Abstract
Adolescent idiopathic scoliosis (AIS) is a complex spinal deformity whose assessment and treatment present many challenges. Computer applications have been developed to assist clinicians. A literature review on computer applications used in AIS evaluation and treatment has been undertaken. The algorithms used, their accuracy and clinical usability were analyzed. Computer applications have been used to create new classifications for AIS based on 2D and 3D features, assess scoliosis severity or risk of progression and assist bracing and surgical treatment. It was found that classification accuracy could be improved using computer algorithms that AIS patient follow-up and screening could be done using surface topography thereby limiting radiation and that bracing and surgical treatment could be optimized using simulations. Yet few computer applications are routinely used in clinics. With the development of 3D imaging and databases, huge amounts of clinical and geometrical data need to be taken into consideration when researching and managing AIS. Computer applications based on advanced algorithms will be able to handle tasks that could otherwise not be done which can possibly improve AIS patients’ management. Clinically oriented applications and evidence that they can improve current care will be required for their integration in the clinical setting.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adolescent idiopathic scoliosis (AIS) is a complex three-dimensional (3D) deformation of the spine. Screening, diagnosis and follow-up of AIS are challenging because the evolution of scoliotic spines does not follow determined patterns [1–4]. The patients require regular evaluation by physicians and imaging to detect any curve progression which has been defined as an increase in Cobb angle >10° between two clinical visits [5]. Yet Cobb angle reliability was shown to be limited. Its inter-observer and intra-observer variability has been estimated to vary up to 9° and 5°, respectively [6–8]. King et al. [9] and Lenke et al. [10] classifications for AIS are the two most widely used classifications but some studies have demonstrated only poor to fair intra- and inter-observer reliability [11, 12]. This lack of reliability in the assessment of AIS may lead to variability in its treatment. In fact, large intra- and inter-observer variability of instrumentation strategy in AIS was documented [13, 14]. With the wide availability of computers used in the clinical setting, researchers are developing applications to improve AIS assessment and treatment. Our working hypothesis is that algorithms and computer applications can improve AIS care by solving AIS enigmas such as variability in evaluation and treatment; unknown progression pattern and classification. The objectives of this review paper are to summarize the applications developed to improve AIS care, evaluate their clinical usability and suggest necessary developments to increase their clinical integration.
Materials and methods
Searching strategy
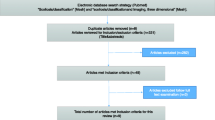
A literature search of articles published between January 2000 and July 2009 was performed in three major electronic databases Medline, Google Scholar, and Ovid using combinations of the following keywords: “adolescent idiopathic scoliosis” alternatively with “algorithms” or “computer” or “artificial intelligence”. All returned abstracts were evaluated.
Inclusion and exclusion criteria
Applications based on algorithms using AIS data and which can have a potential impact on clinical practice have been included in this review. Therefore, articles discussing imaging modalities and reconstruction techniques were not included in this review. All selected articles were thoroughly analyzed in their full content to evaluate the AIS problems solved by the algorithm, the clinical applicability of the applications developed and the elements lacking for their integration into the clinical setting.
Searching results
One hundred and eighty abstracts were retrieved using the selected keywords and screened for computer applications using algorithms aiming at improving AIS assessment and treatment. The main author (PP) analyzed the full content of 73 articles returned by the query in which the abstracts seemed to correspond to this review article interest. Based on the inclusion and exclusion criteria, 47 papers were retained for presentation in this article; 9 studies on applications evaluating scoliosis severity and progression, 12 studies on classification, 7 studies on bracing treatment, and 19 studies on surgical treatment were selected for presentation and discussion in this paper. Summary tables were generated to facilitate the understanding of the methodology and output.
AIS screening and follow-up
Applications have been developed to screen for AIS by automatically detecting its presence and severity on chest X-rays or surface topography. Other applications aim at improving AIS follow-up by limiting patient exposure to irradiation or detecting changes in scoliosis severity with surface topography. Other applications predict changes in AIS severity with artificial intelligence algorithms. All application algorithms and study methodologies are presented in Table 1.
AIS screening method
Tang et al. [15] proposed a computer system to detect scoliosis from chest X-ray by automatically computing the scoliosis classification index (SCI), a measurement of the deviation of each vertebral segment from the vertical spinal line, proposed by Greenspan et al. [16]. There was poor correlation between SCI and Cobb angle for scoliotic curves below 10° but strong correlation was found for curves above 10°. This was attributed to the difficult measurements of Cobb angle from end-vertebrae in small curves highlighting the limitations of the measurements currently used. As opposed to Cobb angle, SCI computation did not show any variability between two measurements. This application shows the potential of automated screening of scoliotic curves from regular chest X-rays but its use is limited because no cut-off values for SCI could be determined to distinguish clinically significant scoliosis with Cobb angles above 10° from those with lesser curves. Therefore, effective screening would require better correlation for lesser curves but it shows the potential to reduce scoliosis measurements variability using automated systems.
Methods to evaluate scoliosis severity using surface topography
Jaremko et al. [17–19] compared Cobb angles measured manually and Cobb angles calculated from 3D reconstruction of the spine using two X-rays with those estimated from 360° torso surface models acquired using four laser scanners. Asymmetry indices were extracted using genetic algorithms on cross-section of the topographic coordinates from the torso surface models. Together with other clinical indices (age, sex, BMI, and treatment status), those asymmetry indices were used as inputs into an artificial neural network (ANN) designed to predict the clinical Cobb angle (Fig. 1). An ANN is a computational model that simulates biological neural networks. It uses interconnected nodes (artificial neurons) that are interconnected by weighted links (analogous to synaptic connections). This network is usually adaptive and learns from training sets [20]. ANN estimation proved to be of comparable precision to computer and clinical measurements. This technique could be of much use in a scoliosis screening clinic, but the costly set-up required to obtain 360° images of the torso limits its use at the moment [21]. Therefore, methods assessing AIS severity with back shape surface topography rather than 360° torso surface models have been proposed to lower equipment cost.
ANN to estimate the Cobb angle (right) from indices to torso asymmetry (left). Each node in the hidden layer takes a weighted sum of inputs and produces an output if the sum is greater than a threshold. The ANN “memory” is distributed through the link weights, which are modified to minimize the difference between actual and estimated output by repeated presentation of input–output pairs in training set. Thus, the network “learns” through experience much as humans do. (From Jaremko et al. [17])
Ramirez et al. [21] combined information from back surface topography and clinical data using a support vector machine classifier to assess scoliotic spines severity accurately while limiting irradiation. Patients were classified into two classes defined as mild and non-mild for Cobb angles <30° and above 30°, respectively. Three types of classifiers SVM (support vector machine), DT (decision tree) and LDA (linear discriminant analysis) were then compared. SVMs are supervised learning methods used for classification or regression. Given a set of points in a multiple dimensional plane, the SVM creates one or several hyperplanes to separate data points from different classes [22]. Decision trees are algorithms represented with trees like graphs where nodes containing conditions split into branches leading to a decision. LDA is a classification method where a discriminant score based on a linear combination of features is computed for each class. A new case is then classified into the class for which it has the highest discriminant score. Of the three types of classifiers, SVM achieved the highest classification accuracy of 85%. Threshold of 30° rather than 10° as defined above as the clinical significant cut-off and the lack of accuracy where up to 15% of patients could be improperly screened are clear limitations of this application.
Methods to evaluate scoliosis progression using artificial intelligence methods
Based on the hypothesis that scoliosis follows progression patterns, Wu et al. [3] used a hybrid learning technique combination of fuzzy c-means clustering and ANN to predict Cobb angles and lateral deviation at follow-up. Fuzzy c-means clustering is a learning method that can be used to classify a data set without supervision. An optimal set of clusters (or classes) is obtained through fuzzy partitioning; which implies iteratively moving the cluster centers and minimizing intra-cluster variance in a given data set. Wu et al. applied those techniques to 72 radiological data sets acquired at successive follow-up clinics from 11 patients and were able to predict Cobb angle at follow-up with accuracy comparable to clinical measurements.
Ajemba et al. [2] have used sequential radiological measurements and included clinical parameters assessing developmental status such as Risser sign and chronological age to predict risk of progression using several models of SVM each of them based on different sets of clinical and radiological parameters. SVM ability to distinguish progressing from non-progressing AIS, was estimated to be between 65 and 80% which is better than former models based on statistical methods of regression.
AIS screening and follow-up rely heavily on the evaluation of the Cobb angle. The applications described above could lower radiation exposure by using surface topography and by optimizing follow-up frequencies. Their use in the clinical setting is limited by their accuracy and the complex setting required for their implementation.
AIS classification
Two major classifications from King et al. [9] and Lenke et al. [23] are used in AIS. Their limited reliability has been described [11, 24, 25], and applications using rule-based algorithms have been developed [26–28].
Stokes and Aronsson [26–28] have developed a rule-based automated algorithm to increase King’s classification reliability. In writing that algorithm, ambiguities and absence of precise definitions in the King et al. classification scheme [9] had to be resolved and permitted the identification and resolution of ambiguities in the definition of curve types. Phan et al. [29] have developed a decision tree to increase curve type classification accuracy using Lenke classification (Fig. 2). Similar findings to Stokes et al. work were found; classification accuracy was increased and the use of those tools has shown potential to increase classification reliability independently of user training. Classification accuracy was proportional to the time spent classifying and did not require more time using the decision tree with Lenke classification.
Decision tree developed to improve classification accuracy of AIS according to the Lenke scheme. MT main thoracic, TL thoraco-lumbar/lumbar, PT proximal thoracic indices, X B Cobb angle for segment X on bending PA X-ray, X H Cobb angle for segment X on sagittal X-ray. From Phan et al. [29]
A limitation of the King and Lenke classifications is their consideration of two-dimensional features extracted from postero-anterior (PA) and lateral (LAT) X-rays for a pathology that is truly three-dimensional. Therefore, several studies have generated classifications using databases with three-dimensional reconstructions of AIS patients.
Using Kohonen self-organizing map, a kind of ANN which can display nodes on a two-dimensional matrix, Mezghani et al. [30] were able to automatically classify 3D reconstructions of AIS spines into three classes based on the severity of the deformation. This self-ordering algorithm showed its ability to generalize three-dimensional features to describe the overall severity of the deformity.
Geometric torsion represents the rate of rotation of the plane formed by the tangent and the normal along the curved spine. Poncet et al. [31] extracted three distinct patterns of torsion, which can classify AIS based on compositions of those three basic torsion patterns. Integration of torsion into spinal classification could add valuable information about points of high geometric torsion correlating with spine stability and therefore influence surgical treatment.
Sangole et al. [32] have performed an unsupervised clustering using 3D reconstruction from patients with Lenke 1 curve type. They extracted three primary subgroups (two surgical and one non-surgical), and were able to determine variations within Lenke curve type 1 that were not evident on plain X-rays, showing that all curve type 1 were not always hypokyphotic and that the orientation of the plane of maximum curvature (a 3D index) was a discriminating factor. This study was limited to a very specific group of curve types; but it demonstrated the possible benefit that cluster analysis can highlight geometrical features, which could influence treatment.
Stokes et al. [33] have performed cluster analysis on 245 X-rays from 110 patients. Four clusters were extracted but of 56 patients followed longitudinally only 25 were consistently grouped at all clinic visits. Therefore, patterns were susceptible to change with repeated observations and cannot be reliably used alone to determine classifications determining treatment strategies.
Duong et al. [34] have developed a 3D classification using an unsupervised learning algorithm, fuzzy K-means clustering, applied to 409 3D AIS spine models. Two classifications with 5 and 12 classes with relevant clinical features and true 3D components were generated. Duong et al. [35] studied several 3D clinical parameters [plane of maximum curvature (PMC), best-fit plane (BFP) and geometric torsion] that could be integrated in the Lenke classification. Performing cluster analysis to evaluate the statistical distribution of those parameters, they showed specific 3D deformation patterns within Lenke 1 type curves using best-fit plane and geometric torsion patterns.
The accuracy of currently used classifications from King and Lenke can be improved with simple algorithms. More advanced algorithms have permitted the development of complex classifications based on large data sets and taking 3D parameters into consideration. While those classifications have focused on geometrical properties, their clinical use is limited because they do not integrate clinical features nor guide surgical decision-making which current classifications actually do. New classifications will need to integrate accepted clinical parameters and focus on guiding treatment to be used by physicians (Table 2).
Methods developed to assist bracing treatment
For patients with moderate AIS curves that are progressing or are between 20° and 40° Cobb angle with remaining growth, orthotic treatment has been advocated [36]. Computer-assisted design (CAD) and computer-aided manufacturing (CAM) have been tested in the fabrication of orthotics and revealed similar improved efficacy in curve correction when compared to traditional manual methods [37–39] while showing potential to save time in adjustments of the casts [40].
A biomechanical study by Perie et al. [41] has evaluated the effectiveness of the Boston brace using finite element model and experimental measurements. It highlighted the contribution of bracing pads to curve reduction but also suggested that other mechanisms participated in orthotic correction. Adaptation of such finite element models was personalized for patients’ specific curve patterns [42]. Bracing simulation with those personalized biomechanical models showed similar results to real in-brace geometry and revealed the potential to optimize bracing treatment of AIS through personalized evaluation and design improvement. A recently developed patient-specific brace simulator was developed based on refinements of the finite element models [43], and allowed to test and assess the efficiency of hundreds of different virtual braces for a given patient, thus optimizing the design of each brace [44]. Labelle et al. [38] undertook a randomized control trial comparing brace design using computer-assisted tool combining surface topography, surface pressure measurement with 3D reconstruction of the trunk (test group) with the conventional manner (control group). At initial visit, the test group had greater diminution of curve deformity in the coronal plane but it also had 3D correction as showed by correction of the plane of maximum deformity, which the control group did not manage to achieve.
A major limitation in orthotic treatment efficacy is patient compliance with timing and tightness when wearing the braces. Recent studies from Katz et al. [45] and Rahman et al. [46] have demonstrated a correlation between patient compliances to bracing treatment and outcome. Lou et al. [47] developed a battery-powered microcomputer system to monitor and guide patient in properly wearing their braces with the prescribed tightness using a feedback module. A limited clinical trial with five patients testing the device on 4 weeks demonstrated improvement in proper wearing of the brace.
These computer applications developed to improve bracing treatment have shown their potential to improve patient care, but cost, time consumption and the lack of clinically integrated systems have limited their use.
Methods developed to assist surgical treatment planning
Despite extensive literature on the surgical treatment of AIS, there is no clear consensus on the optimal treatment, which varies greatly from a patient to another [13, 14]. With the intention to optimize surgical treatment, several computer applications were developed to assist surgeons with their surgical planning.
Fusion levels determination using fuzzy logic
One of the major challenges in AIS treatment planning is to determine whether a curve needs to be fused. In order to assist surgeons in solving this enigma, Nault et al. [48, 49] have developed two fuzzy logic models, one for proximal thoracic curve fusion and another one for lumbar curve fusion. Fuzzy logic is a problem solving methodology based on approximate rather than precise reasoning, it is advantageous in complex systems from which precise mathematical equations cannot be applied which is often the case in medicine [50]. The models developed by Nault et al. output a score of certainty concerning needs for fusion based on rules extracted from the literature. When tested to guide levels of fusion, there was good agreement between those fuzzy logic models and clinicians. Yet the lack of clear justifications for a given output and total contradiction between the model and common agreement between five expert surgeons in spinal deformity for specific examples highlights the limitations of this system.
Surgery simulation
Biomechanical computer modeling offers the possibility to analyze multiple surgical strategies, to assist in decision-making and to compute reaction forces or stresses at different sites in the spine. Due to the complexity of the intervention and the patient characteristics, there are many unknown inputs for the biomechanical analyses. Therefore, appropriate simplifications need to be done.
Finite element analysis
Finite element analyses were commonly used to estimate stresses in internal fixation devices and to analyze the consequences of surgical variables such as the orientation of pedicle screws on the rigidity of the construct. The biomechanics of Harrington instrumentation was analyzed using a wireframe finite element model of the spine [51]. The biomechanics of CD instrumentation also was studied with the same model on an idealized geometry [52] and on 15 surgical cases [53] using patient-specific 3D geometry, built from preoperative stereo X-rays, and intra-operative maneuvers. The simulations of surgical maneuvers showed good agreement with measured effects of surgery in the frontal plane.
Flexible multi-body approach
Aubin et al. [54–58] have developed kinematic model including flexible elements to represent each motion segment, implant-vertebra connections, kinematic joints and sets to model surgical instrumentation of the spine. The spine model is personalized to a specific patient using calibrated radiographs [59]. Recent studies [56, 58] established the validity of this model by simulating the surgical procedures of 10 scoliotic patients who underwent a posterior and anterior instrumentation surgery. Simulation agreed well with documented postoperative results. In comparing simulations of various instrumentations for a same patient, low and high vertebra-implant reaction forces were highlighted. In some cases, those forces were exceeding the experimentally measured pullout values; information that could be valuable in surgical planning. Majdouline et al. [60] simulated 702 different surgical strategies on a computer simulator and demonstrated that strategies with various levels of instrumentation could lead to the same overall correction; such tools could be considered to optimize surgical strategies (Fig. 3).
Representation of a patient with double major AIS spine and instrumentation during a simulation. a Preoperative radiographs, b initial geometry after the installation of the implants, c after the attachment of the first rod on the concave side of the spine deformity, d after the rod rotation maneuver, e final configuration after the installation of the second rod and nut lock up, f postop radiographs
Discussion
To better evaluate AIS, classification reliability can be improved using rule-based algorithms [26–29]. Much of the studies presented have focused on novel 3D measurements parameters such as SCI, PMC or geometric torsion that could be integrated in a classification. Implantation of such measurements to define AIS could lead us to a better understanding of that pathology and its treatment. The measurements to be used are still debated and rely on 3D reconstructions of AIS spines, which are not available in most clinical settings. With the advances in imaging and 3D reconstructions, a classification based on clinically accepted measurements addressing the 3D characteristics of AIS and aimed at guiding its treatment remains to be developed.
Screening and follow-up of AIS patients can impose unnecessary radiation to pediatric patients. Optimization of imaging and its frequency can be achieved. Non-radiation investigations from surface topography using laser scanners can accurately predict Cobb angle [17] and screen for patients requiring further investigation [21]. For screening or longitudinal follow-ups purposes, those radiation free techniques offer attractive alternatives to longitudinal X-ray imaging. Due to the idiopathic nature of the pathology, clinical research has not yet permitted appropriate prediction of its evolution; therefore, applications based on probabilistic or simulative modeling should be developed to guide patient management, in line with the experiments by Ajemba et al. [2] and Wu et al. [3].
Artificial intelligence algorithms were able to take into consideration geometric properties as well as clinical information to predict curve progression [2, 3, 61]. Those applications based on AI algorithms to screen for AIS, evaluate its severity or predict its progression could guide for the need and frequency of follow-up. Being developed in the research setting, their implantation in the clinical setting lacks studies proving their efficacy over current practice. In addition, cost, time consumption and set-up complexity of those systems remain clear limitations.
An application based on fuzzy logic was able to gather and average recommendations from the literature to match a consensus from a panel of experts with adequate accuracy [48, 49]. Such algorithms are able to output solutions around an area of indecision such as AIS surgical planning. Up to now, prediction of surgical outcome during its planning was mainly based on surgeon’s experience learned from past cases. Computer simulations permit an objective prediction of surgical outcome, allowing the clinician to test various options of instrumentation. It gives a quantification of forces resulting from instrumentation, with the calculation of vertebra-implant reaction forces; critical information to predict the risk of screw breakage or pullout. Surgical strategies leading to unstable constructs and under-correction could be avoided and those resulting in proper correction with minimum stress on the spine and materials could be proposed to guide surgical treatment. Yet no applications have demonstrated their ability to guide physicians for surgical indications and optimal surgical strategy Approach and surgical levels of fusion are critical decisions in AIS patient management but remain with high variability amongst surgeons [13].
To our knowledge, none of the applications reviewed are actually implemented in the clinical setting outside research institutions. Common obstacles to clinical use have been noticed. Most of those applications are experimental and lack clinical applicability. Consideration and better understanding of clinician’s need will be required to optimize those applications clinical usability. For example time efficiency when using those applications is a prime requirement. In addition, acceptance of results from computer application by clinicians will require strong evidence about improved gain in patient assessment and treatment over current methods; in our current review, only Labelle et al. passed this critical step by comparing computer-assisted brace design with conventional method. Finally, despite research efforts and proven improved patient care in some cases, the lack of knowledge transfer of such technologies from laboratory to industrial production, limited budgets, and slow adaptation of physicians to health information technologies remain major obstacles to clinical implementation of those applications.
Applications based on past cases should also be used in assisting surgical planning. Despite the development of patient databases [62], a comprehensive application gathering past cases, outputting an optimal instrumentation using AI methods, simulation or statistical analysis with sufficient accuracy and justification to get acceptance from clinicians remains to be developed.
Conclusion
Due to the complexity of AIS geometry, clinical evaluation and treatment, several computer applications have been developed to improve its management. Fuzzy clustering and support vector classifiers can regroup AIS spines having similar curve and curve progression. Applications based on ANN and surface topography algorithms have been able to compute actual and predicted Cobb angle with good accuracy while limiting irradiation. Rule-based algorithms can increase classification reliability. Fuzzy logic can average multiple rules extracted from the literature and output a degree of certainty in domains where no clear consensuses exist such as AIS levels of fusion. The critical question of optimal surgical strategy in selection of approach and levels of fusion remains unanswered and treatment is subject to personal experience and high variability. Applications need to be developed to permit optimization of surgical treatment by improving classification; by developing models based on literature evidence to provide treatment guidelines adapted to each patient and predict outcome based on past cases or simulation.
All those applications have shown potential to improve AIS care, but incomplete consideration of all AIS curve types, unproven benefit over current management, increased cost and time consumption in the clinical setting are clear limitations. Further studies proving their added value to current methods of management of AIS are needed. With proper development for clinical integration, those computer applications could improve the way AIS is currently assessed and treated.
References
Reamy BV, Slakey JB (2001) Adolescent idiopathic scoliosis: review and current concepts. Am Fam Physician 64:111–116
Ajemba PO, Ramirez L, Durdle NG, Hill DL, Raso VJ (2005) A support vectors classifier approach to predicting the risk of progression of adolescent idiopathic scoliosis. IEEE Trans Inf Technol Biomed 9:276–282
Wu H, Ronsky J, Poncet P, Cheriet F, Xue D, Harder J, Zernicke R (2005) Prediction of scoliosis progression in time series using a hybrid learning technique. Conf Proc IEEE Eng Med Biol Soc 6:6452–6455
Villemure I, Aubin CE, Grimard G, Dansereau J, Labelle H (2001) Progression of vertebral and spinal three-dimensional deformities in adolescent idiopathic scoliosis: a longitudinal study. Spine 26:2244–2250
Lonstein JE, Carlson JM (1984) The prediction of curve progression in untreated idiopathic scoliosis during growth. J Bone Joint Surg 66:1061–1071
Goldberg MS, Poitras B, Mayo NE, Labelle H, Bourassa R, Cloutier R (1988) Observer variation in assessing spinal curvature and skeletal development in adolescent idiopathic scoliosis. Spine 13:1371–1377
Morrissy RT, Goldsmith GS, Hall EC, Kehl D, Cowie GH (1990) Measurement of the Cobb angle on radiographs of patients who have scoliosis. Evaluation of intrinsic error. J Bone Joint Surg 72:320–327
Beauchamp M, Labelle H, Grimard G, Stanciu C, Poitras B, Dansereau J (1993) Diurnal variation of Cobb angle measurement in adolescent idiopathic scoliosis. Spine 18:1581–1583
King HA, Moe JH, Bradford DS, Winter RB (1983) The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg 65:1302–1313
Lenke LG, Betz RR, Harms J, Bridwell KH, Clements DH, Lowe TG, Blanke K (2001) Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg 83-A:1169–1181
Richards BS, Sucato DJ, Konigsberg DE, Ouellet JA (2003) Comparison of reliability between the Lenke and King classification systems for adolescent idiopathic scoliosis using radiographs that were not premeasured. Spine 28:1148–1156 discussion 1156–1157
Lenke LG, Betz RR, Clements D, Merola A, Haher T, Lowe T, Newton P, Bridwell KH, Blanke K (2002) Curve prevalence of a new classification of operative adolescent idiopathic scoliosis: does classification correlate with treatment? Spine 27:604–611
Aubin CE, Labelle H, Ciolofan OC (2007) Variability of spinal instrumentation configurations in adolescent idiopathic scoliosis. Eur Spine J 16:57–64
Robitaille M, Aubin CE, Labelle H (2007) Intra and interobserver variability of preoperative planning for surgical instrumentation in adolescent idiopathic scoliosis. Eur Spine J 16:1604–1614. doi:10.1007/s00586-007-0431-x
Tang F-h, Chan LWC, Lau H-p, Tsui P-y, Cheung C-w (2008) Computer-generated index for evaluation of idiopathic scoliosis in digital chest images: a comparison with digital measurement. J Dig Imag Offic J Soc Comput Appl Radiol 21 Suppl 1:S113–S120. doi:10.1007/s10278-007-9050-7
Greenspan A, Pugh JW, Norman A, Norman RS (1978) Scoliotic index: a comparative evaluation of methods for the measurement of scoliosis. Bull Hosp Joint Dis 39:117–125
Jaremko JL, Poncet P, Ronsky J, Harder J, Dansereau J, Labelle H, Zernicke RF (2002) Comparison of Cobb angles measured manually, calculated from 3-D spinal reconstruction, and estimated from torso asymmetry. Comput Methods Biomech Biomed Eng 5:277–281
Jaremko JL, Poncet P, Ronsky J, Harder J, Dansereau J, Labelle H, Zernicke RF (2002) Genetic algorithm-neural network estimation of Cobb angle from torso asymmetry in scoliosis. J Biomech Eng 124:496–503
Jaremko JL, Poncet P, Ronsky J, Harder J, Dansereau J, Labelle H, Zernicke RF (2001) Estimation of spinal deformity in scoliosis from torso surface cross sections. Spine 26:1583–1591
Baxt W (1995) Application of artificial neural networks to clinical medicine. Lancet 346:1075–1079
Ramirez L, Durdle NG, Raso VJ, Hill DL (2006) A support vector machines classifier to assess the severity of idiopathic scoliosis from surface topography. IEEE Trans Inf Technol Biomed 10:84–91
Noble WS (2006) What is a support vector machine? Nat Biotechnol 24:1565–1567. doi:10.1038/nbt1206-1565
Lenke LG, Betz RR, Haher TR, Lapp MA, Merola AA, Harms J, Shufflebarger HL (2001) Multisurgeon assessment of surgical decision-making in adolescent idiopathic scoliosis: curve classification, operative approach, and fusion levels. Spine 26:2347–2353
Cummings RJ, Loveless EA, Campbell J, Samelson S, Mazur JM (1998) Interobserver reliability and intraobserver reproducibility of the system of King et al. for the classification of adolescent idiopathic scoliosis. J Bone Joint Surg 80:1107–1111
Lenke LG, Betz RR, Bridwell KH, Clements DH, Harms J, Lowe TG, Shufflebarger HL (1998) Intraobserver and interobserver reliability of the classification of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg 80:1097–1106
Stokes IA, Aronsson DD (2002) Identifying sources of variability in scoliosis classification using a rule-based automated algorithm. Spine 27:2801–2805
Stokes IA, Aronsson DD (2002) Rule-based algorithm for automated King-type classification of idiopathic scoliosis. Stud Health Technol Inform 88:149–152
Stokes IA, Aronsson DD (2006) Computer-assisted algorithms improve reliability of King classification and Cobb angle measurement of scoliosis. Spine 31:665–670
Phan P, Mezghani N, Nault ML, Aubin CE, Parent S, de Guise J, Labelle H (2010) A decision tree can increase accuracy when assessing curve types according to Lenke classification of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 35:1054–1059. doi:10.1097/BRS.0b013e3181bf280e
Mezghani N, Chav R, Humbert L, Parent S (2008) A computer-based classifier of three-dimensional spinal scoliosis severity. Int J Comput Assist Radiol Surg 3:55–60. doi:10.1007/s11548-008-0163-3
Poncet P, Dansereau J, Labelle H (2001) Geometric torsion in idiopathic scoliosis: three-dimensional analysis and proposal for a new classification. Spine 26:2235–2243
Sangole A, Aubin C, Labelle H, Stokes I, Lenke L, Jackson R, Newton P (2009) Three-dimensional classification of thoracic scoliotic curves. Spine 34:91–99. doi:10.1097/BRS.0b013e3181877bbb
Stokes I, Sangole A, Aubin C (2009) Classification of scoliosis deformity three-dimensional spinal shape by cluster analysis. Spine 34:584–590. doi:10.1097/BRS.0b013e318190b914
Duong L, Cheriet F, Labelle H (2006) Three-dimensional classification of spinal deformities using fuzzy clustering. Spine 31:923–930
Duong L, Mac-Thiong J, Cheriet F, Labelle H (2009) Three-dimensional subclassification of Lenke type 1 scoliotic curves. J Spinal Disord Tech 22:135–143. doi:10.1097/BSD.0b013e31816845bc
Parent S, Newton PO, Wenger DR (2005) Adolescent idiopathic scoliosis: etiology, anatomy, natural history, and bracing. Instr Course Lect 54:529–536
Kessler JI (2008) Efficacy of a new computer-aided design/computer-aided manufacture orthosis in the treatment of adolescent idiopathic scoliosis. J Pediatr Orthop B 17:207–211. doi:10.1097/BPB.0b013e3283046117
Labelle H, Bellefleur C, Joncas J, Aubin C, Cheriet F (2007) Preliminary evaluation of a computer-assisted tool for the design and adjustment of braces in idiopathic scoliosis: a prospective and randomized study. Spine 32:835–843. doi:10.1097/01.brs.0000259811.58372.87
Wong M, Cheng J, Lo K (2005) A comparison of treatment effectiveness between the CAD/CAM method and the manual method for managing adolescent idiopathic scoliosis. Prosthet Orthot Int 29:105–111
Wong M, Cheng J, Wong M, So S (2005) A work study of the CAD/CAM method and conventional manual method in the fabrication of spinal orthoses for patients with adolescent idiopathic scoliosis. Prosthet Orthot Int 29:93–104
Perie D, Aubin C, Petit Y, Beausejour M, Dansereau J, Labelle H (2003) Boston brace correction in idiopathic scoliosis: a biomechanical study. Spine 28:1672–1677. doi:10.1097/01.BRS.0000083165.93936.6D
Perie D, Aubin CE, Petit Y, Labelle H, Dansereau J (2004) Personalized biomechanical simulations of orthotic treatment in idiopathic scoliosis. Clin Biomech (Bristol, Avon) 19:190–195. doi:10.1016/j.clinbiomech.2003.11.003
Clin J, Aubin CE, Labelle H (2007) Virtual prototyping of a brace design for the correction of scoliotic deformities. Med Biol Eng Comput 45:467–473. doi:10.1007/s11517-007-0171-4
Clin J, Aubin CE, Parent S, Sangole A, Labelle H (2010) Comparison of the biomechanical 3D efficiency of different brace designs for the treatment of scoliosis using a finite element model. Eur Spine J. doi:10.1007/s00586-009-1268-2
Katz DE, Herring JA, Browne RH, Kelly DM, Birch JG (2010) Brace wear control of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am 92:1343–1352. doi:10.2106/JBJS.I.01142
Rahman T, Bowen JR, Takemitsu M, Scott C (2005) The association between brace compliance and outcome for patients with idiopathic scoliosis. J Pediatr Orthop 25:420–422
Lou E, Hill D, Raso J, Moreau M, Mahood J (2005) Smart orthosis for the treatment of adolescent idiopathic scoliosis. Med Biol Eng Comput 43:746–750
Nault ML, Labelle H, Aubin CE, Balazinski M (2007) The use of fuzzy logic to select which curves need to be instrumented and fused in adolescent idiopathic scoliosis: a feasibility study. J Spinal Disord Tech 20:594–603
Nault ML, Labelle H, Aubin CE, Sangole A, Balazinski M (2009) Fuzzy-logic-assisted surgical planning in adolescent idiopathic scoliosis. J Spinal Disord Tech 22:263–269. doi:10.1097/BSD.0b013e3181761950
Torres A, Nieto JJ (2006) Fuzzy logic in medicine and bioinformatics. J Biomed Biotechnol 2006:91908. doi:10.1155/JBB/2006/91908
Stokes IA, Gardner-Morse M (1993) Three-dimensional simulation of Harrington distraction instrumentation for surgical correction of scoliosis. Spine 18:2457–2464
Gardner-Morse M, Stokes IA (1994) Three-dimensional simulations of the scoliosis derotation maneuver with Cotrel-Dubousset instrumentation. J Biomech 27:177–181
Gréalou L, Aubin CE, Labelle H (2000) Biomechanical modeling of the C-D instrumentation in scoliosis: a study of correction mechanisms. Arch Physiol Biochem 108(1–2):194
Robitaille M, Aubin CE, Labelle H (2009) Effects of alternative instrumentation strategies in adolescent idiopathic scoliosis: a biomechanical analysis. J Orthop Res 27:104–113. doi:10.1002/jor.20654
Aubin CE, Petit Y, Stokes IA, Poulin F, Gardner-Morse M, Labelle H (2003) Biomechanical modeling of posterior instrumentation of the scoliotic spine. Comput Methods Biomech Biomed Engin 6:27–32. doi:10.1080/1025584031
Desroches G, Aubin CE, Sucato DJ, Rivard CH (2007) Simulation of an anterior spine instrumentation in adolescent idiopathic scoliosis using a flexible multi-body model. Med Biol Eng Comput 45:759–768
Robitaille M, Aubin CE, Labelle H (2006) Biomechanical assessment of variable instrumentation strategies in adolescent idiopathic scoliosis: preliminary analysis of 3 patients and 6 scenarios. Stud Health Technol Inform 123:309–314
Aubin CE, Labelle H, Chevrefils C, Desroches G, Clin J, Eng AB (2008) Preoperative planning simulator for spinal deformity surgeries. Spine (Phila Pa 1976) 33:2143–2152. doi:10.1097/BRS.0b013e31817bd89f
Delorme S, Petit Y, de Guise JA, Labelle H, Aubin CE, Dansereau J (2003) Assessment of the 3-D reconstruction and high-resolution geometrical modeling of the human skeletal trunk from 2-D radiographic images. IEEE Trans Biomed Eng 50:989–998
Majdouline Y, Aubin C-E, Sangole A, Labelle H (2009) Computer simulation for the optimization of instrumentation strategies in adolescent idiopathic scoliosis. Med Biol Eng Comput 47:1143–1154. doi:10.1007/s11517-009-0509-1
Wu H, Ronsky J, Cheriet F, Harder J, Zernicke R (2006) Scoliotic progression patterns in prognostic factors and future prediction of spinal deformity progression. Stud Health Technol Inform 123:40–46
Arlet V, Shilt J, Bersusky E, Abel M (2008) Experience with an online prospective database on adolescent idiopathic scoliosis: development and implementation. Eur Spine J 17(11):1497–1506
Acknowledgments
Federal funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. Supported by the Fonds de la Recherche en santé du Québec and MENTOR, a strategic training program of the Canadian Institutes of Health Research.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Phan, P., Mezghani, N., Aubin, CÉ. et al. Computer algorithms and applications used to assist the evaluation and treatment of adolescent idiopathic scoliosis: a review of published articles 2000–2009. Eur Spine J 20, 1058–1068 (2011). https://doi.org/10.1007/s00586-011-1699-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-011-1699-4