Abstract
Objective
To evaluate whether a synthetic bone chip made of porous hydroxyapatite can effectively extend local decompressed bone graft in instrumented posterior lumbar interbody fusion (PLIF).
Methods
130 patients, 165 segments, who had undergone PLIF with cages and instrumentation for single or double level due to degenerative conditions, were investigated retrospectively by independent blinded observer. According to the material of graft, patients were divided into three groups. HA group (19 patients, 25 segments): with hydroxyapatite bone chip in addition to autologous local decompressed bone, IBG group (25 patients, 28 segments): with autologous iliac crest bone graft in addition to local decompressed bone and LB group (86 patients, 112 segments): with local decompressed bone only. Radiologic and clinical outcome were compared among groups and postoperative complications, transfusion, time and cost of operation and duration of hospitalization were also investigated.
Results
Radiologic fusion rate and clinical outcome were not different. Economic cost, transfusion and hospital stay were also similar. But operation time was significantly longer in IBG group than in other groups. There were no lasting complications associated with HA and LB group with contrast to five cases with persisting donor site pain in IBG group.
Conclusion
Porous hydroxyapatite bone chip is a useful bone graft extender in PLIF when used in conjunction with local decompressed bone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lumbar spinal fusion has been established as an effective treatment modality for proper patients with low back and leg pain suffering from degenerative lumbar spinal disorder. Numerous efforts have been undertaken to fulfill the aim of spinal fusion procedure, which is a solid arthrodesis of unstable segment finally relieving patients from pain and restoring their global spinal function. Among various techniques introduced so far, posterior lumbar interbody fusion (PLIF) with instrumentation is considered as one of most solid and biomechanically sound methods for fusion [1]. Original PLIF with the autograft of the tri-cortical iliac crest [2, 3], however, were associated with non-negligible problems including collapse, retropulsion of the graft, and consequent pseudoarthrosis. To overcome these problems related with mechanical properties of graft, cages were developed and used in combination with autologous cancellous bone from iliac crest [4, 5]. Though autologous iliac crest graft became a gold-standard material for spinal fusion with fusion rate up to 98.9% [6], the morbidities from the donor site including pain, hematoma, infection, fracture and neurovascular injury resulting from the procedure of harvest let patients and surgeons hesitate to accept it as a primary choice for graft material [7–10]. Recently, for short level fusion of lumbar spine, local decompressed bone without supplementary graft material was reported to be enough to get satisfactory result [11, 12]. However, while with insufficient local decompressed bone in quantity and quality, alternative fusion materials are necessary to avoid disadvantages of autologous iliac bone graft. Therefore, various materials have been investigated for many years ranging from allograft, natural and synthetic ceramics, organic and inorganic compounds.
With use of synthetic material, we can elude the potential risk of biologic hazard like transmission of infectious disease following use of allograft as well as morbidities accompanying the harvest of autologous iliac bone graft. Its osteoconductive function and qualification as a graft extender were shown in our previous clinical studies compared to those of the autogenous iliac bone graft in human posterolateral fusion of lumbar spine, which is consistent with results from other studies using synthetic hydroxyapatite in posterolateral fusion [13–16]. However, in interbody fusion, there are contradictory reports on the efficacy of synthetic hydroxyapatite as a graft extender [15–17]. This study was undertaken to evaluate the efficacy of synthetic porous hydroxyapatite bone chips as a graft extender when used with local decompressed bone in PLIF.
Materials and methods
We reviewed consecutive cases of a single or double level posterior lumbar interbody fusion with the diagnosis of degenerative spinal disease including spinal stenosis, spondylolisthesis and recurrent lumbar disc herniation, performed in the department of orthopedic surgery of our hospital during the period from January 2007 to February 2009 after approval of institutional review board (IRB) of our hospital for this study. 130 patients who were followed up at least 1 year postoperatively were included. They were divided into three groups and compared in clinical and radiologic outcome according to the graft material filled in the interbody disc space and cages: (1) a group using only local decompressed bone (LB group), (2) a group using autogenous iliac crest bone graft in addition to local decompressed bone (IBG group), and (3) a group using synthetic porous hydroxyapatite chips as a graft extender in addition to autogenous bone from decompressed bony tissue (HA group). 86 patients (112 fusion levels) were enrolled for LB group. In addition, 25 patients (28 segments) were included for IBG group and 19 patients (25 fusion levels) for HA group. All patients were informed of the possible necessity for additional graft and the information of merits and demerits of each material, and their preference for graft material were learned preoperatively with the surgery consent form. The operative procedure was identical except for the graft materials applied. All patients had standing anteroposterior and lateral radiograph taken at their 3, 6, 12 months’ follow-up visit, including flexion and extension views after 6 months’ visit. The assessment of interbody fusion was judged based on radiography at 1 year postoperatively with consideration of serial changes of radiologic findings reported by Brantigan and Steffee [18] (Table 1). Clinical outcome were assessed 1 year after surgery with modified Japanese Orthopaedic Association (JOA) score [19] (Table 2). In addition, complications, hospital stay, amount of transfused blood and operation time and cost were compared among each group.
Bongros®-HA (Bioalpha Inc., Seongnam, Korea)
Bongros-HA® is a kind of ceramics, or synthetic bone chips made of porous hydroxyapatite developed to be used as a graft extender with focus on osteoconductive function. It is composed of particles with size range from 3.0 to 6.0 mm, made of pure hydroxyapatite (Ca10(PO4)6(OH)2) in a trabecular structure resembling the three-dimensional interconnected pore structure of human cancellous bone with 300 μM of pore size and 80% of porosity. We evaluated its efficacy with combined usage of autologous iliac crest cancellous bone in posterolateral lumbar fusion with a previous study with prospective randomized design [20].
Surgical technique
Standard midline approach to expose proximal and distal facets and interlaminar space between them was followed by insertion of pedicle screws guided by scout intraoperative simple radiography. Decompression including facetectomy of inferior articular process of above level laminae was performed usually by Kerrison punch and osteotome rather than by high-speed burr to save local decompressed bone as much as possible. The local bone was cautiously prepared for grafting with removal of soft tissue and cartilage and then morselized. After thorough discectomy and preparation of interbody disc space to expose bare bone of upper and lower endplate with caution not to break it, the prepared local bone was packed in the disc space as compactly as possible following the general principle of orthopedic surgery expecting bone healing [21], however, allowing room for cages. When the harvested local bone was not enough to fill the interbody space compactly, or is less than 10 cc per level, as the volume of adult lumbar disc space is known to be 10–15 cc [26], 2–5 cc of synthetic bone chip of porous hydroxyapatite or autogenous iliac bone graft from posterior superior iliac spine (PSIS) was mixed with the local bone to fill the void of the interbody disc space in respect for the preference for additional graft material of each patient. But in case with severe osteoporosis, or with T-score of bone mineral density less than −3.0, or other systemic disease affecting the quality of bone, or with previous history of the harvest of iliac crest, synthetic chip bone of porous hydroxyapatite was preferred. Two cages filled with local bone graft were inserted and then rods were fastened to pedicle screws under compression force over cages. Finally, a negative-pressured drain was inserted and wound was closed layer by layer after irrigation and final confirmation of decompression of nerve roots.
Statistical analysis
Using SPSS® 17.0 K (IBM® Corporation, Somers, New York, USA) software, the statistical significance was evaluated by Pearson’s chi-square test, t test and paired t test, and ANOVA (analysis of variance) test for each appropriate variable of interest. In addition, p value less than 0.05 was judged as significant.
Results
The demographic data including age, sex, body-mass-index (BMI) did not show significant difference. And preoperative diagnosis, preoperative clinical score (modified JOA score) was similar among each group. Postoperative follow up period and number of fused segment was also similar (Table 3).
Radiologic results
Based on simple radiography obtained at 1 year postoperatively, according to Brantigan Steffee’s recommended criteria of interbody fusion, the fusion rate were 91.7, 92.9 and 94.6%, respectively in HA, IBG, LB group. In addition, there were no significant statistical differences among groups regarding the fusion rate (p = 0.935) (Table 4).
Clinical results and complications
Postoperative modified JOA score, representing subjective symptoms of patients, was significantly increased then preoperative score in every group (p = 0.000 in every group), however, it was not different among groups: mean of 4.8, 4.8 and 5.0 for HA, IBG and LB group respectively (p = 0.645). Hospital days and the amount of transfusion were also similar among groups (p = 0.614 and 0.718, respectively). In addition, the operative time was significantly longer in IBG group (mean of 234.7 min, significantly longer than HA and LB group, p = 0.012) and average of total cost during hospitalization for surgery was not different among groups (p = 0.447). Although complications were too rare in occurrence to find statistically significant differences among groups, donor site pain was present in five patients of IBG group, which is non-negligible number (20.8%) (Table 5).
Discussion
For successful spinal fusion adequate amount of the graft material is required, which contains (1) osteogenic cells capable to synthesize new bone, (2) osteoinductive factors promoting the osteoblastic differentiation of stem cells and (3) osteoconductive scaffolds facilitating revascularization and ingrowth of bone [8, 22]. From the available options of graft choice, only autogenous bone graft, possesses all these three properties. However, it is too variable in quality and quantity according to each individual patient, furthermore it can accompany the morbidity of the donor site including pain, hematoma, infection, fracture and neurovascular injury resulting from procedure of harvest [7–10]. Recently some authors reported that decompressed local bone from facets, laminae and spinous processes, etc. without any other supplement was not inferior in fusion rate to autologous iliac crest bone graft when used for short level interbody or posterolateral fusion [11, 12]. These results imply that local bone obtained as a by-product of decompression can be enough in quantity and quality to achieve fusion for single or double level lumbar fusion in many cases and pose a question on the usage of cost-effectiveness of additional graft extenders. However, these studies did not clarify the amount of graft material acquired by decompression, although it is one of major factor of successful fusion [15]. There is also a report emphasizing the volume of graft based on the finding that clinical result can be unsatisfactory despite solid radiologic fusion ascribing it to insufficient area of bone bridge for effective load transmission between fused segment [23]. With this point of view, the quality of fusion in PLIF is different according to the quantity of bony bridge between endplates, or fused area ratio, as it is important in the aspect of load transfer and it could be one of factors explaining the well-known mismatch of fusion rate and clinical outcome in PLIF. In addition, this is why we used additional bone graft in the interbody space as well as in the cage to fill the 10–15 cc volume of disc space after thorough discectomy as with local bone alone, the quantity is often not sufficient. Furthermore, the quantity and quality of bone in both donor site and recipient site are various in each individual patient according to his or her age, level of activity, nutritional status and physical constitution. Thus, spinal surgeons still have much chance to be confronted with inappropriate volume or quality of autograft during operation of short spinal fusion as well as during longer fusion.
A synthetic bone made of porous hydroxyapatite was used as supplementary graft material in this study. As other synthetic ceramics, it is non-toxic, non-immunogenic material of uniform quality and unlimited quantity with convenience of sterilization and storage. In addition, it demonstrated its efficacy and safety as a scaffold for osteoconduction in various settings of studies. Inspection of this material with scanning electron microscope (SEM) demonstrated adequate porosity of 80% appropriate pore size of 300 µm and good three-dimensionally interconnected pore structure suitable for osteoconduction [20]. In addition, satisfactory results as a graft extender were reported in animal study and clinical study with application in posterolateral spinal fusion in the mixture with local decompressed bone. However, it has a limitation as a graft material in that it acts purely as an osteoconductive scaffold and contains neither osteogenic cell nor osteoinductive factor. Hence in another published studies using a spongy-form synthetic hydroxyapatite bone it was recommended to be used in combination with adjuvant osteoinductive materials like bone marrow aspirates or bone morphogenic proteins (BMPs) to make up for its limitation [14–16, 24]. Neen et al. found that a collagen-hydroxyapatite spongy with bone marrow aspirate showed equivalent fusion rate in posterolateral gutter but significantly lower fusion rate in interbody space than iliac bone graft, 83–93%. They postulated this discordance of fusion rate might derive from the insufficient volume of osteoinductive and osteoconductive materials in the cages used for interbody fusion. This was supported by another study by Carter et al. [16].
However, current study demonstrated successful spinal interbody fusion with porous hydroxyapatite and local decompressed bone without exogenous osteoinductive enhancers. It is contradictory to results of Neen et al. and Carter et al. using spongy-from collagen-coated hydroxyapatite with bone marrow aspirates. Considering its relatively higher porosity, up to 99%, authors presume that the spongy-form of collagen coated hydroxyapatite used in their study is supposed to be too loose to grab the bone marrow aspirates and osteogenic cells though it can be a good scaffold for bone ingrowth. In addition, extremely high porosity of the spongy form graft is not appropriate for void filler of the gap expected to fuse in that tightly packed, squeezed spongy-form graft will lose soaked adjuvant osteoinductive material like bone marrow aspirates and BMPs. All these combined factors might have negatively influence the studies of Neen et al. and Carter et al. in terms of interbody fusion. However, porous hydroxyapatite is solid and can be compactly packed in the cages retaining its macro and micro structural excellence as scaffolds for osteoconduction. As known in other form of orthopedic surgery, compact void filling of bone defect is essential for effective bone healing [21]. And in addition, the porous hydroxyapatite is known to have affinity to some proteins, which can act in a positive way to osteogenesis providing possibility of adsorption of BMPs [25]. Successful fusions using local decompressed bone alone in interbody space suggested that osteoinductive factors and osteogenic cells from corticocancellous bone acquired by decompression would be enough to utilize the scaffold provided by hydroxyapatite bone chips in interbody space. Thus, authors presume that in current study the adjuvant osteoinductive enhancers were not necessary for successful interbody spinal fusion using synthetic hydroxyapatite bone chips as an extender of local bone graft.
Although this study showed promising result regarding the efficacy of synthetic bone chips of porous hydroxyapatite as an extender of local decompressed bone in PLIF with the successful fusion rate and clinical scores equivalent to those of autologous iliac crest bone graft. It needs further validation to apply this result more generally with additional studies regarding fusion rate in relation to the volume and ratio of local decompressed bone and synthetic bone chips in various clinical settings stratified by factors influencing metabolism of bone. In vitro study of quantitative research on osteoinduction, osteoconduction, and osteogenic cells promoted by local decompressed bone in association with synthetic scaffolds or other osteogenic enhancers will be also helpful to find optimum environment of bony fusion and to determine when the graft extender is necessary in addition to local decompressed bone.
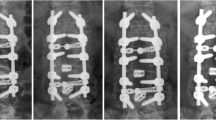
As our study evaluated the radiologic outcome with simple radiography only without computed tomography, bias from the different radio-opacity of corticocancellous bone chips and synthetic hydroxyapatite and from their different rate of resorption cannot be excluded. However, to minimize theses bias radiologic outcome was measured in 1 year postoperatively and we determined the fusion when newly formed bone bridge became more apparent in at least two or more films of serial radiography since usually after 3–6 months postoperatively the initial radio-opacity by cortical bone in graft disappeared as the graft material resorbed and then a new trabecular pattern of radio-opacity is observed with replacement of resorbed graft by newer bone bridge in up to 1 or 2 years postoperatively (Fig. 1). Nevertheless prospective study using computed tomography with quantitative measurement of fused area will add more evidences for the efficacy of a synthetic hydroxyapatite as extender of local bone graft.
Lateral simple radiography of the 57 years of female patient with degenerative spondylolisthesis and spinal stenosis at L3-4-5 level, who undergone double level PLIF with porous hydroxyapatite bone chip in addition to local decompressed bone. a Immediate post-operative image showing clotty radio-opaque shadow of hydroxyapatite bone chips in interbody space. b 3 months after operation, irregular pattern of mixed radio-opaque and -lucent shadow is seen presumably due to uneven resorption of graft materials. c 12 months postoperatively, more organized shadow is observed representing trabecular bridging across interbody space
Conclusion
Local bone acquired during decompression procedure is as good source of graft material for lumbar interbody fusion as autogenous iliac crest bone graft. However, when the quality and quantity of local bone acquired is not enough a synthetic bone chips made of porous hydroxyapatite can extend the local bone graft resulting successful spinal interbody fusion.
References
Turner JA, Ersek M, Herron L, Haselkorn J, Kent D, Ciol MA, Deyo R (1992) Patient outcomes after lumbar spinal fusions. JAMA 268:907–911
Jaslow IA (1946) Intercorporal bone graft in spinal fusion after disc removal. Surg Gynecol Obstet 82:215–218
Cloward RB (1953) The treatment of ruptured lumbar intervertebral discs by vertebral body fusion. I. Indications, operative technique, after care. J Neurosurg 10:154–168. doi:10.3171/jns.1953.10.2.0154
Brantigan JW, Steffee AD (1993) A carbon-fiber implant to aid interbody lumbar fusion—2-year clinical-results in the 1st 26 patients. Spine 18:2106–2117
Brantigan JW, Steffee AD, Geiger JM (1991) A carbon-fiber implant to aid interbody lumbar fusion—mechanical testing. Spine 16:S277–S282
Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM (2000) Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine (Phila Pa 1976) 25:1437–1446
Boden SD (2002) Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine (Phila Pa 1976) 27:S26–S31
Whang PG, Wang JC (2003) Bone graft substitutes for spinal fusion. Spine J 3:155–165 (S1529943002005399)
Kessler P, Thorwarth M, Bloch-Birkholz A, Nkenke E, Neukam FW (2005) Harvesting of bone from the iliac crest—comparison of the anterior and posterior sites. Br J Oral Maxillofac Surg 43:51–56. doi:10.1016/j.bjoms.2004.08.026
Kim DH, Rhim R, Li L, Martha J, Swaim BH, Banco RJ, Jenis LG, Tromanhauser SG (2009) Prospective study of iliac crest bone graft harvest site pain and morbidity. Spine J 9:886–892. doi:10.1016/j.spinee.2009.05.006
Ito Z, Matsuyama Y, Sakai Y, Imagama S, Wakao N, Ando K, Hirano K, Tauchi R, Muramoto A, Matsui H, Matsumoto T, Kanemura T, Yoshida G, Ishikawa Y, Ishiguro N (2010) Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion. Spine (Phila Pa 1976) 35:E1101–E1105. doi:10.1097/BRS.0b013e3181de4f2e
Ohtori S, Suzuki M, Koshi T, Takaso M, Yamashita M, Yamauchi K, Inoue G, Orita S, Eguchi Y, Ochiai N, Kishida S, Kuniyoshi K, Nakamura J, Aoki Y, Ishikawa T, Arai G, Miyagi M, Kamoda H, Toyone T, Takahashi K (2010) Single-level instrumented posterolateral fusion of the lumbar spine with a local bone graft versus an iliac crest bone graft: a prospective, randomized study with a 2-year follow-up. Eur Spine J. doi:10.1007/s00586-010-1656-7
Thalgott JS, Giuffre JM, Fritts K, Timlin M, Klezl Z (2001) Instrumented posterolateral lumbar fusion using coralline hydroxyapatite with or without demineralized bone matrix, as an adjunct to autologous bone. Spine J 1:131–137 (S1529-9430(01)00011-0)
Kraiwattanapong C, Boden SD, Louis-Ugbo J, Attallah E, Barnes B, Hutton WC (2005) Comparison of Healos/bone marrow to INFUSE(rhBMP-2/ACS) with a collagen-ceramic sponge bulking agent as graft substitutes for lumbar spine fusion. Spine (Phila Pa 1976) 30:1001–1007; discussion 1007 (00007632-200505010-00003)
Neen D, Noyes D, Shaw M, Gwilym S, Fairlie N, Birch N (2006) Healos and bone marrow aspirate used for lumbar spine fusion: a case controlled study comparing healos with autograft. Spine (Phila Pa 1976) 31:E636–E640. doi:10.1097/01.brs.0000232028.97590.12
Carter JD, Swearingen AB, Chaput CD, Rahm MD (2009) Clinical and radiographic assessment of transforaminal lumbar interbody fusion using HEALOS collagen-hydroxyapatite sponge with autologous bone marrow aspirate. Spine J 9:434–438. doi:10.1016/j.spinee.2008.11.004
Hashimoto T, Shigenobu K, Kanayama M, Harada M, Oha F, Ohkoshi Y, Tada H, Yamamoto K, Yamane S (2002) Clinical results of single-level posterior lumbar interbody fusion using the Brantigan I/F carbon cage filled with a mixture of local morselized bone and bioactive ceramic granules. Spine (Phila Pa 1976) 27:258–262
Brantigan JW, Steffee AD (1993) A carbon fiber implant to aid interbody lumbar fusion. Two-year clinical results in the first 26 patients. Spine (Phila Pa 1976) 18:2106–2107
Association JO (1986) Assessment of treatment of low back pain. J Jpn Orthop Assoc 60:391–394
Lee JH, Hwang CJ, Song BW, Koo KH, Chang BS, Lee CK (2009) A prospective consecutive study of instrumented posterolateral lumbar fusion using synthetic hydroxyapatite (Bongros-HA) as a bone graft extender. J Biomed Mater Res A 90:804–810. doi:10.1002/jbm.a.32113
van Loon CJ, de Waal Malefijt MC, Buma P, Stolk T, Verdonschot N, Tromp AM, Huiskes R, Barneveld A (2000) Autologous morsellised bone grafting restores uncontained femoral bone defects in knee arthroplasty. An in vivo study in horses. J Bone Joint Surg Br 82:436–444
Lee C, Dorcil J, Radomisli TE (2004) Nonunion of the spine: a review. Clin Orthop Relat Res 2004:71–75 (00003086-200402000-00012)
Lee JH, Jeon DW, Lee SJ, Chang BS, Lee CK (2010) Fusion rates and subsidence of morselized local bone grafted in titanium cages in posterior lumbar interbody fusion using quantitative three-dimensional computed tomography scans. Spine (Phila Pa 1976) 35:1460–1465. doi:10.1097/BRS.0b013e3181c4baf5
Ylinen P, Kinnunen J, Laasonen EM, Lamminen A, Vainionpaa S, Raekallio M, Rokkanen P, Tormala P (1991) Lumbar spine interbody fusion with reinforced hydroxyapatite implants. Arch Orthop Trauma Surg 110:250–256
Ripamonti U, Ma SS, van den Heever B, Reddi AH (1992) Osteogenin, a bone morphogenetic protein, adsorbed on porous hydroxyapatite substrata, induces rapid bone differentiation in calvarial defects of adult primates. Plast Reconstr Surg 90:382–393
Bilgic S, Sahin B, Sonmez OF, Odaci E, Colakoglu S, Kaplan S, Ergur H (2005) A new approach for the estimation of intervertebral disc volume using the Cavalieri principle and computed tomography images. Clin Neurol Neurosurg 107:282–288. doi:10.1016/j.clineuro.2004.08.001
Acknowledgments
This work was supported by Research Settlement Fund for the new faculty of Seoul National University.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, H., Lee, CK., Yeom, JS. et al. The efficacy of porous hydroxyapatite bone chip as an extender of local bone graft in posterior lumbar interbody fusion. Eur Spine J 21, 1324–1330 (2012). https://doi.org/10.1007/s00586-011-2092-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-011-2092-z