Abstract
Purpose
We prospectively investigated whether high intramedullary SI and contrast [gadolinium-diethylene-triamine-pentaacetic acid (Gd-DTPA)] enhancement in magnetic resonance imaging (MRI) are associated with postoperative prognosis in cervical compressive myelopathy (CCM) patients.
Methods
Seventy-four patients with ventral cord compression at one or two levels underwent anterior cervical discectomy and fusion (ACDF) for CCM between March 2006 and June 2009. The mean follow-up period was 39.7 months (range, 12.7–55.7 months). The cervical cord compression ratio and clinical outcomes were measured using Japanese Orthopedic Association (JOA) scores for cervical myelopathy. Patients were classified into three groups based on the SI change in T2WI, T1-weighted images (T1WI), and contrast (Gd-DTPA) enhancement.
Results
The mean preoperative and postoperative JOA scores were 10.5 ± 2.9 and 15.0 ± 2.1 (P < 0.05), respectively. The mean recovery ratio of the JOA score was 70.9 ± 20.2%. There were statistically significant differences in postoperative JOA and recovery ratio among three groups. However, post-surgical neurological outcomes were not associated with age, symptom duration, preoperative JOA, and cord compression.
Conclusions
We found that intramedullary SI change is a poor prognostic factor and the intramedullary contrast (Gd-DTPA) enhancement on preoperative MRI should be viewed as the worst predictor of surgical outcomes in cervical myelopathy. Contrast (Gd-DTPA) enhancement and postoperative MRI are useful for identifying the prognosis of patients with poor neurological recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cervical myelopathy is a diagnosis with a basis in radicular and myelopathic syndromes of degeneration. Magnetic resonance imaging (MRI) has been used, not only to depict how the spinal cord is compressed anatomically, but also to reflect the pathological changes within the spinal cord through changes in signal intensity (SI) in patients with cervical myelopathy [8, 13]. Additionally, contrast [gadolinium-diethylene-triamine-pentaacetic acid (Gd-DTPA)] enhanced MRI provides information about the integrity of the spinal cord. However, the application of any contrast medium has not been commonly utilized in the imaging of patients with cervical degenerative diseases.
Several studies have reported that patients with increased SI in T2WI tend to have poorer prognoses after surgery [8, 9, 13, 17]; however, other studies have reported no such association [22]. Several studies have reported contrast (Gd-DTPA) enhancement in MR images in patients with cervical myelopathy [3, 11]. While previous studies have examined both high SI changes in T2WI and contrast enhancement in T1WI, there have been few studies comparing the prognostic significance of these imaging parameters.
This study prospectively evaluates the relevance of signal intensity changes to the neurological outcomes of patients with cervical compressive myelopathy (CCM) caused by intervertebral disc herniation or osteophyte formation at one or two levels. Additionally, this study investigates the usefulness of contrast-enhanced MRI in patients with cervical myelopathy.
Patients and methods
One hundred and forty-three consecutive patients with cervical myelopathy were treated at our institution from March 2006 to June 2009. Of the 143 patients treated, 74 (51.7%) consecutive patients with ventral cord compression at one or two levels of the spine caused by intervertebral disc herniation or bony spur were enrolled in this study. The concomitant diagnoses causing CCM were cervical spondylotic myelopathy in 48 patients and cervical disc herniation in 26 patients. Patients with cervical myelopathy because of cervical ossification of the posterior longitudinal ligament were excluded. The 74 patients (48 men, 26 women; mean age, 51.3 years; from 26 to 69 years) underwent anterior cervical discectomy and fusion (ACDF). Of these patients, 45 underwent one level ACDF as these patients had a diagnosis of one level CCM at C3-4 (8 patients), C4-5 (9), C5-6 (21), or C6-7 (7). One iliac bone and Atlantis plate (Medtronic Sofamor-Danek, Memphis, TN) were used in 29 cases, while a Cervios cage (Synthes, Oberdorf, Germany) and Atlantis plate were used in 16 cases. Twenty-nine patients underwent a two level ACDF, as these patients were diagnosed with a two level CCM at C3-4-5 (7), C4-5-6 (8), or C5-6-7 (14). For this ACDF procedure, two autologous iliac bones and an Atlantis plate were used in each of the cases. A diagnosis of cervical myelopathy was assigned with radiological confirmation MRI, and the diagnosis was required to be in one or more “upper motor neuron” domains (e.g., spasticity, hyperreflexia, positive Babinski sign) based on a neurological examination.
Patients with central cord syndrome, other acute traumatic cord injuries, other neurological disorders (e.g., cerebral palsy, multiple sclerosis), and instability of the cervical spine as diagnosed with radiographs were excluded from this study. Patients whose symptoms of myelopathy were precipitated following traumatic injury with a pre-existing CCM diagnosis were also excluded.
Radiological assessment
All patients underwent high resolution MRI using a 1.5 T Signa (Siemens Medical System) unit with a surface coil. T1- and T2-weighted sagittal views of the cervical spine were obtained using a spin echo sequence for the T1-weighted images, and a first spin echo sequence was used for the T2-weighted images. The slice thickness was 3 mm in the sagittal and axial planes, and the acquisition matrix was 448 × 269. The sequence parameters were repetition time (TR) 450 ms/echo time (TE) 9.5 ms for T1-weighted and TR 3,790 ms/TE 114 ms for T2-weighted MRI. After acquisition of the precontrast MRI, an intravenous injection of Gd-DTPA (Magnevist, Schering; 0.1 mmol/kg body weight) was administered. The postcontrast MR images were performed using the same parameters and protocol as the noncontrast MRI. Additional parameters included TR 690 ms/TE 11 ms for the Gd-DTPA enhancement in the T1-weighted images. Increased signal intensity was defined as a high-intensity area in contrast with the adjacent isointensity portion of the spinal cord in the sagittal and axial plane [25]. We defined Gd-DTPA enhancement as a demarcation relatively clear from the surrounding cord parenchyma where it was found [18].
In the case of preoperative cord signal changes, follow-up MRIs were completed at 3 or 6, and again at 12 months after surgery. If the intramedullary contrast (Gd-DTPA) enhancement appeared preoperatively, follow-up assessments were performed with contrast-enhanced MRI. The interval change of intramedullary high SI and contrast enhancement on follow-up MRI was compared with that on preoperative images.
MR imaging factors
Three patterns of spinal cord signal intensity on T1-weighted images, T2-weighted images, and Gd-DTPA enhanced MR images were observed. Specifically, group A (N/N) displayed normal intensity on both T1- and T2-weighted images; group B (N/Hi) displayed no intramedullary signal intensity abnormalities on the T1-weighted images and high SI on the T2-weighted images; and group C (N/Hi/Gd) displayed no intramedullary signal intensity abnormalities on the T1-weighted images, high SI on the T2-weighted images, and an enhanced contrast (Gd-DTPA) image (Figs. 1, 2, 3).
Magnetic resonance imaging of a 34-year-old man with cervical myelopathy (11 points on JOA score). The sagittal T1-weighted image displays no intramedullary SI change. And T2-weighted image reveals increased SI at C4/5, where the cord compression can be seen. The signal intensity is grade 2. The patient was arranged into group B (N/Hi)
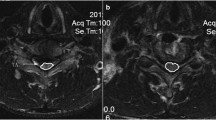
Magnetic resonance imaging of a 50-year-old man with cervical compressive myelopathy (10 points on JOA score). The sagittal T1-weighted image shows no signal abnormality on the spinal cord. The sagittal T2-weighted image shows a high signal change at C4/5, where the signal intensity grade 1 is seen. Intramedullary Gd-DTPA enhancement reveals a demarcation from the surrounding cord parenchyma. The patient was classified into group C (N/Hi/Gd)
By comparing the preoperative and postoperative signal change on T2-weighted images, patients with high SI before surgery were classified into two groups after the surgery: reversible group (those whose SI had decreased) and stationary group (those whose SI was unchanged). We evaluated the degree of signal intensity according to grade 0, for no change in signal intensity on T2-weighted MR images; grade 1, for light intensity change; and grade 2 for a bright signal, clearly distinguishable from that of grade 1 [15]. Spinal cord signal intensity changes were evaluated by two neuroradiologists (J.H.L. and W.H.C.) who were blinded to the patients’ clinical data. They independently interpreted the T2 and T1-weighted images to determine the presence of signal intensity changes and contrast enhancement in the spinal cord. If they disagreed on the radiological finding in any patient, the case was excluded from this study.
Compression ratio
The compression ratio was measured by dividing the smallest anteroposterior dimension of the spinal cord by the broadest transverse diameter at the same level on axial MRI scans (Fig. 4).
Functional outcome measures
Patient neurological outcomes were evaluated according to the Japanese Orthopedic Association (JOA) scale for cervical myelopathy (Table 1) [7].
Statistical analysis
For nonparametric analysis, the Mann–Whitney U test was used to analyze differences between the two groups, and the Kruskal–Wallis test was used to analyze differences among the three groups. All statistical analyses were conducted using MedCalc version 11.1 software (MedCalc, Mariakerke, Belgium). P values <0.05 were considered statistically significant.
Results
All 74 participants underwent anterior cervical discectomy and fusion. The mean follow-up time was 39.7 months (range, 12.7–55.7 months) after surgery. The mean preoperative and postoperative JOA scores were 10.5 ± 2.9 and 15.0 ± 2.1, respectively (P < 0.05). Neurological outcomes for all patients with CCM were improved through ACDF. The mean recovery rate was 70.9 ± 20.2%.
Fifty (67.6%) of the 74 participants experienced increased signal intensity in the spinal cord on the T2-weighted MRI, while 24 (32.4%) did not. Additionally, 16 (32%) of the 50 patients experiencing increased intramedullary signal changes displayed contrast (Gd-DTPA) enhancements on the MR images. All contrast-enhancement MR findings were observed in the intramedullary signal change on the T2WI MR images.
Age, preoperative JOA, symptom duration, and spinal cord compression
The mean ages of the participants were 50.0 ± 8.3 years in group A (N/N), 53.5 ± 9.9 years in group B (N/Hi), and 48.4 ± 8.9 years in group C (N/Hi/Gd). The preoperative JOA scores were 11.6 ± 2.2 for group A, 10.1 ± 3.2 for group B, and 9.8 ± 3.0 for group C. The duration of preoperative symptoms ranged from 3 to 60 weeks (mean, 9.8 ± 10.6 weeks). The duration of symptoms was 6.2 ± 7.9 weeks in group A, 10.4 ± 11.5 weeks in group B, and 13.9 ± 10.9 weeks in group C. The mean cord compression ratios for groups A, B, and C were 34.5 ± 7.8, 31.0 ± 7.6, and 35.0 ± 10.4%, respectively. Statistical analyses revealed no significant group differences in age, preoperative JOA scores, symptom duration, or cord compression (Table 2).
Postoperative JOA and neurological outcomes
The respective postoperative JOA scores and recovery ratios (%) were 16.2 ± 0.8/82.4 ± 16.7% for group A, 14.6 ± 2.5/69.1 ± 20.8% for group B, and 13.9 ± 1.7/57.5 ± 14.0% for group C. Significant differences in postoperative JOA scores and recovery ratios were found in the three groups (P < 0.05) (Table 2). That is, neurological outcomes improved significantly according to the level of preoperative intramedullary signal changes or contrast enhancements experienced by the group.
Follow-up magnetic resonance images
All patients who experienced intramedullary signal changes on their preoperative MR images were re-examined at 3 or 6, and 12 months after surgery. At the time of the postoperative MRI, 19 of the 50 participants with intramedullary SI exhibited a reversal of SI, while 31 patients did not. The mean recovery ratio of the reversal group was 75.9 ± 14.3%, which was better than that of the other 31 patients (59.0 ± 19.7%), and the difference between these groups was statistically significant (P < 0.05) (Table 3). Additionally, in 17 (50%) of the 34 participants displaying preoperative intramedullary high signal intensity the signal intensity decreased substantially postoperatively, while only 2 (12.5%) of the 16 patients experiencing intramedullary high SI and contrast enhancement preoperatively had the signal intensity decrease postoperatively.
At postoperative contrast enhanced MRI, 11 (68.8%) of the 16 participants displaying preoperative contrast (Gd-DTPA) enhancement experienced a substantial reduction in the intramedullary contrast enhancement. Intramedullary contrast enhanced outcomes were reduced generally within 1 year (average 8.4 months) following surgery. In five cases, the contrast-enhanced outcomes remained, but decreased slightly in the extent of the detected area and the degree of signal intensity 1 year following decompressive surgery. These changes could not be evaluated statistically due to the small number of patients undergoing follow-up contrast enhanced MRI.
Discussion
Many authors have recommended surgical decompression for the treatment of CCM [1, 7, 10]. Although the “best practice” approach to the treatment of CCM remains controversial, for patients with both kyphosis and ventral compression the use of a ventral surgical approach may be the most beneficial [6, 23]. Improvement rates following decompression surgery for the treatment of cervical myelopathy have been reported to range from 51 to 85% [6, 12]. In the current study, cervical myelopathic patients who experienced ventral cord compression at either one or two levels, who then underwent ACDF were followed prospectively. The overall recovery ratio based on the JOA scores of these participants was 70.9%, which is compatible with estimates of recovery published in other studies [6].
Many authors have investigated the association between SI changes and surgical outcomes following the surgical treatment of CCM [8, 9, 13, 15, 25]. Yukawa et al. [25] reported that MRI outcomes associated with intramedullary high SI are indicators of poor prognosis. According to several other studies, the signal changes on preoperative MR images have been observed variously range from 28.7 to 83% [16, 24]. In this study, we found that preoperative intramedullary signal changes were present in 67.6% of patients enrolled in this study. Clinical outcomes were significantly worse for patients whose MR images demonstrated intramedullary high signal intensity changes.
The purpose of this study was to clarify the usefulness of contrast (Gd-DTPA) enhancement on preoperative MRI and the relationship between the changes in signal intensities and the neurological outcomes. Gd-DTPA is a biologically inert substance, and it diffuses passively through the blood–cord barrier (BCB) into the extracellular space [21]. Gd-DTPA enhancement can indicate a disruption to the parenchyma of the spinal cord and a disturbance in the BCB of the spinal cord [2]. Generally, intramedullary Gd-DTPA enhanced MRI reveal intramedullary lesions, including intramedullary tumors, multiple sclerosis, sarcoidosis, myelitis, and spinal cord infarction [2, 14, 19]. Recently, intramedullary Gd-DTPA enhanced MRI findings were known to appear in spinal cord injuries [14, 19]. Shimada et al. [14] observed a contrast enhancement of the injured cords of patients who had experienced a spinal cord injury. However, contrast enhancement of the spinal cord occurs even more rarely in patients with chronic degenerative processes of the spinal cord [3, 4, 18]. To our knowledge, Takahashi et al. [17] were first to describe MR outcomes of intramedullary Gd-DTPA enhancement in patients with cervical myelopathy. There are few reports of the prognostic value of enhancement MRI with respect to clinical outcomes in patients with cervical myelopathy [11]. Ozawa et al. [11] discussed mainly the prevalence and morphologic findings of intramedullary enhancement in cervical myelopathy and reported intramedullary contrast enhancement was indicative of worse prognosis in cervical myelopathy rather than the severity of preoperative symptoms. However, they evaluated the cervical myelopathic patients treated at various affected levels and by treatment modalities with either anterior fusion or posterior laminoplasty using Kurokawa’s method.
Many authors demonstrated that the surgical outcomes of cervical myelopathy have been reported variously depending on affected level number, treatment modality, and surgical options including anterior cervical fusion, posterior fusion, or cervical laminoplasty [5, 20]. There has been no study to compare the clinical outcome of none SI change and intramedullary increased SI change to that of contrast (Gd-DTPA) enhancement. In present study, we evaluated the neurological outcomes in patients with cervical myelopathy and ventral cord compression at one or two levels, who underwent ACDF. Patients with contrast (Gd-DTPA) enhancement on T1-weighted MRI had lower postoperative JOA scores and JOA recovery ratios in comparison with patients with intramedullary high signal changes on T2-weighted MRI or without enhanced signal intensity preoperatively (Fig. 5). Our findings suggest that the intramedullary contrast enhancement on preoperative MRI should be considered as the worst predictor of surgical outcomes in cervical myelopathy.
a Preoperative T2-weighted MRI of a 48-year-old male patient with spinal cord compression at C5–C6 and a JOA score of 14. b Intramedullary Gd-DTPA enhancement below the region of the marked compressed cord within intramedullary SI. c MRI of the patient from B, 6 months following the cervical anterior discectomy and fixation with successful decompression of the spinal cord. The intramedullary SI change remained. The patient’s JOA score was 16. The recovery rate was 66.7%. d The intramedullary Gd-DTPA enhancement regressed after decompressive surgery leaving a partial enhancement at C5–C6
The mechanism of contrast (Gd-DTPA) enhancement has been not fully explained in patients with cervical myelopathy. We speculate that a breakdown of the BCB may develop due to compression leading to spinal cord swelling and to leakage of Gd-DTPA. We expected that the preoperative symptom duration would be correlated with the existence of intramedullary SI and contrast enhancement. In this study, all contrast (Gd-DTPA) enhancement was observed below the region of the compressed cord; however, the preoperative mean cord compression ratio and symptom duration were not significantly associated with group status. Thus, we believe that contrast (Gd-DTPA) enhancement was derived from the compression of the spinal cord, but not associated with time course or cord compression severity. Cabraja et al. [4] reported that the contrast enhancement of cervical myelopathy was likely due to a mild reactive gliosis on histopathological findings.
Based on the results of this study, it appears that intramedullary high SI and contrast enhancement may disappear or decrease in intensity due to improvement in the pathological condition as a result of the decompression surgery. The regression of intramedullary signal changes in patients with hyperintensity T2WI can be regarded as a predictor of improved outcome following surgery. Several studies have reported that patients whose SI decreased following surgery had better neurological outcomes postoperatively than patients whose SI did not decrease [16, 24]. However, other studies have reported that postoperative alterations in SI were uncorrelated with postoperative outcomes [22]. To investigate the relationship between the neurological outcomes and the changes in signal intensities at follow-up MRI, patients having high SI before surgery were classified into two groups depending on the post-surgical regression of high SI. After the decompression surgery, intramedullary high SI was lessened in 19 patients (38%) and remained unchanged in 31 (62%). The reversal group displayed more improvements in JOA recovery ratios than the non-reversal group. Additionally, the reversal rate of the signal change of patients with preoperative intramedullary high signal intensity showed greater changes than those with contrast enhancement. Thus, it can be concluded that intramedullary high signal intensity reflects greater recuperative potential and contrast enhancement indicates less recuperative potential in patients with CCM.
Conclusion
Preoperative intramedullary high SI change and contrast (Gd-DTPA) enhancement on MR images were significantly associated with neurological status after surgery. Intramedullary SI change is a poor prognostic factor and the intramedullary contrast enhancement on preoperative MRI should be viewed as the worst predictor of surgical outcomes in cervical myelopathy. Contrast (Gd-DTPA) enhancement in CCM implies a severe form of cervical myelopathy. The regression of signal changes in patients with intramedullary high SI before surgery reflects improved neurological outcomes after surgery. We observed that the reversal rate of the signal change of patients with preoperative intramedullary high signal intensity showed greater changes than those with contrast enhancement. We can conclude that intramedullary high signal intensity indicates greater recuperative potential and contrast enhancement less recuperative potential in cervical myelopathy. We consider that contrast (Gd-DTPA) enhancement and postoperative MRI are useful for identifying the prognosis of patients with poor neurological recovery.
References
Bapat MR, Chaudhary K, Sharma A, Laheri V (2008) Surgical approach to cervical spondylotic myelopathy on the basis of radiological patterns of compression: prospective analysis of 129 cases. Eur Spine J 17:1651–1663
Bilgen M, Abbe R, Narayana PA (2001) Dynamic contrast-enhanced MRI of experimental spinal cord injury: in vivo serial studies. Magn Reson Med 45:614–622
Boet R, Chan YL, King A, Mok CT, Poon WS (2004) Contrast enhancement of the spinal cord in a patient with cervical spondylotic myelopathy. J Clin Neurosci 11:512–514
Cabraja M, Abbushi A, Costa-Blechschmidt C, van Landeghem FK, Hoffmann KT, Woiciechowsky C, Kroppenstedt S (2008) Atypical cervical spondylotic myelopathy mimicking intramedullary tumor. Spine (Phila Pa 1976) 33:E183–E187
Chatley A, Kumar R, Jain VK, Behari S, Sahu RN (2009) Effect of spinal cord signal intensity changes on clinical outcome after surgery for cervical spondylotic myelopathy. J Neurosurg Spine 11:562–567
Fessler RG, Steck JC, Giovanini MA (1998) Anterior cervical corpectomy for cervical spondylotic myelopathy. Neurosurgery 43:257–265 (discussion 265–267)
Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K (1981) Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976) 6:354–364
Matsuda Y, Miyazaki K, Tada K, Yasuda A, Nakayama T, Murakami H, Matsuo M (1991) Increased MR signal intensity due to cervical myelopathy. Analysis of 29 surgical cases. J Neurosurg 74:887–892
Mehalic TF, Pezzuti RT, Applebaum BI (1990) Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery 26:217–226 (discussion 226–227)
Nagashima H, Dokai T, Hashiguchi H, Ishii H, Kameyama Y, Katae Y, Morio Y, Morishita T, Murata M, Nanjo Y, Takahashi T, Tanida A, Tanishima S, Yamane K, Teshima R (2011) Clinical features and surgical outcomes of cervical spondylotic myelopathy in patients aged 80 years or older: a multi-center retrospective study. Eur Spine J 20(2):240–246
Ozawa H, Sato T, Hyodo H, Ishii Y, Morozumi N, Koizumi Y, Matsumoto F, Kasama F, Aizawa T, Itoi E, Kokubun S (2010) Clinical significance of intramedullary Gd-DTPA enhancement in cervical myelopathy. Spinal Cord 48:415–422
Papadopoulos CA, Katonis P, Papagelopoulos PJ, Karampekios S, Hadjipavlou AG (2004) Surgical decompression for cervical spondylotic myelopathy: correlation between operative outcomes and MRI of the spinal cord. Orthopedics 27:1087–1091
Ramanauskas WL, Wilner HI, Metes JJ, Lazo A, Kelly JK (1989) MR imaging of compressive myelomalacia. J Comput Assist Tomogr 13:399–404
Shimada K, Tokioka T (1999) Sequential MR studies of cervical cord injury: correlation with neurological damage and clinical outcome. Spinal Cord 37:410–415
Shin JJ, Jin BH, Kim KS, Cho YE, Cho WH (2010) Intramedullary high signal intensity and neurological status as prognostic factors in cervical spondylotic myelopathy. Acta Neurochir (Wien) 152:1687–1694
Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM (2003) Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J 3:33–45
Takahashi M, Sakamoto Y, Miyawaki M, Bussaka H (1987) Increased MR signal intensity secondary to chronic cervical cord compression. Neuroradiology 29:550–556
Takahashi M, Yamashita Y, Sakamoto Y, Kojima R (1989) Chronic cervical cord compression: clinical significance of increased signal intensity on MR images. Radiology 173:219–224
Terae S, Takahashi C, Abe S, Kikuchi Y, Miyasaka K (1997) Gd-DTPA-enhanced MR imaging of injured spinal cord. Clin Imaging 21:82–89
Wada E, Yonenobu K, Suzuki S, Kanazawa A, Ochi T (1999) Can intramedullary signal change on magnetic resonance imaging predict surgical outcome in cervical spondylotic myelopathy? Spine (Phila Pa 1976) 24:455–461 (discussion 462)
Weinmann HJ, Laniado M, Mutzel W (1984) Pharmacokinetics of GdDTPA/dimeglumine after intravenous injection into healthy volunteers. Physiol Chem Phys Med NMR 16:167–172
Yone K, Sakou T, Yanase M, Ijiri K (1992) Preoperative and postoperative magnetic resonance image evaluations of the spinal cord in cervical myelopathy. Spine (Phila Pa 1976) 17:S388–S392
Yonenobu K, Fuji T, Ono K, Okada K, Yamamoto T, Harada N (1985) Choice of surgical treatment for multisegmental cervical spondylotic myelopathy. Spine (Phila Pa 1976) 10:710–716
Yukawa Y, Kato F, Ito K, Horie Y, Hida T, Machino M, Ito ZY, Matsuyama Y (2008) Postoperative changes in spinal cord signal intensity in patients with cervical compression myelopathy: comparison between preoperative and postoperative magnetic resonance images. J Neurosurg Spine 8:524–528
Yukawa Y, Kato F, Yoshihara H, Yanase M, Ito K (2007) MR T2 image classification in cervical compression myelopathy: predictor of surgical outcomes. Spine (Phila Pa 1976) 32:1675–1678 (discussion 1679)
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cho, Y.E., Shin, J.J., Kim, K.S. et al. The relevance of intramedullary high signal intensity and gadolinium (Gd-DTPA) enhancement to the clinical outcome in cervical compressive myelopathy. Eur Spine J 20, 2267–2274 (2011). https://doi.org/10.1007/s00586-011-1878-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-011-1878-3