Abstract
Purpose
Disc degeneration, and associated low back pain, are a primary cause of disability. Disc degeneration is characterized by dysfunctional cells and loss of proteoglycans: since intervertebral tissue has a limited capacity to regenerate, this process is at present considered irreversible. Recently, cell therapy has been suggested to provide more successful treatment of IVD degeneration. To understand the potential of cells to restore IVD structure/function, tissue samples from degenerated IVD versus healthy discs have been compared.
Methods
Discal tissue from 27 patients (40.17 ± 11 years) undergoing surgery for degenerative disc disease (DDD), DDD + herniation and congenital scoliosis, as controls, was investigated. Cells and matrix in the nucleus pulposus (NP) and annulus fibrosus (AF) were characterized by histology. AF- and NP-derived cells were isolated, expanded and characterized for senescence and gene expression. Three-dimensional NP pellets were cultured and stained for glycosaminoglycan formation.
Results
Phenotypical markers of degeneration, such as cell clusters, chondrons, and collagen disorganization were seen in the degenerate samples. In severe degeneration, granulation tissue and peripheral vascularization were observed. No correlation was found between the Pfirrmann clinical score and the extent of degeneration.
Conclusion
The tissue disorganization in degenerate discs and the paucity of cells out of cluster/chondron association, make the IVD-derived cells an unreliable option for disc regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Degeneration of the disc, naturally occurring during aging and accelerated by environmental or genetic factors, is one of the major causes of low back pain (LBP). Indeed, the degenerative process of the disc begins as early as the second decade of life, with most of the lumbar discs showing some evidence of degeneration by the fifth decade [1, 2].
Conservative therapies are current treatments to relieve of pain. When such therapies are not effective, with persistent pain and dysfunction, or in case of further degeneration, an emerging approach is the cell-based regeneration of the intervertebral disc [3, 4].
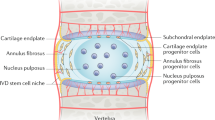
Embryogenetic origin and structure of the intervertebral disc (IVD) are well known: it is macroscopically made of a fibrous coat (annulus fibrosus, AF) surrounding a gel-like matrix with cells (nucleus pulposus, NP). Two thin endplates of hyaline cartilage extend superiorly and inferiorly to interface with the vertebral bodies, and regulate nutrient diffusion. The fibrillar matrix is made up of tension-resistant collagen type-I, in outer annulus, and compression-resistant collagen type-II, in inner annulus, nucleus and end-plate [5].
Intervertebral disc is a relatively acellular environment, with ~5,000 cells/mm3 in the NP region and 9,000 cells/mm3 in the AF region.
Nucleus pulposus cells are rounded with a chondrocyte-like phenotype, while AF cells are thin, elongated and aligned with the collagen fibers [6].
It has been shown that phenotype and morphology of both cell types is interchangeable, dependent on culture conditions: NP cells in monolayer become fibroblastic, decrease type-II collagen and increase type-I collagen production. Conversely, AF cells cultured in alginate increase type-II collagen production and chondrocyte differentiation factor SOX-9 [7].
The disc is the largest avascular tissue in the body, and the cells are nourished by diffusion through the matrix. Therefore, the NP matrix is rich in glycosaminoglycans (GAGs) and highly hydrated to support cell viability and to resist mechanical loads transferred through the AF. The rare NP cells are located within a glycan-rich niche, like cartilage cells, express chondrocyte markers, including SOX-9, collagen type-II and aggrecan, and are able to withstand severe conditions, such as hypoxia. Unfortunately, due to its avascular nature, the disc has a very low self-repair ability. The cell population changes dramatically with age, with a progressive loss by apoptosis of the large notochordal cells in the NP, and a shift to small sparse chondrocyte-like cells; since the composition of the matrix is determined by the cells within it, there is a net loss of large matrix proteoglycans, and a change in collagen secretion.
Severe disc degeneration is associated with increased vascularization and innervation of peripheral tissue, changes of extracellular matrix (ECM) composition, appearance of cell clusters, and chondrons.
Summarizing, changes in ECM composition, swelling, and paucity of NP cells are mutually influencing processes, which progressively cause disc degeneration.
Thus, the characterization of the cells and matrix in the degenerate IVD tissue, in comparison with healthy discs, is useful in order to understand/predict the feasibility of a cell therapy to restore IVD structure/function. Several tissue engineering (TE) protocols of the disc have been developed, and the use of mesenchymal cells (MSC) has been shown to be promising, but MSC introduction in the degenerate tissue should be accurately designed using TE strategies, because some adverse effects have been reported [8, 9].
This study is the first part of an investigation on the tissue features of IVD retrievals, and ability of disc cell to expand in vitro. Human IVD tissues were taken at surgery from patients suffering from LBP (degenerative disc disease, DDD) or DDD plus herniation following informed consent of the donors.
Disc tissue from patients undergoing surgery for scoliosis was used as control tissue [10]. The histology of degenerate and ‘healthy’ discs was investigated to describe cellularity and matrix organization, whereas the disc-derived cells were cultured and assayed, with a view to using one or other of these cells in future experiments.
Materials and methods
Following institutional review board approval and patient informed consent, tissue specimens of human IVD were obtained from 27 patients (40.17 ± 11 years) undergoing surgery at the Orthopedic-Trauma Spine Surgery Dept. of the Istituto Ortopedico Rizzoli, due to DDD, or DDD plus herniation of the disc or congenital scoliosis (Table 1).
Tissue collection
Separated AF and NP tissues, collected in Dulbecco’s modified minimum essential medium (D-MEM), were dissected, and a ~50 mg-fragment of each tissue fixed and stained for histology, while another part was finely minced and seeded in separate 75 cm2 flasks for cell expansion.
Histology
After AF and NP fixation and embedding, 5 μm-thick sections were cut. Alcian Blue, 1% in 0.1 M HCl, pH 1.0, for 1 h, and Toluidine blue, 0.04% in 0.1 M sodium acetate buffer, pH 4.0, for 10 min, were used for the glycosaminoglycan-rich matrix. Alcian blue–Sirius red–hematoxylin (ASH) were combined: after nuclear staining (Weigert-haematoxylin, 10 min), proteoglycans were stained with Alcian blue (0.5% in 1% acetic acid, 5 min), followed by molybdophosphoric acid (1%, 20 min) and Sirius-red F3B (0.1% in 30% picric-acid, 1 h) for collagen.
AF and NP cells
AF and NP fragments were cultured with D-MEM containing 10% FBS, and 1% antibiotic/antimycotic. Cells were expanded for 3–4 weeks: at confluence, 3 × 105 NP cells were centrifuged at low speed and pellets were cultured in loosely capped tubes at 37°C, 5% CO2, with Alamar-Blue assay used for cell proliferation. At the end of cell proliferation, pellets were fixed and stained with toluidine blue for the matrix production by 3D-arranged cells.
AF and NP monolayers were stained for β-galactosidase, i.e. an intracellular green-blue, insoluble precipitate, using the Senescence Detection Kit β-Gal (Calbiochem, QIA117) [11].
Gene expression
AF and NP gene expression was measured by real time PCR (Table 2). RNA was extracted from 1st and 2nd pass cells using RNeasy mini kit (Qiagen, GmbH, Germany), and cDNA obtained by MuLV Reverse Transcriptase (Applied Biosystems, CA, USA). 1 μg of cDNA was amplified with Light Cycler and Universal ProbeLibrary system (Roche Applied Science, Italy) [12].
Probes and primers were selected using a web-based assay software (ProbeFinder https://www.roche-applied-science.com) (Table 2). Housekeeping gene GAPDH was used as reference to normalize PCR data, expressed as a gene of interest/GAPDH ratio. A ‘variation index’ was calculated for each experiment and for each ‘gene of interest/GAPDH’ by relating the result of degenerate disc cells with that of healthy discs.
Results
Histology
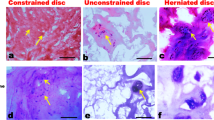
Morphology of non-degenerate discs was observed in young scoliotic patients: the cells were smaller than in adults, cell clusters were absent and cartilage was hypertrophic. Regularly arrayed collagen-rich lamellae with fibro-chondrocytes were seen in the AF (Fig. 1).
In the AF/NP transition zone, type-I collagen fibers crossed diagonally each other, and cells were included within GAG-rich niches (Fig. 2). In the NP area single cells or islets of few chondrocyte-like cells surrounded by a capsule were seen among sparse collagen fibers. The cartilage endplate showed the typical amorphous matrix with sparse single and grouped chondrocytes (Fig. 3).
Degenerate discs showed highly fibrotic lamellae and interlamellar cells with a flattened “pancake” morphology in the AF. Layers become more irregularly distributed with increased interbundle spaces (Fig. 4): the loss of distinct layers carries with it the inability for a sustained response to loads.
Chondrons, i.e. aggregates of three or more cells within a common territorial matrix whose frequency seems to be related to the grade of degeneration [13], were seen in AF and NP tissue of degenerate discs (Figs. 5, 6, 7, 8, 9).
IVD cell culture
Cell outgrew after 3–4 days from AF and NP seeding in culture flask (Fig. 10).
After a lag phase (7–14 days) before proliferation, the mean time to confluence in 75 cm2-flasks was 40.9 days for AF and 44.9 days for NP cells, independent from the donor age or sex.
Cells from DDD-patients reached the first confluence later than cells from young patients (NP: 42 ± 3 and 28 days, p = 0.05; AF: 35 ± 5 and 28 days, respectively). The two cell types were fibroblast-like, likely due to the 2D-culture (Fig. 10). Interestingly, cytochemical ALP was negative for AF cells, while ALP-positive cells were found in NP monolayers, confirming that some osteoprogenitors can be found in the NP tissue.
Beta-galactosidase is a biomarker of cell senescence, as senescent cells produce increased amounts of SA-β-Gal at pH 6.0. By L.M. no correlation was found between the frequency of positive cells and the severity of the score at diagnosis or the histological degeneration; the apparently increased number of senescent cells from pass 1 to pass 2/3 cells is due to the increased confluency (Fig. 11).
Cell viability in 3D pellet was measured by Alamar Blue assay: after 15–18 days cells stop proliferating, due to inability of oxygen and nutrients to reach the inner cells. With toluidine blue, cells in the 3D arrangement were shown to produce GAGs (Fig. 12).
Five NP and five AF from degenerate discs and 1 NP-1 AF from a young patient were tested for gene expression: a slight decrease of all transcripts was observed in AF cells of degenerate discs, but the changes were not significant (Fig. 13). Major changes were observed in NP: SOX-9 and ACAN were significantly decreased in degenerate disc cells (unpaired t test: p < 0.0001, and p = 0.005, respectively), while COL2A1 expression was slightly diminished. On the contrary, COL1A1 was more expressed, but the increase was not statistically significant.
Discussion
Low back pain, a predominant cause of disability in adults, is associated with degeneration of the intervertebral disc. Among genetic, biologic and mechanical factors involved in the progress of disc degeneration, the loss of the gel-like consistency of the nucleus pulposus matrix, due to a decreased proteoglycan content, is recognized as a main factor [14]. This phenomenon progresses towards fibrosis of NP, altered transmission of the intervertebral forces, damage to the annulus fibrosus and various degenerative processes.
The purpose of this study was to understand the phenotypic features of a degenerative IVD tissue versus IVD tissue of young subjects. The more we understand the cell biology of discal tissue and discover the mechanisms underlying degenerative processes, the greater is the potential to manipulate biological systems and intervene to prevent disease and pain in the future.
The normal IVD is hypocellular, which amplifies the effect of increased senescence and declining matrix production associated with disc degeneration [15].
During degeneration, and aging too, intervertebral disc cells undergo multiple changes, including altered cell phenotype and senescence.
Actually, in early degeneration, cell proliferation is upregulated in an attempt to combat progressive ECM loss. This was observed in our samples, where cell clusters and chondrons try to restore matrix synthesis and mechanical function. In our study, the proportion of clustered cells was similar in AF and NP of DDD samples, as well as in herniated tissues.
Being intervertebral disc avascular, it is distinctly different from other tissues. When there is an annular tear or injury, repair response activates from the vascularized AF outer layer to inner layer along the tear/injury, but if it occurs in the inner layer of AF or NP, the process of wound healing cannot be initiated [16]. This may account for NP fibrosis and loss of delineation between AF and NP observed in the latter stages of degeneration.
The ECM constituents are not static: though continually degraded by matrix metalloproteases, they are promptly refreshed by newly-synthesized components produced by cells: while cells constitute only 1% of the adult disc tissue by volume, their role in matrix synthesis and metabolic turnover is vital.
Intervertebral disc tissue and cells undergo continuous adaptive changes during aging, regardless of any concurrent injury, which may stimulate degeneration; senescent cells cannot divide, and the disc ability to generate new cells to replace those lost to necrosis or apoptosis is reduced. Moreover, if senescent cells accumulate over time, their metabolic products may contribute to pathologic changes seen in degenerating discs [17].
Other authors found a greater proportion of senescent cells in herniated than non-herniated discs, and more senescent cells in NP compared to AF [5]: this was not observed in our study, where a slight increase of β-gal positive cells was seen with cell passaging, indicating a certain grade of replicative senescence.
Annulus cells have shown to lose their phenotype during two-dimensional (2D) culturing. Chou et al. showed that up to passage two, both inner and outer annulus cells are not different from freshly isolated cells. At later passages, however, both cell types became indistinguishable fibroblast-like with similar type-I collagen expression and protein elaboration [18].
An altered gene expression in senescent cells was recently reported [19]; therefore, the expression of transcripts coding for IVD cell phenotypic markers was assessed in degenerate discs in comparison to healthy discs. Currently, there are no specific markers for NP cells, and they have often been considered as chondrocyte-like cells due to ECM similarity. NP matrix mainly contains aggrecan and type-II collagen, while AF cells prevalently produce type-I collagen; SOX-9 is one of the key-regulators of MSC differentiation to chondrocyte-like cells [20]. The increase in COL1A1 expression with a concomitant decrease in COL2A1 and ACAN is consistent with the IVD aging, and suggest that NP cells undergo dedifferentiation [21].
We are aware of the different behavior of IVD-derived cells when cultured in a 3D-arrangement versus 2D culture.
Protein synthesis of the key molecules produced by NP cells, including collagen II and aggrecan, were shown to be reduced during monolayer culture. But NP cells in 3D were found to have an increased matrix protein synthesis with only limited cell proliferation, and matrix protein synthesis was re-induced in monolayer-expanded cells by further culture as a pellet, i.e. expanded NP cells maintain the functional integrity [22].
Better understanding of the biology of IVD degeneration and discogenic low back pain will likely lead to the discovery of biomarkers that may help diagnose LBP more accurately, thereby leading to more biologic and mechanistic management strategies.
Undifferentiated precursors, including ALP-positive connective-tissue precursors and notochordal cells, have been found in young subjects. Unfortunately, such precursors are lost with aging, therefore a cell therapy relying on them to drive disc regeneration is unreliable in old subjects. ‘Biologics’ have been already described some years ago as the future in spinal surgery, and more recently cell-based therapies with mesenchymal stromal cells have been suggested. The efficacy of MSC in inducing IVD regeneration has been shown in several ex vivo systems, either by induction of the endogenous NP cells to regain a non-degenerate phenotype through interactions between MSC and degenerate NP cells or by direct injection [23, 24]. Unfortunately, repair mechanisms in animal studies may differ compared to patients with disc diseases, due to different pathophysiological changes within the IVD and different biomechanics.
Nonetheless, the feasibility of engineering a functional spinal motion segment using biological therapies for degenerative disc diseases is under extensive research, and clinical application is foreseen [25].
References
Miller JA, Schmatz M, Schultz AB (1988) Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine 13:173–178
Hadjipavlou AG, Tzermiadianos MN, Bogduk N, Zindrick MR (2008) The pathophysiology of disc degeneration: a critical review. J Bone Jt Surg Br 90:1261–1270
Anderson DG, Risbud MV, Shapiro IM, Vaccaro AR, Albert TJ (2005) Cell-based therapy for disc repair. Spine J 5:297S–303S
Nerurkar NL, Elliot DM, Mauck RL (2010) Mechanical design criteria for intervertebral disc tissue engineering. J Biomech 43(6):1017–1030
Roberts S, Evans H, Trived J, Menage J (2006) Histology and pathology of the human intervertebral disc. J Bone Joint Surg A 88(S2):10–14
Sive JI, Baird P, Jeziorsk M, Watkins A, Hoyland JA, Freemont AJ (2002) Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. J Clin Pathol: Mol Pathol 55:91–97
Richardson SM, Mobasheri A, Freemont AJ, Hoyland JA (2007) Intervertebral disc biology, degeneration and novel tissue engineering and regenerative medicine therapies. Histol Histopathol 22:1033–1041
Bertolo A, Thiede T, Aebli N, Baur M, Ferguson SJ, Stoyanov JV (2011) Human mesenchymal stem cell co-culture modulates the immunological properties of human intervertebral disc tissue fragments in vitro. Eur Spine J 20(4):592–603
Vadalà G, Sowa G, Hubert M, Gilbertson LG, Denaro V, Kang JD (2011) Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med 13. doi:10.1002/term.433
Ford JL, Jones P, Downes S (2002) Cellularity of human annulus tissue: an investigation into the cellularity of tissue of different pathologies. Histopathology 41:531–537
Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O (2009) Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4(12):1798–1806
Mouritzen P, Noerholm M, Nielsen PS, Jacobsen N, Lomholt C, Pfundheller HM, Tolstrup N (2005) Probe library: a new method for faster design and execution of quantitative real-time PCR. Nat Methods 4:313–316
Johnson WE, Eisenstein SM, Roberts S (2001) Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res 42(3):197–207
Livshits G, Popham M, Malkin I, Sambrook PN, Macgregor AJ, Spector T, Williams FM (2011) Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis 70(10):1740–1745
Kepler CK, Anderson DG, Tannoury C, Ponnappan RK (2011) Intervertebral disk degeneration and emerging biologic treatments. J Am Acad Orthop Surg 19(9):543–553
Peng B, Hao J, Hou S, Wu W, Jiang D, Fu X, Yang Y (2006) Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976) 31(5):560–566
Stephan S, Johnson WE, Roberts S (2011) The influence of nutrient supply and cell density on the growth and survival of intervertebral disc cells in 3D culture. Eur Cell Mater 22:97–108
Chou AI, Bansal A, Miller GJ, Nicoll SB (2006) The effect of serial monolayer passaging on the collagen expression profile of outer and inner anulus fibrosus cells. Spine (Phila Pa 1976) 31(17):1875–1881
Gruber HE, Hoelscher GL, Ingram JA, Zinchenko N, Hanley EN Jr (2010) Senescent vs. non-senescent cells in the human annulus in vivo: cell harvest with laser capture microdissection and gene expression studies with microarray analysis. BMC Biotechnol 10:1–10
Yang Z, Huang CY, Candiotti KA, Zeng X, Yuan T, Li J, Yu H, Abdi S (2011) Sox-9 facilitates differentiation of adipose tissue-derived stem cells into a chondrocyte-like phenotype in vitro. J Orthop Res 29:1291–1297
Zhao CQ, Wang LM, Jiang LS, Dai LY (2007) The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev 6:247–261
Preradovic A, Kleinpeter G, Feichtinger H, Balaun E, Krugluger W (2005) Quantitation of collagen I, collagen II and aggrecan mRNA and expression of the corresponding proteins in human nucleus pulposus cells in monolayer cultures. Cell Tissue Res 321(3):459–464
Strassburg S, Richardson SM, Freemont AJ, Hoyland JA (2010) Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med 5(5):701–711
Le Maitre CL, Baird P, Freemont AJ, Hoyland JA (2009) An in vitro study investigating the survival and phenotype of mesenchymal stem cells following injection into nucleus pulposus tissue. Arthritis Res Ther 11(1):R20
Bron JL, Helder MN, Meisel HJ, Van Royen BJ, Smit TH (2009) Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur Spine J 18(3):301–313
Acknowledgment
Excellent technical assistance for histology was provided by Michela Greco.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ciapetti, G., Granchi, D., Devescovi, V. et al. Ex vivo observation of human intervertebral disc tissue and cells isolated from degenerated intervertebral discs. Eur Spine J 21 (Suppl 1), 10–19 (2012). https://doi.org/10.1007/s00586-012-2234-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-012-2234-y