Abstract
Ineffective erythropoiesis in patients with thalassemia intermedia drives extramedullary hematopoietic tumor formation in several parts of the body. Paraspinal involvement has received increasing attention due to the associated morbidity secondary to spinal cord compression. Although the history and physical examination may help narrow the differential diagnosis, radiographic imaging remains essential to confirm the existence of hematopoietic tissue. Characteristic appearance has been observed mainly on magnetic resonance imaging. Several treatment options have been described, including transfusion therapy, laminectomy, radiotherapy, and the use of fetal hemoglobin inducing agents that decrease the hematopoietic drive. However, the ideal management scheme remains controversial. Until large prospective trials evaluate the efficacy and safety of the available treatment options, both in single and in combination therapy, an individualized approach should be entertained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The β-thalassemias are a group of autosomal recessive disorders characterized by absence or reduced synthesis of the red cell β-globin chains. As a group, they are the most common single gene disorder in the world and are found at high frequencies in many populations worldwide [1]. Extremely diverse phenotypes exist within the homozygous and compound heterozygote states for β-thalassemia. The terms thalassemia major (TM) and thalassemia intermedia (TI) lack specific molecular correlates, but encompass a wide spectrum of clinical, as well as laboratory abnormalities [1]. At the severe end of the spectrum are patients whose clinical course is characterized by profound anemia, who present to medical attention in the first year of life, and who subsequently require regular transfusions for survival, the condition known as TM. But many patients with inheritance of two mutant beta alleles have a milder illness, with a broad range of severity including, at least in early childhood, a virtually asymptomatic state. Patients in this group who present to medical attention in later childhood and remain largely transfusion-independent are said to have TI [1]. However, transfusion-independence in TI does not come without its own side effects. Ineffective red cell production by the bone marrow (ineffective erythropoiesis) forces expansion of the hematopoietic tissue outside the marrow medulla and leads to hematopoietic compensatory involvement, mostly in the form of masses, of other regions in the body—the phenomenon termed extramedullary hematopoiesis (EMH) [2]. Among the various body regions reported, paraspinal involvement deserves special attention due to the debilitating clinical consequences and challenges in diagnosis and management. Experience to date comes from scattered case reports and small cases series.
This review comprehensively revisits these reports for data on the pathophysiology, clinical consequences, diagnosis, and treatment of paraspinal EMH in patients with TI, aiming to orient spinal surgeons to this increasingly reported problem.
Pathophysiology and paraspinal involvement
Extramedullary hematopoiesis is a physiological compensatory phenomenon occurring because of insufficient bone marrow function that becomes unable to meet circulatory demands [2]. EMH is seen in many hematological diseases, such as myelofibrosis, polycythemia vera, leukemia, lymphoma or after bone marrow irradiation [3–11]. However, its occurrence in chronic hemolytic anemias remains highest, particularly in transfusion independent-TI [2, 12]. Almost all body sites may be involved including the spleen, liver, lymph nodes, thymus, heart, breasts, prostate, broad ligaments, kidneys, adrenal glands, pleura, retroperitoneal tissue, skin, peripheral and cranial nerves, and the spinal canal [13–17]. These sites are believed to normally engage in active hematopoiesis in the fetus during gestation. This pathway normally stops at birth, but the extramedullary hematopoietic vascular connective tissues retain the ability to produce red cells under conditions of longstanding ineffective erythropoiesis [18] (Fig. 1).
The origin of the spinal epidural hematopoietic tissue is still controversial. It has been hypothesized that this tissue could be extruded through the trabecular bone of the vertebral body with a circumferential involvement of the vertebra, or it may have extended through the thinned trabeculae at the proximal rib ends [19–21]. Others have proposed some embryological hematopoietic cell remnants within the epidural space, which would be stimulated along the course of chronic anemia. Development of hematopoietic tissue from branches of the intercostal veins has also been suggested [22], while others still attribute the masses to embolic phenomena [23, 24]. Early in its evolution, the paraspinal extramedullary site of hematopoiesis reveals immature and mature cells mainly of the erythroid and myeloid series and dilated sinusoids containing precursors of red cells. The lesions eventually become inactive and reveal some fatty tissue and fibrosis or massive iron deposits [19].
There is some predilection for the site of spinal cord involvement by the hematopoietic tissue. The thoracic region and to a lesser extent the lumbar region are the most commonly involved sites. The reason for this predilection is uncertain, but because the subarachnoid space and the spinal canal are narrow in the thoracic region, which also has limited mobility [25, 26], small intraspinal hematopoietic tissue may cause compression of the spine at this level. This is in contrast with other regions of the cord in which such tissues must reach larger sizes to exert enough pressure on the spinal cord and cause symptoms [27, 28].
Clinical incidence, presentation, and consequences
The incidence of EMH in patients with TI may reach up to 20% compared to polytransfused TM patients where the incidence remains <1% [2, 29, 30]. A paraspinal location for the hematopoietic tissue occurs in 11–15% of cases with EMH [12, 31]. Since the first case described by Gatto et al. [32] in 1954, a large number of cases has been reported in the literature [12, 18–20, 23–28, 30, 31, 33–69]. Paraspinal EMH mainly presents as pseudotumors, which may cause a variety of neurological symptoms due to spinal compression. However, it is believed that more than 80% of cases may remain asymptomatic and the lesions are usually discovered incidentally by radiologic techniques [26, 47, 48]. Probably the development of neurologic symptoms depends on the chronicity of the disease with neurologic symptoms most frequently being reported during the third and fourth decades of life [64], although few reports described presentation as early as the first decade of life [23, 63, 69]. The male to female ratio reaches 5:1 [64] (Fig. 2). Various clinical presentations have been reported including: back pain, lower extremity pain, parasthesia, abnormal proprioception, exaggerated or brisk deep tendon reflexes, Babinski response, Lasegue sign, paraparesis, paraplegia, ankle clonus, spastic gate, urgency of urination, and bowl incontinence. The size and location of lesions and the extent of spinal cord involvement determine the severity, acuteness, and multiplicity of signs and symptoms.
Diagnosis
Early diagnosis of EMH will affect the course of management and may reduce the incidence of irreversible neurologic damage that would otherwise occur with prolonged undiagnosed cord compression [70]. The medical history remains important to rule out other entities in the differential diagnosis of epidural masses including metastatic malignant disease, lymphoma, multiple myeloma, vascular anomalies, or epidural abscess [71].
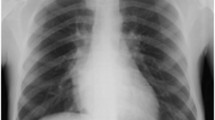
In the past, the diagnosis of paraspinal EMH in patients with TI was suspected from the typical osseous abnormalities found on chest radiographs [37, 72] or was confirmed after surgical removal of the mass [62]. Plain radiographs often reveal well-demarcated paraspinal masses [36, 44] and bony changes associated with chronic anemia such as trabeculation, widened ribs, or thickened calvaria [36]. Bony destruction or pathological fractures are usually absent (Fig. 3a). In the early 1980s, several reports demonstrated that computed tomography was a more preferred diagnostic imaging method (Fig. 3b). 99-mTc bone scan has also been used to diagnose paraspinal EMH [73] but the diagnosis within the epidural space may be difficult due to the proximity to bone marrow [49]. Myelography is declining in popularity because of its invasiveness, the need for cisternal puncture in cases of complete block preventing passage of radiographic contrast [40, 73] and reports of neurological deterioration following the procedure [24, 38].
Imaging of extramedullary hematopoiesis. a Chest X-ray demonstrating expanded anterior rib ends consistent with medullary hyperplasia. A paraspinal mass is seen in the right lower zone (white arrow). With permission from reference [62]. b Computed tomography scan showing inactive paraspinal extramedullary hematopoietic lesion with increased density compared to soft tissue due to iron deposition (black arrowheads). With permission from reference [18]. c Magnetic resonance image of cervical and thoracic spine. T2-weighted sagittal image showing thoracic cord compression by extramedullary intraspinal epidural hematopoietic mass from T2 to T10 (white arrows). With permission from reference [83]
Currently, magnetic resonance imaging (MRI) has eventually replaced all these methods and is considered the method of choice for the diagnosis and follow-up evaluation of spinal cord compression cases resulting from EMH [42, 43, 45, 49, 55, 72, 75]. MRI can clearly show anatomical details with high quality including both site and extent of the masses within the spinal canal, while producing soft tissue delineation with high sensitivity. Active recent hematopoietic extramedullary lesions have rich vasculature while inactive older lesions have more fatty tissue and iron deposits [18, 19, 49, 76]. If the patient is treated with blood transfusions, the lesion may decrease in size and appear on MRI with massive iron deposition [18, 77, 78]. Fatty degeneration is most probably related to oxidative stress leading to lipid peroxidation of cell membranes and production of oxygen free radicals. This is probably the reason why foci with fatty content are observed in non-transfused, non-chelated patients in whom conditions of oxidative stress occur more often than in transfused and iron-chelated TM patients. Although iron deposition and fatty replacement of the foci are inactive procedures, they seem to never coexist, probably because of the different oxidative stress conditions [18]. Active lesions show intermediate signal intensity in both T1 and T2 weighted MR images (Fig. 3c). Gadolinium enhancement is minimal or absent differentiating it from other epidural lesions such as abscesses or metastases [49, 55]. Older inactive lesions show high signal intensity in both T1 and T2 weighted MR images due to fatty infiltration or low signal intensity in both T1 and T2 weighted MR images due to iron deposition [71, 79–81].
Differential diagnosis is often easy, when the lesion is multifocal (paravertebral and epidural) or bilateral, due to characteristic iron deposition or fatty replacement and the characteristic topography. The only diagnostic problem exists with the solitary, unilateral active lesion. Mesenchymal tissue tumors or tumors from neural tissue elements should be included in the differential diagnosis but the clinical history of congenital hemolytic anemia should lead to the correct diagnosis [18, 76].
Although biopsy remains the gold standard for establishing a tissue diagnosis [82], it is an invasive procedure that carries the risk of catastrophic hemorrhage and is therefore not usually advocated [22, 74]. It should be reserved for older patients with a high probability of malignant disease and for cases in which the clinical and radiological picture is equivocal [69].
Management
Because of its rarity, no evidence-based guidelines for the treatment of paraspinal pseudotumors caused by EMH exist. Management options include blood transfusion, radiotherapy, surgical decompression, hydroxyurea, or a combination of these modalities. Therapy usually depends on the severity of symptoms, size of the mass, patient’s clinical condition, and previous treatment.
Because the EMH encountered in TI patients is only a compensatory mechanism for the chronic anemia, correction of this anemia with initiation of recurrent blood transfusions can decrease the need for EMH; thus resulting in relative inactivity of these tissues, and leading to the shrinkage of the mass size, decompression of the spinal cord, and neurologic improvement. The initial response results primarily from a decrease in blood flow to these tissues even before reduction in the size of the mass can be detected [26, 59]. It has been used as the principal treatment modality. Some authors have reported cases of EMH treated exclusively by this treatment as a first choice [26, 34, 43, 51, 59, 83] or in cases where surgical decompression or radiotherapy were contraindicated, e.g. pregnancy [34, 58] or severe anemia [46]. The target hemoglobin level is usually >10 g/dl. Blood transfusion was even considered as a diagnostic test since only cases of cord compression secondary to EMH could respond transfusion therapy [26]. However, blood transfusion is not a harmless procedure with infectious risks, iron overload, and alloimmunization being clearly recognized. Moreover, several reports showed that improvement is usually, slow, insufficient and only temporary [23, 44, 53, 55, 63, 83]. Moreover, while blood transfusion may prevent further progression of EMH, some proposed that it is unable to reverse preexisting cord compression. Its role in the management of patients with symptoms of acute onset may therefore be limited [26, 46]. Thus, many advocate using blood transfusion only as an adjunct to surgery (preparation and/or postoperative course) as correction of the hemoglobin level can be helpful in the immediate pre-operative period in order to insure an optimal oxygenation of the spinal cord during the surgery [38, 39, 49, 64]. The combination of blood transfusion and radiotherapy has also been reported as a therapeutic option, either for cases of recurrence after using either treatment method alone or as an initial treatment regimen [44, 52].
Low-dose radiation as a monotherapy has been reported to yield excellent results in up to 50% of patients with neurological improvement observed soon as 3–7 days after initiation of treatment [27, 40, 44, 66]. Hematopoietic tissue is extremely radiosensitive and undergoes shrinkage after radiotherapy, with a decrease in volume by as much as 16.4% [67]. Dosages reported in the literature range from 900 to 3,500 cGy [27, 37, 40, 43, 64]. A high risk of recurrence [37], up to 19–37%, is the main drawback of radiotherapy [40]. Fortunately, these recurrences are often amenable to further doses. The risks of radiotoxicity on an already compressed and injured spinal cord remain a concern [44, 84–86]. Tissue edema associated with radiation could sometimes result in neurological deterioration during the initial phase of treatment [38, 82], which is minimized by concomitant high-dose steroid therapy. The immunosuppressive effect of radiotherapy should be monitored with frequent peripheral blood counts as the resultant pancytopenia may further aggravate the condition [12, 37, 58, 82]. In patients who need rapid therapeutic response due to severe neurological symptoms, radiotherapy might be considered the primary treatment. In addition to primary treatment, radiotherapy is commonly employed as a post-operative adjunct following laminectomy to reduce the likelihood of recurrence [27, 28, 30, 31, 49, 64]. Moreover, successful combination therapy of low-dose radiation and blood transfusion or hydroxyurea has also been reported [26, 37, 40, 43, 69].
Laminectomy is currently indicated in cases of acute presentation which do not respond to adequate transfusion or radiotherapy [12, 40, 49, 74]. Surgery confers the benefits of immediate relief of cord compression and histological diagnosis [49]. Disadvantages include risk of bleeding associated with the high vascularity of the mass in question and the risks of operating on anemic individuals who are predisposed to shock, incomplete excision in cases of diffuse involvement [24, 35, 38], instability [24] and kyphosis associated with multilevel laminectomy [55, 62, 64]. Another drawback is that the procedure is not always possible or desirable due to diffuse nature of the mass and the possibility of recurrence. Furthermore, immediate total resection of extramedullary hematopoietic masses can lead to clinical decompensation and deterioration because these masses play a crucial role in maintaining an adequate hemoglobin level [40]. For these reasons, surgery should be reserved for cases of acute, progressive, and severe neurologic deterioration.
Hydroxyurea is a ribonucleotide reductase enzyme inhibitor. By reducing the globin chain imbalance through stimulating synthesis of fetal hemoglobin and cytoreduction, hydroxyurea contributes to a decrease ineffective erythropoiesis and the associated EMH [57, 61]. Patients with spinal EMH have been successfully treated with hydroxyurea alone [56, 61], especially in thalassemic patients who are unable to receive blood transfusions due to alloimmunization [87]. It was also used in conjunction with transfusion [56, 57, 88] and radiotherapy [53, 57].
Although treatment recommendations may be derived from the aforementioned reports (Fig. 4), management should remain individualized. Whereas significant improvement has been made in our understanding of the molecular, pathophysiological, and clinical characteristics of TI, the lack of clear treatment guidelines still presents a challenge. Large randomized clinical trials are thus called for to evaluate the efficacy and safety of different treatment modalities, as mono- or combined therapy, to control paraspinal EMH in this patient population and prevent disabling morbidity.
References
Weatherall DJ, Clegg JB (2001) The thalassemia syndromes, 4th edn. Blackwell Scientific Publications, Oxford
Taher A, Ismaeel H, Cappellini MD (2006) Thalassaemia intermedia: revisited. Blood Cells Mol Dis 37:12–20
Cromwell LD, Kerber C (1978) Spinal cord compression by extramedullary hematopoiesis in myeloid metaplasia. Radiology 128:118
Stahl SM, Ellinger G, Baringer JR (1979) Progressive myelopathy due to extramedullary hematopoiesis: case report and review of the literature. Ann Neurol 5:485–489
Rice GP, Assis LJ, Barr RM, Ebers GC (1980) Extramedullary hematopoiesis and spinal cord compression complicating polycythemia rubra vera. Ann Neurol 7:81–84
Cook G, Sharp RA (1994) Spinal cord compression due to extramedullary haemopoiesis in myelofibrosis. J Clin Pathol 47:464–465
Price F, Bell H (1985) Spinal cord compression due to extramedullary hematopoiesis. Successful treatment in a patient with longstanding myelofibrosis. JAMA 253:2876–2877
Jackson A, Burton IE (1990) Retroperitoneal mass and spinal cord compression due to extramedullary haemopoiesis in polycythemia rubra vera. Br J Radiol 42:91–92
McDonald AC, Cook G, Sharp RA, Bissett D (1993) Spinal cord compression in myelofibrosis—a case report. Acta Oncol 32:692–693
de Morais JC, Spector N, Lavrado FP, Nobre LF, de Mattos JP, Pulcheri W, Nucci M, Novis S, de Oliveira HP (1996) Spinal cord compression due to extramedullary hematopoiesis in the proliferative phase of polycythemia vera. Acta Haematol 96:242–244
Haran M, Ni S (2001) Recurrent reversible paraplegia. Lancet 357:1092
Dore F, Cianciulli P, Rovasio S, Oggiano L, Bonfigli S, Murineddu M, Pardini S, Simonetti G, Gualdi G, Papa G et al (1992) Incidence and clinical study of ectopic erythropoiesis in adult patients with thalassemia intermedia. Ann Ital Med Int 7:137–140
Aessopos A, Tassiopoulos S, Farmakis D, Moyssakis I, Kati M, Polonifi K, Tsironi M (2006) Extramedullary hematopoiesis-related pleural effusion: the case of beta-thalassemia. Ann Thorac Surg 81:2037–2043
Chuang CK, Chu SH, Fang JT, Wu JH (1998) Adrenal extramedullary hematopoietic tumor in a patient with beta-thalassemia. J Formos Med Assoc 97:431–433
Kumar A, Aggarwal S, de Tilly LN (1995) Case of the season. Thalassemia major with extramedullary hematopoiesis in the liver. Semin Roentgenol 30:99–101
Brannan D (1927) Extramedullary hematopoiesis in anemias. Bull Johns Hopkins Hosp 41:104–135
Ross P, Logan W (1969) Roentgen findings in extramedullary hematopoiesis. AJR Am J Roentgenol 106:604–613
Tsitouridis J, Stamos S, Hassapopoulou E, Tsitouridis K, Nikolopoulos P (1999) Extramedullary paraspinal hematopoiesis in thalassemia: CT and MRI evaluation. Eur J Radiol 30:33–38
Pantongrag-Brown L, Suwanwela N (1992) Case report: chronic spinal cord compression from extramedullary haematopoiesis in thalassemia BMRI findings. Clin Radiol 46:281–283
Da Costa JL, Loh YS, Hanam E (1974) Extramedullary hematopoiesis with multiple tumour-simulating mediastinal masses in hemoglobin E-thalassemia disease. Chest 65:210–212
Lyall A (1935) Massive extramedullary bone marrow formation in a case of pernicious anaemia. J Pathol Bacteriol 41:469–472
Cone SM (1925) Bone marrow occurring in intercostal veins. JAMA 84:1732–1733
Cardia E, Toscano S, La Rosa G, Zaccone C, d’Avella D, Tomasello F (1994) Spinal cord compression in homozygous beta thalassemia intermedia. Pediatr Neurosurg 20:186–189
Amirjamshidi A, Abbassioun K, Ketabchi SE (1991) Spinal extradural hematopoiesis in adolescents with thalassemia. Childs Nerv Syst 7:223–225
Abbassioun K, Amir-Jamshidi A (1982) Curable paraplegia due to extradural hematopoietic tissue in thalassemia. Neurosurgery 11:804–807
Parsa K, Oreizy A (1995) Nonsurgical approach to paraparesis due to extramedullary hematopoiesis. J Neurosurg 82:657–660
Singhal S, Sharma S, Dixit S, De S, Chander S, Rath GK, Mehta VS (1992) The role of radiation therapy in the management of spinal cord compression due to extramedullary hematopoiesis in thalassemia. J Neurol Neurosurg Psychiatry 55:310–312
Luyendjik W, Went L, Schaad HD (1975) Spinal cord compression due to extramedullary hematopoiesis in homozygous thalassemia: case report. J Neurosurg 42:212–216
Logothetics J, Constantoulakis M, Exonomidou J, Stefanis C, Hakas P, Augoustaki O, Sofroniadou K, Loewenson R, Bilek M (1972) Thalassaemia major (homozygous eta thalassemia). A survery of 138 cases with emphasis on neurologic and muscular aspects. Neurology 22:249–304
Prabhakar S, Chopra J, Khosla VK, Dash S, Banerijee AK (1980) Spinal compression in homozygous beta thalassemia. Surg Neurol 13:351–354
Shin KH, Sharma S, Gregoritch SJ, Lifeso RM, Bettigole R, Yoon SS (1994) Combined radiotherapeutic and surgical management of a spinal cord compression by extramedullary hematopoiesis in a patient with hemoglobin E beta thalassemia. Acta Haematol 91:154–157
Gatto I, Terrana V, Biondi L (1954) Compression of the spinal cord due to proliferation of bone marrow in epidural space in a splenectomized person with Cooley’s disease. Haematologica 38:61–75
Cross JN, Morgan OS, Gibbs WN, Cheruvanky I (1977) Spinal cord compression in thalassaemia. J Neurol Neurosurg Psychiatry 40:1120–1122
Issargisil S, Piankijagum A, Prawase W (1981) Spinal cord compression in thalassemia. Report of 12 cases and recommendations for treatment. Arch Intern Med 141:1033–1036
Luitjes WF, Braakman R, Abels J (1982) Spinal cord compression in a new homozygous variant of beta-thalassemia. Case report. J Neurosurg 57:846–848
David CV, Balasubramaniam P (1983) Paraplegia with thalassemia. Aust NZ J Surg 53:283–284
Papavasiliou C, Sandilos P (1987) Effect of radiotherapy in symptoms due to heterotopic marrow in beta-thalassemia. Lancet 1:13–14
Mann KS, Yue CP, Chan KH, Ma LT, Ngan H (1987) Paraplegia due to extramedullary hematopoiesis in thalassemia. Case report. J Neurosurg 66:938–940
Issardgrisil S, Piankijagum A, Wasi P (1987) Spinal cord compression in thalassemia. Arch Intern Med 141:1033–1036
Jackson DV Jr, Randall ME, Richards F 2nd (1988) Spinal cord compression due to extramedullary hematopoiesis in thalassemia: long-term follow-up after radiotherapy. Surg Neurol 29:388–392
Martinez-Rodrigo MA, Sanjuanbenito L, Rodriguez del Barrion E, Martinez-San Millan J, Saldana D (1988) Spinal cord compression secondary to epidural extramedullary hematopoiesis in thalassemia: a clinical case and review of literature. Rev Neurol 27:998–1004
Singounas EG, Sakas DE, Hadley DM, Chalevelakis G, Sfakianos G, Raptis S, Karvounis PC (1991) Paraplegia in a pregnant thalassemic woman due to extramedullary hematopoiesis: successful management with transfusions. Surg Neurol 36:210–215
Hassoun H, Lawn-Tsao L, Langevin ER, Lathi ES, Palek J (1991) Spinal cord compression secondary to extramedullary hematopoiesis: a noninvasive management based on MRI. Am J Hematol 37:201–203
Kaufmann T, Coleman M, Giardina P, Nisce LZ (1991) The role of radiation therapy in the management of hematopoietic neurologic complications in thalassemia. Acta Haematol 85:156–159
Chaljub G, Guinto F, Crow W, Kumar R (1991) MRI diagnosis of spinal cord compression in beta thalassemia. Spine 16:583–584
Mancuso P, Zingale A, Basile L, Chiaramonte I, Tropea R (1993) Cauda equina compression syndrome in a patient affected by thalassemia intermedia: complete regression with blood transfusion therapy. Childs Nerv Syst 9:440–441
Richter E (1993) Extramedullary hematopoiesis with intraspinal extension in thalassemia. Aktuelle Radiol 3:320–322
Dore F, Pardini S, Gaviano E, Longinotti M, Bonfigli S, Rovasio S, Tomiselli A, Cossu F (1993) Recurrence of spinal cord compression from extramedullary hematopoiesis in thalassemia intermedia treated with low doses of radiotherapy. Am J Hematol 44:148
Lau SR, Chan O, Chow Y (1994) Cord compression due to extramedullary hematopoiesis in a patient with thalassemia. Spine 19:2467–2470
Gouliamos AD, Plataniotis GA, Michalopoulos ES, Vlahos LS, Papavasiliou CG (1995) Magnetic resonance imaging spinal cord compression in thalassaemia before and after radiation treatment. Clin Radiol 50:504–505
Lee AC, Chiu W, Tai KS, Wong V, Pech WC, Lau YL (1996) Hypertransfusion for spinal cord compression secondary to extramedullary haematopoiesis. Pediatr Hematol Oncol 13:89–94
Pistevou Gompaki K, Skaragas G, Papaskevopoulos P, Kotsa K, Repanta E (1996) Extramedullary hematopoiesis in thalassemia, results of radiotherapy: a report of three patients. Clin Oncol 8:120–122
Cianciulli P, Sorrentino F, Morino L, Massa A, Sergiacomi GL, Donato V, Amadori S (1996) Radiotherapy combined with erythropoietin for the treatment of extramedullary hematopoiesis in an alloimmunized patient with thalassemia intermedia. Ann Hematol 72:379–381
Boyacıgil S, Ardiç S, Tokog˘lu F, Pasaog˘lu E, Karakas HM (1996) Intrathoracic extramedullary haematopoiesis. Australas Radiol 40:179–181
Coskun E, Keskin A, Suzer T, Sermez Y, Kildaci T, Tahta K (1998) Spinal cord compression secondary to extramedullary hematopoiesis in thalassemia intermedia. Eur Spine J 7:501–504
Saxon BR, Rees D, Olivieri NF (1998) Regression of extramedullary haemopoiesis and augmentation of fetal haemoglobin concentration during hydroxyurea therapy in beta thalassemia. Br J Haematol 101:416–419
Cianciulli P, di Toritto TC, Sorrentino F, Sergiacomi L, Massa A, Amadori S (2000) Hydroxyurea therapy in paraparesis and cauda equina syndrome due to extramedullary haematopoiesis in thalassaemia: improvement of clinical and haematological parameters. Eur J Haematol 64:426–429
Phupong V, Uerpairojkij B, Limpongsanurak S (2000) Spinal cord compression: a rareness in pregnant thalassemic woman. J Obstet Gynaecol Res 26:117–120
Aliberti B, Patrikiou A, Terentiou A, Frangatou S, Papadimitriou A (2001) Spinal cord compression due to extramedullary hematopoiesis in two patients with thalassemia: complete response with blood transfusion therapy. J Neurol 248:18–22
Chourmouzi D, Pistevou-Gompaki K, Plataniotis G, Skaragas G, Papadopoulos L, Drevelegas A (2001) MRI findings of extramedullary haemopoiesis. Eur Radiol 11:1803–1806
Cario H, Wegener M, Debatin KM, Kohne E (2002) Treatment with hydroxyurea in thalassemia intermedia with paravertebral pseudotumors of extramedullary hematopoiesis. Ann Hematol 81:478–482
Tan TC, Tsao J, Cheung FC (2002) Extramedullary haemopoiesis in thalassemia intermedia presenting as paraplegia. J Clin Neurosci 9:721–725
Chehal A, Aoun E, Koussa S, Skoury H, Koussa S, Taher A (2003) Hypertransfusion: a successful method of treatment in thalassemia intermedia patients with spinal cord compression secondary to extramedullary hematopoiesis. Spine 28:E245–E249
Salehi SA, Koski T, Ondra SL (2004) Spinal cord compression in betathalassemia: case report and review of the literature. Spinal Cord 42:117–123
Zamanizadeh B, Eghbali SS, Taghipour M, Haghnegahdar A, Zare Z (2006) Spinal cord compression secondary to extramedullary hematopoiesis: case report and summary. Neurosurg Q 16:144–146
Malik M, Pillai LS, Gogia N, Puri T, Mahapatra M, Sharma DN, Kumar R (2007) Paraplegia due to extramedullary hematopoiesis in thalassemia treated successfully with radiation therapy. Haematologica 92:e28–e30
Goerner M, Gerull S, Schaefer E, Just M, Sure M, Hirnle P (2008) Painful spinal cord compression as a complication of extramedullary hematopoiesis associated with β-thalassemia intermedia. Strahlenther Onkol 4:224–226
Moncef B, Hafedh J (2008) Management of spinal cord compression caused by extramedullary hematopoiesis in beta-thalassemia. Inter Med 47:1125–1128
Ileri T, Azik F, Ertem M, Uysal Z, Gozdasoglu S (2009) Extramedullary hematopoiesis with spinal cord compression in a child with thalassemia intermedia. J Pediatr Hematol Oncol 31:681–683
Fetell MR, Stein BM (1989) Spinal tumors. In: Rowland LP (ed) Merritt’s textbook of neurology, 8th edn. Lea & Febiger, Philadelphia, pp 350–364
Dibbern DA, Loevner LA, Lieberman AP, Salhany KE, Freese A, Marcotte PJ (1997) MR of thoracic cord compression caused by epidural extramedullary hematopoiesis in myelodysplastic syndrome. AJNR Am J Neuroradiol 18:363–366
Sorsdahl O, Taylor PE, Noyes WD (1964) Extramedullary hematopoiesis, mediastinal masses and spinal cord compression. JAMA 189:343–347
Bronn LJ, Paquelet JR, Tetalman MR (1980) Intrathoracic extramedullary hematopoiesis: appearance on 99mTc sulfur colloid marrow scan. AJR Am J Roentgenol 134:1254–1255
Oustwani MB, Kurtides ES, Christ M, Ciric I (1980) Spinal cord compression with paraplegia in myelofibrosis. Arch Neurol 37:389–390
Guermazi A, Miaux Y, Chiras J (1997) Imaging of spinal cord compression due to thoracic extramedullary haematopoiesis in myelofibrosis. Neuroradiology 39:733–736
Kalina P, Zaheer W, Drehobl KE (1995) Cord compression by extramedullary hematopoesis in polycythemia vera. AJR Am J Roentgenol 164:1027–1028
Wolman IJ, Ortolani M (1969) Some clinical features of Cooley’s anemia patients as related to transfusion schedules. Ann N Y Acad Sci 165:407–414
Lawson J, Ablow R, Pearson H (1981) The ribs in thalassemia. I the Relationship to therapy. Radiology 140:663–672
Yamato M, Fuhrman C (1987) Computed tomography of fatty replacement in extramedullary hematopoiesis. J Comput Assisted Tomogr 11:541–542
Savader S, Otero R, Savader B (1988) MR imaging of intrathoracic extramedullary hematopoiesis. J Comput Assist Tomogr 12:878–880
Mesurolle B, Sayag E, Meingan P, Lasser P, Duvillard P, Vanel D (1996) Retroperitoneal extramedullary hematopoiesis: sonographic, CT and MR imaging appearance. AJR Am J Roentgenol 167:1139–1140
De Klippel N, Dehou MF, Bourgain C, Schots R, De Keyser J, Ebinger G (1993) Progressive paraparesis due to thoracic extramedullary hematopoiesis in myelofibrosis. Case report. J Neurosurg 79:125–127
Tai SM, Chan JS, Ha SY, Young BW, Chan MS (2006) Successful treatment of spinal cord compression secondary to extramedullary hematopoietic mass by hypertransfusion in a patient with thalassemia major. Pediatr Hematol Oncol 23:317–321
Bolling T, Schuck A, Rube C, Hesselmann S, Pape H, Dieckmann K, Pöllinger B, Kortmann RD, Speiser-Held I, Meyer FM, Martini C, Asadpour B, Timmermann B, Beck JD, Langer T, Paulides M, Schmidt B, Willich N (2006) Therapy-associated late effects after irradiation of malignant diseases in childhood and adolescence, Feasibility analyses of a prospective multicenter register study. Strahlenther Onkol 182:443–449
Rades D, Dahm-Daphi J, Rudat V, Schulte R, Stalpers LJ, Veninga T, Hoskin PJ (2006) Is short-course radiotherapy with high doses per fraction the appropriate regimen for metastatic spinal cord compression in colorectal cancer patients? Strahlenther Onkol 182:708–712
Trott KR, Kamprad F (2006) Estimation of cancer risks from radiotherapy of benign diseases. Strahlenther Onkol 182:431–436
Olivieri NF, Rees DC, Ginder GD, Thein SL, Brittenham GM, Waye JS, Weatherall DJ (1997) Treatment of thalassemia major with phenylbutyrate and hydroxyurea. Lancet 350:491–492
Konstantopoulos K, Vagiopoulos G, Kantouni R, Lymperi S, Patriarcheas G, Georgakopoulos D, Fessas P (1922) A case of spinal cord compression by extramedullary haematopoiesis in a thalassaemia patient: a putative role for hydroxyurea? Haematologica 77:352–354
Conflict of interest statement
The authors have no conflicts of interest to disclose. This study did not receive external funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haidar, R., Mhaidli, H. & Taher, A.T. Paraspinal extramedullary hematopoiesis in patients with thalassemia intermedia. Eur Spine J 19, 871–878 (2010). https://doi.org/10.1007/s00586-010-1357-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-010-1357-2