfaces of lubrication, possibly resulting in adhesion of these surfaces and a potential worst-case scenario for wear. Group 2a was tested using the ASTM load and motion profiles until steady state wear occurred (4 Mc) followed by the ISO load and motion profiles until steady state wear occurred (3 Mc) (Group 2b), both at a test frequency of 1 Hz. The test fluid was maintained at 37 ± 3 °C. For this group of devices, the test was stopped at every 0.5 Mc to minimize protein degradation. Group 3 was tested using the ISO load and motion profiles, with a 5 g/L protein content lubricant. For this group of devices, the temperature of the lubricant was maintained at 23 ± 3 °C and the test stopped at every 0.5 Mc to even further minimize protein degradation, with the test terminated when steady state wear occurred (3 Mc).
For Groups 4 and 5, wear testing was performed to assess third body wear potential due to the HA coating. Group 4 had the coating removed by submerging in 10 % phosphoric acid at 100 °C for 30 min, placed in a sodium bicarbonate solution to neutralize the acid and double rinsed with DI water. This group served as a control. Group 5 was tested with the HA coating intact. The ISO load and motion profiles were used (Fig. 2). However, the test fluid for these groups consisted of PBS to minimize premature dissolution of the HA coating. A test frequency of 2 Hz was utilized for these groups, with the test fluid temperature kept at 37 ± 3 °C. Since the test fluid consisted of PBS, this allowed for the cycle intervals to be decreased to minimize handling of the coated devices (Table 1).
Prior to testing with saline as a lubricant, a feasibility wear test without the addition of EDTA to the test fluid was explored as another testing parameter. EDTA is utilized in wear testing fluid in order to bind Ca++ present within the testing serum, and being a known food preservative, to help prevent protein denaturization. However, as a Ca++ chelator, prior experience with EDTA has shown that this will result in premature removal and dissolution of the coating from the device. Therefore, two non-coated devices were tested under the same testing parameters as Group 1b (Table 1) to determine the effect of not using EDTA.
The average wear rates were determined using linear regression analysis with a generalized estimating equation (GEE). A parametric statistical analysis using the Wald’s test was used to determine if significant differences (p < 0.05) in the wear rates within or between groups was present. Wear volume was calculated using the density of PEEK, 1.30 g/mL3. Light microscopy was used to characterize the articulating wear surfaces using a Nikon Multizoom AZ100.
Results
All implants for each group maintained full functionality throughout each test duration. The exception to this was the samples in the feasibility test. Significant protein precipitates were noted to form along with severe delamination of the articulating surfaces (Fig. 3), so the test was terminated at 0.5 Mc. Given the significant formation of the protein precipitates and severity of the delaminations, a mass loss assessment was not performed. The mass loss and wear rates for all other groups are presented in Figs. 4, 5. For Group 1 (1a, b), the results showed only a slight, but significant variation in the wear rates over the course of 20 Mc. The wear rate for the first 10 Mc was approximately 0.26 ± 0.01 mm3/Mc. For the interval Group 1b (10–20 Mc), the wear rate increased slightly, 0.32 ± 0.02 mm3/Mc. For Groups 2a and 2b, the wear rate again showed only a slight variation (non-significant) at the measured time points. From 0.0 to 4.0 Mc, the wear rate was 0.58 ± 0.07 mm3/Mc and from 4.0 to 7.0 Mc was 0.57 ± 0.03 mm3/Mc. For Group 3, the wear rate increased to 0.67 ± 0.10 mm3/Mc. For Groups 4 and 5, the wear rates were 0.89 ± 0.08 and 1.23 ± 0.07 mm3/Mc, respectively.
Visual and light microscopy revealed no evidence of gross deformation, delamination or fatigue cracks in the implants after testing. Closer examination under light microscopy revealed an abrasive wear mechanism occurring, with scratches and highly polished surfaces for all groups (Fig. 6). The articulating surfaces for Group 3 appeared to be more polished than that of any other group (Fig. 7). For Groups 4 and 5, there were microscopic delaminations noted in addition to burnishing and what appeared to be micro-fatigue cracks. There were no notable differences in the images that third body wear was present (Fig. 8), which implied that no third body wear was occurring with this material couple.
Representative top component and bottom component for Group 1a (a, b) at 10 million cycles. Highly polished surfaces are noted with mild abrasions. Representative top component and bottom component for Group 1b (c, d) at 20 million cycles. The abrasions have given way to a more polished surfaced with folds (“hand mark” on d)
Representative top component and bottom component for Group 4 (a, b) and Group 5 (c, d) at 10 million cycles. Highly polished surfaces are noted with mild abrasions. Unlike Groups 1a–3, microscopic delaminations and what appears to be fatigue cracks (b) are also noted. These varied by specimen and are thought to be one of the reasons for the high variance in mass loss
Discussion
The results of this study support our hypothesis that the simulated wear behavior of a self-mating PEEK TDR is highly dependent on the simulator testing environment, where decreasing the cyclic loading frequency resulted in approximately a doubling of the amount of wear when slowed from 2 to 1 Hz. While a fourfold decrease in the amount of protein in the simulator fluid increased the wear by approximately 15 % (Groups 2b vs. 3), the elimination of protein from the simulator fluid resulted in nearly a threefold increase in the wear rate (Groups 1b vs. 4), demonstrating the protective effect of protein in the simulator fluids. Past investigations of other materials have shown that the wear behavior of arthroplasty devices can be critically influenced by various testing parameters, along with material selection and device design. For example, an increase in the sliding distance a bearing surface travels can result in increased wear [15, 16]. Decreasing the quantity of protein in the lubricant can increase the wear rate of UHMWPE on CoCr and decrease the wear rate of PTFE on CoCr, but testing both in a zero protein fluid yields unrealistically low wear rates for both as compared to clinical retrieval analyses [17, 18]. In addition, multidirectional or “cross-shear” motion can increase the wear rate by an order of magnitude [19–22] or not have an effect at all [23]. Moreover, a test frequency of 1 versus 2 Hz has been shown to yield a lower wear rate for hip arthroplasty devices [24], whereas the opposite effect has been seen in total disc replacements [25, 26]. Therefore, the selection of the proper input parameters and the potential effect of the testing environment should be an important part in the assessment of the wear behavior of a TDR device.
The influence of various testing parameters in this study is consistent with the knowledge gained from previous wear testing, where kinematic and loading conditions, protein content, test frequency and lubricant were shown to influence the results. There was a statistical, although slight, difference (0.06 mm3/Mc), in the wear rate when utilizing either the selected ASTM or ISO load and motion parameters (Groups 1a, 1b), which suggests that either a dynamic (ISO) or static (ASTM) compressive load may potentially be used to evaluate wear performance. However, testing at a frequency of 1 versus 2 Hz approximately doubled the wear rate for the Group 2 implants. This may be the result of thermal heating at the articulating surfaces when testing at 2 Hz. PEEK is considered a thermal insulator (thermal conductivity of 0.25 W/mK) as compared to the more common material of CoCr (thermal conductivity of 18 W/mK), and this may have resulted in denaturization of the proteinaceous lubricant, potentially forming a protective triobolayer [27–29]. This is supported by the results for Group 3, where a reduced protein content of 5 g/L and a decrease in the lubricant temperature to 23 °C was performed to reduce any thermal artifact and further minimize protein denaturization. This resulted in a similar wear rate to that of the Group 2b implants tested at 1 Hz. The Group 4 implants, without any tribo-protective protein, resulted in a nearly a threefold increase in wear. Physiologically extreme testing using only saline as a lubricant (Groups 4, 5) was required to minimize dissolution of the HA coating and thus minimize any mass loss assessment artifact. EDTA is utilized in wear testing fluid to bind Ca++, and being a known food preservative, to help prevent protein denaturization. As a Ca++ chelator, EDTA will result in premature removal and dissolution of the HA coating from the device. Therefore, wear testing without the addition of EDTA to the test fluid was explored. A feasibility wear test was performed consistent with the test methodology of Group 1b. However, unacceptable wear of the device occurred at only 0.5 Mc. It is speculated that Ca++/protein precipitates rapidly formed and became entrapped within the articulating surfaces leading to severe adhesion (Fig. 3). Subsequently, Groups 4 and 5 were tested in saline, primarily to give a percent increase or decrease in the wear rate, and along with visual and microscopic inspection, to assess the potential and subsequent results of third body wear. The use of saline resulted in the highest wear rate of 0.89 ± 0.08 and 1.23 ± 0.07 mm3/Mc (Groups 4 and 5, respectively). The higher wear rate for Group 4 was expected, given that saline lacks any lubricating proteins [30], and yielded the opposite result of polymer on metal articulations [17, 18]. Given the lack of evidence for third body wear from the imaging assessment for Group 5 as compared to Group 4, it is believed that slow dissolution of the coating and/or physical removal of the HA coating during specimen handling during testing is responsible for the difference in mass loss; the cycle intervals were decreased to minimize handling of the coated devices. However, this is just speculation. A control device to account for the dissolution of the coating would have been ideal.
A limitation to this study was that unused samples would have been the best method for testing Groups 1b and 2a, b. In addition, all groups were not tested to 10 Mc, as recommended by ASTM and ISO. However, the main goal of this study was to assess the effect of various parameters on the wear rate and not the overall longevity of the articulation, therefore we measured the effects of the testing parameters up to the point that steady state wear occurred. Steady state wear was noted to occur within 3–4 Mc in all tests. The decision to reuse samples or proceed with testing passed this point was based upon the results and resources available at the time. This was the reasoning for testing to 10 Mc in saline, as this was considered the physiological extreme and resulted in the highest wear rate of all groups tested.
The use of self-mating PEEK was evaluated previously as an alternative bearing material in comparison to conventional UHMWPE on metal and other self-mating polymer combinations in the form of the Active C device [31]. The testing methodology utilized the load and motion profiles of the ISO standard at a test frequency of 1 Hz in a lubricant of 30 g/L bovine serum with the addition of 20 mM of EDTA and amphotericin to retard bacterial degradation. The overall wear rate of PEEK-on-PEEK was similar to the UHMWPE/CoCr bearing couple, 1.08 versus 1.07 mm3/Mc, respectively. In addition, the overall volume loss was similar; 11.3 versus 10.3 mm3, respectively. Interestingly, these wear rates were comparable despite significant pitting and delamination, which occurred between 2.0 and 5.0 Mc on the Active C device manufactured from PEEK. The authors therefore questioned the use of self-mating PEEK as an alternative bearing material, but gave no reason for the source of this phenomenon. It is not clear why there is a discrepancy with these results and our results, but it may lie in the testing methodology or device design. However, it is worthy to note that the same severe delaminations seem to have occurred during our feasibility testing without the addition of EDTA (Fig. 3), that this phenomenon was not observed when utilizing self-mating PEEK under multidirectional pin-on-plate testing [32], and in the current test, only microscopic delaminations at 10 Mc were seen while testing in physiologically extreme saline at 2 Hz. It may be possible that the use of a higher protein content (30 g/L) leading to significant protein precipitation could be the cause, as seen in our feasibility test.
The experimental wear rates found in this investigation are consistent with the other tribological investigations of TDR devices that are used clinically. The wear rate of the Prodisc C, an UHWMPE/CoCr device with a ball and socket articulation, has been reported to range between 2.11 ± 0.16 [33] and 2.74 ± 0.38 mm3/Mc [15] using the ISO method. The Discover, another ball and socket implant design, clinically utilizes cross-linked UHMWPE/Ti and reportedly has an experimental wear rate of 2.3 mm3/Mc using the ISO method, but when paired with CoCr and a custom motion waveform, can also have an experimental wear rate of up to 4.5 mm3/Mc [34]. The Bryan cervical disc device comprises two Ti alloy clam shell shaped endplates with an articulating polycarbonate polyurethane (Bionate-S) core enclosed within a polyether-polyurethane sheath bathed in saline. Testing was based on the neutral zone ROM of the cervical spine without a lateral bending component (±4.9° flexion/extension and ±3.8° axial rotation), a constant 130 N compressive load and a test frequency of 4 Hz. This yielded a wear rate of 0.97 mm3/Mc [9]. The Prestige ST, a self-mating SS ball and trough device, was tested to 10 Mc of flexion/extension (±9.7°, 148 N load, 2 Hz) followed with an additional 5 Mc of coupled lateral bending (±4.7°, 49 N load, 2 Hz) and axial rotation (±3.8°, 49 N load, 2 Hz) [35]. The reported wear rates were 0.01 ± 0.01 and 0.73 ± 0.25 mm3/Mc, respectively. The device was also tested with the motion order reversed, with reported wear rates of 0.53 ± 0.21 and 0.07 ± 0.02 mm3/Mc, respectively. The Kineflex C, a self-mating CoCr device, was reportedly tested under the ASTM guidelines [36]. The wear rate for this device was 0.38 mm3/Mc. The M6, an elastomeric TDR was also testing under the ASTM guidelines and was reported to have a wear rate of 0.44 mm3/Mc [37]. In perspective, the range of wear rates that were determined for the self-mating PEEK device in the current study was 0.26 ± 0.01 to 0.89 ± 0.10 mm3/Mc.
In summary, the simulated wear behavior of a self-mating PEEK TDR demonstrated it was dependant on the simulated testing environment, where protein content more than decreasing the cyclic loading frequency resulted in increased wear. The results of the testing performed in the current investigation compared with the previously published data on the wear behavior of other devices demonstrates that a single set of parameters cannot fully predict the performance of a TDR device. This highlights the importance of understanding the various potential factors within a broad spectrum of testing conditions that can influence the wear rate, especially when the ability to properly match simulator results with retrieval analysis is not available at this time. In this regard, a more realistic worst-case testing scenario could be possible than testing in saline solution. For instance, the addition of third body wear particulate to the testing serum would undoubtedly result in a higher wear rate than those found in the current study, or impingement testing would be another parameter to investigate. To this end, the importance of clinical retrievals cannot be emphasized enough in this regard. Despite the differences in test parameter-dependent wear rates, it is not known what the clinical consequence would be, if any, for the increased wear rates observed in the best- and worst-case rates observed in the current study. In comparison to previous experimentally established rates of wear for other TDR devices, the results of this study indicates comparable or better wear performance of self-mating PEEK in cervical arthroplasty. However, long-term clinical results and ongoing implant retrievals are required for further validation between clinical performance and inputs, and will eventually validate which, if any, of these testing conditions is more clinically relevant than others.
References
Fernstrom U (1966) Arthroplasty with intercorporal endopothesis in herniated disc and in painful disc. Acta Chir Scand Suppl 357:154–159
Reitz H, Joubert MJ (1964) Intractable headache and cervico-brachialgia treated by complete replacement of cervical intervertebral discs with a metal prosthesis. S Afr Med J 38:881–884
Murrey D, Janssen M, Delamarter R, Goldstein J et al (2009) Results of the prospective, randomized controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J 9(4):275–286
Heller JG, Sasso RC, Papadopoulos SM, Anderson PA et al (2009) Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: clinical and radiographic results of a randomized, controlled, clinical trial. Spine 34(2):101–107
Mummaneni PV, Burkus JK, Haid RW, Traynelis VC et al (2007) Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled clinical trial. J Neurosurg Spine 6(3):198–209
Nabhan A, Ahlhelm F, Pitzen T, Steudel WI et al (2007) Disc replacement using Pro-Disc C versus fusion: a prospective randomised and controlled radiographic and clinical study. Eur Spine J 16(3):423–430
American Society of Testing and Materials (2006) Designation: F 2423-05. Standard guide for functional, kinematic, and wear assessment of total disc prostheses. American Society of Testing and Materials, West Conshohocken
International Organization for Standardization (2008) ISO 18192-1: implants for surgery—wear of total intervertebral spinal disc prostheses: Part 1. Loading and displacement parameters for wear testing and corresponding environmental conditions for tests. International Organization for Standardization
Anderson PA, Rouleau JP, Toth JM et al (2004) A comparison of simulator-tested and -retrieved cervical disc prostheses. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine 1(2):202–210
Pitzen T, Kettler A, Drumm J et al (2007) Cervical spine disc prosthesis: radiographic, biomechanical and morphological post mortal findings 12 weeks after implantation: a retrieval example. Eur Spine J 16(7):1015–1020
Tumialán LM, Gluf WM (2011) Progressive vertebral body osteolysis after cervical disc arthroplasty. Spine 36(14):E973–E978
Cavanaugh DA, Nunley PD, Kerr EJ et al (2009) Delayed hyper-reactivity to metal ions after cervical disc arthroplasty: a case report and literature review. Spine 34(7):E262–E265
Guyer RD, Shellock J, MacLennan B et al (2011) Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction: diagnosis and treatment experience in four cases. Spine 36(7):E492–E497
American Society of Testing and Materials (2006) Designation: F 2025-06. Standard practice for gravimetric measurement of polymeric components for wear assessment. American Society of Testing and Materials, West Conshohocken
Bushelow M, Nechtow W, Hinter M et al (2006) Wear testing of a cervical total disc replacement: effect of motion and load parameters on wear rate and particle morphology. Transactions of the 54th Orthopedic Research Society, No. 1925
Pare PE, Chan FW, Bhattacharya S et al (2009) Surface slide track mapping of implants for total disc arthroplasty. J Biomech 42(2):131–139
Wang A, Essner A, Schmidig G (2004) The effects of lubricant composition on in vitro wear testing of polymeric acetabular components. J Biomed Mater Res B Appl Biomater 68(1):45–52
Good VD, Clarke IC, Gustafson GA et al (2000) Wear of ultra-high molecular weight polyethylene and polytetrafluoroethylene in a hip simulator: a dose-response study of protein concentration. Acta Orthop Scand 71(4):365–369
Grupp TM, Yue JJ, Garcia R et al (2009) Biotribological evaluation of artificial disc arthroplasty devices: influence of loading and kinematic patterns during in vitro wear simulation. Eur Spine J 18:98–108
Nechtow W, Hintner M, Bushelow M et al (2006) IVD replacement mechanical performance depends strongly on input parameters. Orthopedic Research Society, Chicago
Wang A, Sun DC, Yau SS et al (1997) Orientation softening in the deformation and wear of ultra-high molecular weight polyethylene. Wear 203–204:230–241
Bradgon CR, O’Connor DO, Lowenstein JS et al (1996) The importance of multidirectional motion on the wear of polyethylene. Proc Inst Mech Eng [H] 210:157–165
Brown T, Bao QB, Kilpela T et al (2010) An in vitro biotribological assessment of NUBAC, a polyetheretherketone-on-polyetheretherketone articulating nucleus replacement device: methodology and results from a series of wear tests using different motion profiles, test frequencies, and environmental conditions. Spine 35(16):E774–E781
Bragdon CR, O’Connor DO, Weinberg EA et al (1999) The effect of cycle rate on the wear of conventional and highly crosslinked UHMWPE acetabular components using the Boston AMTI hip simulator. Transactions of the 45th Orthopedic Research Society, No. 831
Center of Devices and Radiological Health [DoHaHS P060018] (2011) Summary of safety and effectiveness [Food and Drug Administration]. http://www.accessdata.fda.gov/cdrh_docs/pdf6/P060018b.pdf. Accessed 22 June 2011
Kettler A, Bushelow M, Wilke HJ (2010) Influence of the loading frequency on the wear rate of a polyethylene-on-metal lumbar intervertebral disc replacement. Eur Spine J Oct 10 [Epub ahead of print]
Liao YS, McKellop H, Lu Z et al (2003) The effect of frictional heating and forced cooling on the serum lubricant and wear of UHMW polyethylene cups against cobalt–chromium and zirconia balls. Biomaterials 24(18):3047–3059
Lu Z, McKellop H, Liao P et al (1999) Potential thermal artifacts in hip joint wear simulators. J Biomed Mater Res 48(4):458–464
Lu Z, McKellop H (1997) Frictional heating of bearing materials tested in a hip joint wear simulator. Proc Inst Mech Eng H 211(1):101–108
Heuberger MP, Widmer MR, Zobeley E et al (2005) Protein-mediated boundary lubrication in arthroplasty. Biomaterials 26(10):1165–1173
Grupp TM, Meisel HJ, Cotton JA et al (2010) Alternative bearing materials for intervertebral disc arthroplasty. Biomaterials 31(3):523–531
Scholes SC, Unsworth A (2008) Comparison of PEEK, PEK and CFR-PEEK in self-mating wear couples for use in orthopaedics. Transactions of the 8th World Congress of Biomaterials, No. P-SAT-I-598
Nechtow W, Bushelow M, Hintner M et al (2007) Cervical disc prosthesis polyethylene wear following the ISO cervical test. Transactions of the 54th Orthopedic Research Society, No. 1926
Dooris A, Hester D, Albert T et al (2007) Cervical disc wear depends on bearing materials and test parameters. In: Global Symposium on Motion Preservation Technology
Center of Devices and Radiological Health [DoHaHS P060023b] (2009) Summary of safety and effectiveness [Food and Drug Administration]. http://www.accessdata.fda.gov/cdrh_docs/pdf5/P050010b.pdf. Accessed 1 June 2009
Rappaport JR Kineflex C cervical artificial disc. In: Yue JJ, Bertagnoli R, McAfee PC, An HS (eds) Motion preservation surgery of the spine. Advanced techniques and controversies, 1st edn. Saunders Elsevier, Pennsylvania, pp 258–266
Kim DH, Reo ML, Robinson JC et al (2007) Wear properties and biological response of a novel total artificial disc with compressible artificial nucleus and fiber annulus. Transactions of the 7th Global Symposium on Motion Preservation Technology
Conflict of interest
Tim Brown is a Employee Pioneer Surgical.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, T., Bao, QB. The use of self-mating PEEK as an alternative bearing material for cervical disc arthroplasty: a comparison of different simulator inputs and tribological environments. Eur Spine J 21 (Suppl 5), 717–726 (2012). https://doi.org/10.1007/s00586-012-2252-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-012-2252-9