Abstract
Astrocytomas affect a significant portion of patients with intramedullary tumors. These infiltratively growing tumors are treated by a variety of methods—biopsy and decompressive surgery, maximal safe resection, adjuvant oncological therapy. Also, numerous prognostic factors are reported in the literature. Better understanding of factors that influence prognosis may help in treatment planning with the goal of prolonging survival. We have thus undertaken an extensive literature review in order to define factors affecting prognosis. A total of 38 articles were studied. Only tumor grade was consistently reported as the major factor affecting prognosis. The influence of other clinical factors (age, gender, history length, functional status, tumor location or extent, syrinx or cyst presence) can be speculated upon, but cannot be assessed adequately from the available literature. For both low- and high-grade (HG) astrocytomas, maximal safe tumor resection should be the primary treatment objective but is often not feasible in contrast to other intramedullary and spinal neoplasms. Since the biological nature of spinal cord HG glioma is identical to that of the brain, the same treatment algorithm of maximal safe resection followed by concomitant radio- and chemotherapy would be sensible to implement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intramedullary spinal cord tumors (IMSCTs) are relatively rare neoplasms accounting for 2–4% of all central nervous system tumors and for 20–25% of all spinal tumors [4, 23, 68, 69]. Astrocytomas and ependymomas represent approximately 80% of all IMSCTs [23]. Whereas ependymoma is the most common IMSCT in the middle-aged population, its diagnosis is relatively rare among children. On the other hand, astrocytomas constitute the majority of IMSCTs found in children and adolescents [9, 17, 19, 23, 67]. The majority of IMSCTs are low-grade (LG) neoplasms. On the other hand, high-grade (HG) tumors, e.g. anaplastic astrocytomas (WHO grade III) and intramedullary glioblastomas (WHO grade IV), together account for approximately 8–13% of intramedullary tumors of astrocytic origin [7, 29, 65, 68] and are usually characterized by rapid progression.
Gross total resection (GTR) has been established as the gold standard in the treatment of intramedullary ependymoma, with series reporting 70–100% of ependymomas being radically resected [6, 16, 20, 45, 57] and long-term survival being the rule rather than exception. On the contrary, the therapeutical spectrum in infiltratively growing intramedullary astrocytomas ranges from histological verification and decompressive surgery with subsequent radiotherapy [64] to GTR, with 6–70% of intramedullary astrocytomas undergoing GTR [27, 28, 34, 49, 56, 58, 61, 64].
Besides surgery, radiotherapy is a widely employed therapeutical option in the treatment of intramedullary astrocytoma. Its use varies considerably; some centers use it as a standard part of treatment protocol [24, 30, 37], others irradiate patients according to the rapidity of preoperative disease progression [61] and other institutions irradiate patients depending upon resection extent [34, 64].
Despite advances in diagnostic imaging, microsurgical techniques, surgical adjuncts and adjuvant oncological therapy, the prognosis of intramedullary astrocytoma is not as well known contrary to their intracranial counterparts [63]. Due to its relative rarity, it is very probable that a randomized controlled trial comparing various management strategies in the treatment of intramedullary astrocytoma will never be conducted. Better understanding of factors that influence prognosis may help in treatment planning with the goal of prolonging survival. We have thus undertaken an extensive literature review in order to define factors affecting recurrence rate and survival with the goal of clarifying an optimal therapeutical strategy for intramedullary astrocytoma, since its management is controversial, contrary to ependymoma.
Methods
The Medline database was searched in February 2008 using the keywords “spinal cord neoplasm”, “spinal cord tumor”, “spinal cord astrocytoma”, “intramedullary tumor”, “intramedullary astrocytoma”, “intramedullary glioma”, “spinal cord glioma”, “intramedullary glioblastoma”, “spinal cord glioblastoma”, “surgery”, “radiotherapy”, “prognosis”, “prognostic factor”, “treatment”, “resection”, “chemotherapy” and their combinations. The “What’s new” Medline function was applied for the described search strategy to update the list of articles during the time of manuscript preparation. Full texts of relevant English language articles (review articles included) were studied by two readers and their reference lists searched for additional articles which were in turn studied as well. This traditional approach is more prone to bias than a meta-analysis or systematic review; however, we endeavored to unreservedly include all studies. Such an approach has been successfully used before also in other topics [21, 31, 50]. Moreover, studies of intramedullary astrocytoma report patient characteristics and results in a very varying fashion, practically precluding a meta-analysis or systematic review. Many valuable studies would then be excluded if strict inclusion criteria were applied. Particularly, articles dealing primary with intramedullary tumors, where astrocytomas constitute a varying portion, would have to be excluded, because astrocytoma patients are described as part of the whole group and crude data extraction is not always possible.
The point of our review was factors that influence progression-free (PFS) and overall survival (OS). We concentrated on patient demographics (age, gender, history length, functional status), tumor characteristics (location, extension, syrinx/cyst presence, tumor grade), surgical therapy (extent or resection, EOR) and adjuvant oncological therapy (radiotherapy, RT). Particular attention was turned to studies published after 1980 and reporting 10 or more patients and presenting data in a statistical fashion where the influence of the above described factors proved to be statistically (in)significant. Each article was read by two independent reviewers and assessed both for quality (see bellow) and for presence of relevant information with regard to prognostic factors of interest.
Quality assessment
In order to assess quality of the reviewed studies, we first defined what attributes an ideal single institution study should have (Table 1). Number of patients was assessed as follows: less than 20 patients, 0 points; 21–50 patients, 1 point; more than 51 patients, 2 points. Every other parameter listed was assigned 1 point if clearly reported, 0 if missing or unable to ascertain, and occasionally 0.5 if present, but information incomplete. Such methodology was used successfully before [74]. IMSCT studies were assessed not only by their overall methodology, but also by astrocytoma-specific results. Possible maximum number of points gained is 30. Based on number of points gained, studies were divided into two categories: A, 15 points and more; B, 14 points and less (Tables 1, 2, last column). Two independent readers assigned points to each article, discrepancies were resolved through consensus.
Results
The described search strategy yielded a total of more than 2,400 articles. After excluding clearly irrelevant articles by title, a total of 290 studies dealing with intramedullary astrocytomas or IMSCTs were left for further study. Abstracts of these articles were reviewed and full texts of 170 articles were subsequently obtained. Of these, 19 intramedullary astrocytoma articles reported one or more of the prognostic factors of interest and form the basis of this review (Table 2). Additional 19 articles dealing with IMSCTs also reported one or more prognostic factors of interest for astrocytoma patients and form the basis of this review as well (Table 3). One IMSCT study [2] is a follow-up of a previous one [1], only the latter was used in this review [2].
Quality assessment
There were 21 category A and 17 category B studies. The mean number of point reached was 15.7 (range 9–22.5). Altogether, 7 articles scored 20 points or more [8, 9, 24, 46, 56, 59, 60].
Age
Results of a statistical analysis of age as a prognostic factor were reported in 15 studies [2, 5, 24, 27, 28, 30, 34, 41, 46, 49, 56, 59, 60, 64, 65] (Table 4). Of these, 13 were classified as category A, 8 failed to find a significant relationship between age and prognosis [2, 24, 28, 30, 34, 46, 56, 59], as did one additional category B study [27]. Four category A studies reported increased age to negatively affect prognosis: Sandler et al. [64] found patients with tumor recurrence to be older (mean 38 years, median 31 years) than those without recurrence (mean 19 years, median 17 years), furthermore the oldest three patients in his study all experienced recurrence within 1 year. Similarly, Lee et al. [41] reported that older age adversely affected local control, PFS and OS, finding confirmed by others in terms of both PFS and cause-specific survival (CSS) [60]. A large multicenter review of pediatric patients with intramedullary astrocytomas conducted in France reported age <7 years to correlate with better outcome. Ten-year OS for younger patients was 76% compared with 38% for patients older than 7 years (p = 0.04) [5]. Contrary to these four reports, the last category A study [49] reported age over 20 to be associated with increased survival. In addition, one category B study dealing exclusively with malignant astrocytomas reported advanced age to decrease median survival [65].
Gender
Results of a statistical analysis of gender as a prognostic factor were reported in 11 studies [2, 5, 24, 27, 30, 34, 41, 49, 59, 60, 65] (Table 5). Of these, nine were classified as category A, six failed to find a significant association between gender and prognosis [2, 34, 41, 49, 59, 60], as did two additional category B studies [27, 65]. Two category A studies reported female patients to have better prognosis: Huddart [24] found female patients to have statistically better 5-year OS (100%) compared with male patients (34%; p < 0.01), even after stratification by grade. Jyothirmayi [30] reported better 5-year PFS for female patients (90%) when compared to male patients (65%; p = 0.03), although difference in 5-year OS was not significant (58 vs. 52%, p = 0.6). On the other hand, in the already mentioned pediatric French cooperative study, where gender representation was almost equal, boys fared better than girls (10-year OS 79 vs. 39%, p = 0.04) [5].
History length
Results of a statistical analysis of history length as a prognostic factor were reported in 10 studies [5, 22, 24, 27, 30, 34, 49, 59, 60, 64] (Table 6). Of these, eight were classified as category A, five failed to identify significant relationship between history length and outcome [24, 30, 34, 59, 64], as did one additional category B study [22]. Three category A studies in agreement reported increased survival in patients with history length longer than 2 months [5] and 6 months [49, 60]. In addition, one category B study reported decreased 5-year OS in patients with history length shorter than 1 year [27].
Functional status
Statistical analysis reporting functional status as a prognostic factor was reported in eight studies [24, 27, 30, 34, 41, 46, 56, 59] (Table 7). All but one [27] were classified as category A, five did not report any significant relationship between functional status and survival [24, 30, 46, 56, 59]. Two category A studies in agreement reported increased survival in patients with favorable functional status: mean and median survivals were increased for patients with higher preoperative functional status (149.5 and 184 vs. 22.5 and 6 months; p < 0.05) and functional status was indentified as the only important prognostic factor among LG astrocytomas [34]. Five-year OS was also significantly increased in patients with favorable neurological function (73 vs. 22%, p = 0.04), although the difference in local control and PFS was not significant [41]. In addition, one category B study reported increased survival rates for patients with higher Karnofsky Performance Status [27].
Tumor location
Statistical analysis reporting tumor location as a prognostic factor was reported in nine studies [2, 24, 27, 28, 30, 34, 49, 51, 52] (Table 8). Of these, five were classified as category A, four did not report any significant relationship between tumor location and outcome [24, 28, 30, 34], only Minehan [49] found significantly “increased” survival in patients with thoracic spinal cord involvement. In addition, in one category B study, survival of patients with astrocytomas in thoracic location was significantly higher than in cervical location in both LG and HG tumors [51].
Tumor extent
Statistical analysis reporting tumor extent as a prognostic factor was reported in nine studies [2, 5, 24, 27, 28, 34, 41, 46, 59], all but one [27] classified as category A (Table 9). Only Kim et al. [34] found tumor extension of four and more segments to be associated with shorter mean survival (46.1 months) than tumors spanning less than four segments (mean survival 119.6 months; p < 0.05). In the other mentioned studies, tumor extent was not associated with prognosis.
Syrinx/cyst presence
Intramedullary peritumoral syrinx or tumor-associated cysts were reported as a prognostic factor in five studies, all were classified as category A [2, 24, 30, 59, 62] (Table 10). The presence of intramedullary cysts was associated with improved 5-year OS (88 vs. 44%, p < 0.05) [24] and improved 5-year PFS (100 vs. 70%, p = 0.03), although not 5-year OS (67 vs. 43%, p = 0.06) [30]. In the other studies, the presence of syrinx was not found to be a statistically significant factor.
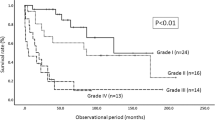
Tumor grade
There were 35 studies reporting outcomes for patients according to histological grade, 20 were classified as category A, 15 as category B (Table 11). Comparison between grades was made in 30 articles, 1 study referred to histology as “astrocytoma” [18], 1 study reported outcomes for HG tumors only [7] and 3 for LG tumors only [28, 59, 61]. In 14 studies (10 category A [2, 5, 24, 30, 34, 41, 46, 49, 60, 66], 4 category B [47, 51, 52, 58]), statistical analysis comparing histological grades was performed. Only Shirato et al. [66] did not find statistical significant difference in 3-year OS between LG and HG tumor (80 vs. 40%, p = 0.0861). All other articles reported more favorable outcome for LG tumors when compared to HG. One study [46] compared grade III patients with grade IV patients; increased survival rates were found for grade III histology. Results from the remaining studies are summarized in Table 11; LG histology was predictive of better outcome, although formal statistical analysis was not always performed.
Extent of resection
Results for EOR (as assessed by the authors) regardless of histology were reported in 14 studies, 10 were classified as category A [2, 5, 24, 30, 34, 41, 49, 56, 60, 64], 4 as category B [22, 25, 27, 58] (Table 12). In two category A studies, GTR in comparison to lesser resection was associated with significantly reduced risk of recurrence (0 vs. 69%, p = 0.029) [56] and with significant risk reduction of 15-year PFS, although not 15-year OS [2]. One additional category B study reported better 7-year OS in patients with subtotal resection (STR) compared to biopsy (100 vs. 42%, p = 0.02) [58].
Results for EOR specifically for LG histology were reported in 13 studies, 6 were classified as category A [5, 9, 15, 28, 34, 59], 7 as category B [11, 20, 26, 27, 51, 52, 61] (Table 13, upper part). Only one category A study found significantly increased 10-year PFS for patients receiving GTR or STR when compared to partial resection (PR) defined as less than 80% tumor resection, although no such difference was found when comparing GTR with STR [9]. Also, one category B study reported significantly better 10-year OS in patients receiving GTR or PR when compared to biopsy only [51]. In addition, in the study of Epstein (category A), where all 17 patients received GTR, no recurrence was observed during a mean follow-up of 50 months [15].
Results for EOR specifically for HG histology were reported in nine studies, four were classified as category A [5, 15, 34, 46], five as category B [11, 27, 51, 52, 65] (Table 13, lower part). Only one category A study reported increased 4-year OS in anaplastic astrocytoma (grade III) patients [46]; those receiving GTR had significantly improved survival (78% at 5 years) when compared to patients with STR (38% at 5 years, p = 0.028). Likewise, no patient with completely resected tumor developed disseminated disease as compared with nine patients (60%) of those receiving STR (p = 0.01). However, after adjusting for multiple comparisons, the difference in surgery extent only trended toward significance (p < 0.007). Regarding residual tumor volume, the authors reported that increase in each 10% of tumor residual is associated with a 40% increased risk of mortality. Remarkably, radical resection of anaplastic astrocytoma was not associated with increase in postoperative decline in neurological function [46]. In addition, one category B study reported increased 10-year OS in patients receiving GTR or PR when compared to biopsy (80 vs. 32%, p = 0.0251) [51].
Radiotherapy
Results for RT regardless of histology were reported in five studies [2, 5, 18, 56, 64], all but one [18] were classified as category A (Table 14, upper part). Statistical analysis comparing irradiated and non-irradiated patients was performed in the four category A studies. No significant relationship was identified in any of them.
Results for RT specifically for LG histology were reported in 16 studies, 9 were classified as category A [2, 5, 10, 28, 34, 49, 59, 60, 66], 7 as category B [25, 27, 37, 42, 47, 51, 54] (Table 14, middle part). Six category A and one category B study reported statistical analysis comparing irradiated and non-irradiated patients, only one (category A) study found statistical significance. Abdel-Wahab et al. [2] found the addition of RT to surgery to significantly reduce adjusted hazard ratio (0.24, p = 0.02) in a multivariate model when compared to surgery alone. This difference was significant only for 15-year PFS and not 15-year OS.
Results for RT specifically for HG histology were reported in 12 studies, 7 were classified as category A [2, 5, 7, 10, 49, 60, 66], 5 as category B [27, 36, 47, 51, 65] (Table 14, lower part). Altogether, five studies (3 category A, 2 category B) reported statistical analysis comparing radiated and non-irradiated patients; only one (category A) found statistically significant advantage in survival for irradiated patients with non-pilocytic astrocytoma [49].
Results for RT specified for EOR were reported in six studies, three were classified as category A [5, 24, 30], three as category B [20, 25, 42] (Table 15). Two studies (one category each) reported comparison for radiated and non-irradiated patients. Guidetti et al. [20] (category B) did not find any relationship. Bouffet (category A) found the addition of RT to be associated with decreased 10-year OS for patients receiving GTR or STR (39 vs. 100%, p = 0.02). However, no such relationship was found for irradiated/non-irradiated patients receiving PR or biopsy [5].
Dose response
Dose–response relationship was analyzed in 11 studies, 7 were classified as category A [2, 24, 30, 34, 49, 60, 64], 4 as category B [18, 38, 42, 47] (Table 16). Only the study of Garcia et al. (category B) found decreased recurrence rates in patients treated with more than 40 Gy (2 recurrences out of 10) in comparison to patients treated with lesser dose (5 recurrences in 5 patients). Although no formal statistical analysis was performed, the authors recommend dose greater than 45 Gy for radiotherapy of intramedullary astrocytoma [18]. The other studies did not report any statistical significant dose–response relationship.
Discussion
We are forced to admit that the ultimate goal of defining an optimal therapeutic strategy for intramedullary astrocytoma was not answered by our literature review. Natural history of intramedullary astrocytoma is believed to be slowly progressive, although no studies to support this have been conducted. Contemporary treatment of intramedullary astrocytoma starts with surgery which plays several crucial roles. Representative histological sample is obtained allowing a detailed analysis (including genetical tumor characteristics in the near future). Tumor debulking relieves possible mass effect on the surrounding spinal cord tissue allowing functional recovery [8, 9, 15, 28, 34]. Additional dura- and laminoplasty provide space for possible subsequent tumor growth. Smaller residual tumor is present for adjuvant oncological treatment. Intuitively, it seems reasonable to believe that more extensive resection would bring about extended survival; however, more radical resection must not be achieved at the cost of neurological function. Since histological studies have shown the presence of normal neurons within the tumor itself, the possibility of radical resection in infiltratively growing intrinsic tumors is at best dubious [15]. A parallel situation exists in intracranial gliomas, where more or less well-conducted trials have shown that prolonging life expectancy with more radical resection is an achievable goal [63]. On the other hand, intracranial gliomas need not be surrounded by such an eloquent tissue as those in spinal cord, where the concentration of functionally important tracts and cells is much higher per unit of tissue. Thus, no “safety margin” of non-eloquent tissue is available. Decreased functional status as a tax for more extensive resection is associated with decreased quality of life, and even shorter survival [27, 34, 41]. In such context, the advantage gained by more extensive tumor removal is lost. Radical resection can be safely pursued when the tumor shows clearly delineated margin. Further safety from intraoperative neurological injury is brought by the addition of neurophysiological monitoring into the surgical armamentarium [39, 75]. More extensive resection was not associated with neurological decline after surgery [9, 11, 34, 46] (Table 17), preoperative neurological function was the most important parameter for favorable functional outcome [6, 8, 9, 11, 13–15, 26, 41, 51, 52, 57, 62].
Another problem encountered with surgery is the assessment of EOR and definition of GTR. Obviously, pre-MRI studies had to rely on intraoperative impression, sometimes supplemented by ultrasound. Nowadays, only “clean” early postoperative MRI scan should be the standard of GTR assessment, as it is in intracranial glioma surgery. Intraoperative MRI may be the promise of near future in centers with such capability.
One reason why few studies support the advantage of GTR is small number of patients within this treatment group. For statistical purposes, these patients are frequently grouped together with those undergoing less extensive resection (STR or PR) [5, 9, 22, 30, 34, 49, 51, 52, 59] to increase sample size and statistical power. Clearly, treatment efficacy of GTR cannot be detected this way, although inferior results of less extensive resection or biopsy may be proven [51]. Similarly, combining patients with less extensive resection together may overestimate the importance of GTR [2], because the non-radical cohort contains a wide spectrum of surgical results [63]. Obviously, large cohorts of patients where postoperative residual tumor volume would be assessed by MRI may answer this question.
The addition of radiotherapy to the treatment protocol of LG astrocytoma is controversial. Its application can be rationalized because the predominant pattern of failure is local [60]. A dose–response relationship was identified for patients receiving more than 40 Gy [18], whereas no significant advantage was demonstrated for patients receiving more than 50 Gy (Table 16) [2, 24, 30, 49]. Radiotherapy after non-radical resection in intracranial astrocytomas was shown to prolong PFS, although not OS [32, 70]. Progression of an intramedullary tumor is associated with clinical deterioration and decreased quality of life, thus prolonging disease-free interval is an appealing endeavor. On the other hand, LG astrocytomas are slow growing tumors and radiotherapy may not be as effective on tumor cells which are currently not undergoing mitosis [26]. Furthermore, radiation tolerance of spinal cord is limited, particularly in the presence of an intrinsic tumor, which makes the tissue more vulnerable to injury [35, 44, 71]. Long-term survivors treated with mere biopsy and external decompression were reported in some studies [20, 27, 56]. Following radical resection, reserving radiotherapy for recurrent disease may be a reasonable option [15, 28]. Withholding radiation for infants where spinal cord and skeleton are still developing is also necessary [9]. Long-term survivors after radiotherapy may also be exposed to increased risk of development of a second malignant tumor; 13% risk at 20 years as reported [54]. Some studies also reported decreased survival rates for irradiated patients [56, 64]; referral bias for patients in poorer condition to RT may play a role. Currently, there is no sufficient data in the literature to soundly support or discourage radiotherapy in LG intramedullary astrocytomas and resolve this dilemma.
Adjuvant radiotherapy in HG tumors could be viewed as a necessity in the setting of a known highly malignant and progressive tumor, where the treating physician is trying to extend survival by all possible and available means. Best example is patients treated with radiocordectomy, in which case spinal cord function is sacrificed for extended survival [7, 66]. Although some studies of HG astrocytomas did not find benefit for radiotherapy [2, 5, 51, 65], this can be explained by the aggressive nature of HG astrocytoma rather than as a lack of treatment efficacy.
Chemotherapy in the treatment of intramedullary astrocytoma is not widely used. Few case series reported anecdotal long-term survivors [3, 12, 43, 53, 73]. Chemotherapy was used as a part of a complex oncological treatment regimen after surgery and radiotherapy, not infrequently for recurrent tumor. In the setting of extensive or unresectable tumor, chemotherapy can be considered as a first line of treatment and may avoid or delay the use of radiotherapy for young children and infants [12]. For HG astrocytomas, additional chemotherapy did not lead to extended survival [46, 65]. Hopefully, new chemotherapeutical agents will show more promise in the future.
Despite the wealth of literature concerning IMSCTs, only a small portion reports prognostic factors, while the majority of studies are concerned with functional outcome following more or less radical surgery. Among the factors studied, some findings need to be addressed or emphasized.
As expected, higher tumor grade was consistently reported to be associated with poorer prognosis in nearly all studies reviewed. Likewise, short history length correlated with decreased survival in some studies [5, 27, 49, 60], finding best explained by short history in HG tumors [7]. McGirt [46] also found patients with grade III tumors to have longer symptom duration than those with grade IV tumors. Quite the opposite situation can be found in children presenting with spinal deformities. These take considerable time to develop, with symptoms spanning several years, suggesting slow and indolent tumor growth. Since 10-year survival was reported to be 87% in these patients, aggressive and risky management may not be justified in this patient subgroup [5].
Tumor location in the thoracic spinal cord [49] was associated with increased survival when compared to cervical location [51]. Tumors growing from the thoracic spinal cord take longer time to reach respiratory center leading to respiratory failure (common cause of death among intramedullary astrocytoma patients) than tumors initially located in the cervical spinal cord [51]. On the other hand, thoracic spinal cord has been reported to be more susceptible to radiation damage [40, 55], which may be a cause of considerable morbidity in long-term survivors.
On the first sight, controversial results were found for the prognostic importance of age. Minehan et al. reported age over 20 years at diagnosis to be associated with increased survival contrary to other studies (Table 4). The authors also report better survival for patients with pilocytic astrocytoma when compared to diffuse fibrillary astrocytoma or astrocytoma not otherwise specified. However, only 5 of 14 patients (36%) younger than 20 years had pilocytic astrocytoma, whereas 38 of 65 older patients (58%) had this histological finding. The difference in survival may then be influenced by the greater proportion of more favorable histology among older patients [49].
The French multicenter review of pediatric patients also reported adverse effect of age over 7 years when compared to younger patients. The study did not report histological composition or specified therapy within each age group which could have possibly introduced bias [5]. Other studies that did not find age to be of importance did not report separate results for pediatric and adolescent patients [24, 27, 30] owing to small numbers of patients in this age group. Instead, these patients were compared to adults. This comparison obviously cannot detect subtle difference within the youngest group, particularly when sample is small. In addition, pediatric male patients also had significantly better 10-year survival than females in the French study [5], contrary to others [24, 30] where adult female patients fared better. Other authors also reported long-term survival in children with HG tumors (over 10 years) [5, 9, 46, 48, 54, 56] which is rather unusual among adult patients [7, 15, 27, 30, 34, 35, 46, 49, 51, 65]. Thus, it may be possible that pediatric astrocytomas are biologically diverse and the large French study [5] reflected this phenomenon.
Our search strategy and subsequent exclusions yielded 38 articles for detailed study and analysis. Limiting our search to articles published in English inadvertently lead to exclusion of some studies published in other languages. However, we do not believe that these excluded studies would shed any more light on the controversial topic of intramedullary astrocytoma. If a fundamentally important article was missed in the initial search, it would surely be encountered in the reference lists of the reviewed papers and as such would be cited extensively. In fact, articles not found on Medline were discovered by this strategy [19, 48, 72] and neither proved to substantially influence the resulting review. Any newly published article is today most likely to appear on Medline, we used the “What’s new” function to ascertain that no such article [46] escaped our attention. The classic work of Slooff et al. [68], although thoroughly studied, was not included in the review, since most of the patients were treated in the first half of previous century, not reflecting modern management methods. This was also the reason why we chose to concentrate on articles published after 1980. In addition, statistical analysis before this date was not as elaborate, and rarely performed, as it is in the recent articles. Furthermore, our intent was to reflect contemporary management of intramedullary astrocytoma, just as did others in intracranial glioma surgery [63]. Performing a historical comparison would be beyond the scope of this article and of limited value to present clinical practice anyway.
Other problem encountered was populations overlapping among numerous articles. For example, we included eight papers from New York University Medical Center or Beth Israel Institute for Neurology and Neurosurgery reporting patients treated by F.J. Epstein and his group [7–10, 15, 19, 46]. Study periods in all of the papers were different and overlapping, numerous patients had to be included in more than one article. Similar situation came around with University of Rome [20, 26, 27, 57] and Massachusetts General Hospital [37, 38]. We were able to ascertain that all patients reported by Abdel-Wahab et al. [1] were included in the follow-up enlarged study [2], allowing us to concentrate on the more recent study only (Abdel-Wahab, personal communication). The overlapping articles were also the reason why we chose not to perform a systematic meta-analysis, patient double (or perhaps multiple) counting would be unavoidable. Another problem was the inclusion of IMSCTs studies, astrocytoma patients were in many instances not reported as a separate group. Whenever possible, we included these papers, because the reported results were deemed important. The alternative would be inclusion of astrocytoma studies only, such strategy would inadvertently lead to limiting the number of studied articles by half. Our suggestion for future articles dealing with IMSCTs is to report survival and recurrence rates separately for each histological diagnosis and grade, which is of utmost importance for this outcome measure. Surgical results with regard to resection extent, morbidity–mortality and long-term functional outcome may be reported across the whole histological spectrum of all IMSCTs. Surgical technique would be better reflected and comparison across histology is valuable in the context of expected postoperative deterioration and long-term functional outcome (Table 17).
The presented review can only be as good as the studies that form its basis. We have been unable to identify a study with prospective data collection. Moreover, patient exclusion because of incomplete chart review was not infrequent. Such an approach is obviously prone to bias. Thus, the best available level of evidence derived from the presented review is level III. The above statement is not meant as a critique of the reviewed papers, rather it is a reflection of the rarity of intramedullary astrocytoma where series usually span decades and treatment strategies and surgical techniques evolve making comparison in patient subgroups particularly difficult and subject to bias. We have thus adopted and slightly modified (to better reflect nature of intramedullary astrocytoma) previously successfully used methodology [74] to further assess the quality of the reviewed articles. The usually applied levels of evidence would not allow for more detailed discrimination. This approach is not without its failing. The division between category A and B studies was chosen arbitrary at the middle of the scale. We could have chosen more “severe” limit for category A studies (e.g. 20), this would limit the number of articles in this category to 7. To control for this, we summarize major conclusions drawn from these “top” articles in Table 18. Of note, of these seven articles, five dealt with homogenous population—LG or HG tumors, pediatric or infant patients only. Such homogenous population was subject of study in 17 articles, 11 classified as category A (52%) and 6 classified as category B (35%), although this difference was not statistically significant (p = 0.36, Fisher exact test).
In order to obtain a larger cohort of patients, the obvious answer is a multicenter cooperation which would ideally, but unlikely, result into a randomized controlled trial. This could evaluate, e.g., the addition of radiotherapy after appropriate patient stratification with regard to resection extent. Results of such a trial would not be available for years (maybe decades) and until that time we have to counsel and treat our patients according to the best knowledge available. An interim solution could be the creation of an international registry of intramedullary astrocytic tumors, where patient and tumor characteristics, surgical complications, resection extent based on MRI, central histology review and survival would be reported in a standardized fashion.
Conclusion
Successful treatment of intramedullary astrocytoma remains a formidable and elusive task. Although patients with LG tumors may enjoy long years without disease progression, recurrence and tumor progression are almost unavoidable. Maximal safe resection as guided by intraoperative neurophysiological monitoring helps to prolong disease-free interval but must not be achieved at the cost of neurological function. Withholding adjuvant radiotherapy after MRI-confirmed radical resection is a reasonable option. Radiotherapy is likely to prolong disease-free interval after non-radical resection; however, there is insufficient data in the literature to further clarify its role in the treatment of LG intramedullary astrocytoma.
Treatment of HG intramedullary astrocytomas is unsatisfactory. More extensive resection possibly delays disease progression. Adjuvant oncological therapy fails to control this aggressive tumor, with the possible exception of a subset of pediatric patients. Since the biological nature of spinal cord HG glioma is identical to those of the brain, it would probably be sensible to implement the same treatment algorithm—maximal safe resection followed by concomitant radiotherapy and chemotherapy.
Although many prognostic factors are reported in the literature, the one and only truly important is tumor grade.
Abbreviations
- IMSCT:
-
Intramedullary spinal cord tumor
- LG:
-
Low grade
- IMG:
-
Intermediate grade
- HG:
-
High grade
- WHO:
-
World Health Organization
- GTR:
-
Gross total resection
- STR:
-
Subtotal resection
- PR:
-
Partial resection
- Bio:
-
Biopsy
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- CSS:
-
Cause-specific survival
- LC:
-
Local control
- EOR:
-
Extent of resection
- RT:
-
Radiotherapy
- CHT:
-
Chemotherapy
- MRI:
-
Magnetic resonance imaging
- FU:
-
Follow-up
- MM:
-
Morbidity and mortality
- NR:
-
Not reported
- NS:
-
Not significant
- KPS:
-
Karnofsky Performance Status
- HR:
-
Hazard ratio
- aHR:
-
Adjusted hazard ratio
References
Abdel-Wahab M, Corn B, Wolfson A, Raub W, Gaspar LE, Curran W Jr, Bustillo P, Rubinton P, Markoe A (1999) Prognostic factors and survival in patients with spinal cord gliomas after radiation therapy. Am J Clin Oncol 22:344–351. doi:10.1097/00000421-199908000-00004
Abdel-Wahab M, Etuk B, Palermo J, Shirato H, Kresl J, Yapicier O, Walker G, Scheithauer BW, Shaw E, Lee C, Curran W, Thomas T, Markoe A (2006) Spinal cord gliomas: a multi-institutional retrospective analysis. Int J Radiat Oncol Biol Phys 64:1060–1071. doi:10.1016/j.ijrobp.2005.09.038
Allen JC, Aviner S, Yates AJ, Boyett JM, Cherlow JM, Turski PA, Epstein F, Finlay JL (1998) Treatment of high-grade spinal cord astrocytoma of childhood with “8-in-1” chemotherapy and radiotherapy: a pilot study of CCG-945. Children’s Cancer Group. J Neurosurg 88:215–220
Barker DJ, Weller RO, Garfield JS (1976) Epidemiology of primary tumours of the brain and spinal cord: a regional survey in southern England. J Neurol Neurosurg Psychiatry 39:290–296. doi:10.1136/jnnp.39.3.290
Bouffet E, Pierre-Kahn A, Marchal JC, Jouvet A, Kalifa C, Choux M, Dhellemmes P, Guerin J, Tremoulet M, Mottolese C (1998) Prognostic factors in pediatric spinal cord astrocytoma. Cancer 83:2391–2399. doi:10.1002/(SICI)1097-0142(19981201)83:11<2391::AID-CNCR20>3.0.CO;2-0
Brotchi J, Dewitte O, Levivier M, Baleriaux D, Vandesteene A, Raftopoulos C, Flament-Durand J, Noterman J (1991) A survey of 65 tumors within the spinal cord: surgical results and the importance of preoperative magnetic resonance imaging. Neurosurgery 29:651–656, discussion 656–657. doi:10.1097/00006123-199111000-00002
Cohen AR, Wisoff JH, Allen JC, Epstein F (1989) Malignant astrocytomas of the spinal cord. J Neurosurg 70:50–54
Constantini S, Houten J, Miller DC, Freed D, Ozek MM, Rorke LB, Allen JC, Epstein FJ (1996) Intramedullary spinal cord tumors in children under the age of 3 years. J Neurosurg 85:1036–1043
Constantini S, Miller DC, Allen JC, Rorke LB, Freed D, Epstein FJ (2000) Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg 93:183–193
Cooper PR (1989) Outcome after operative treatment of intramedullary spinal cord tumors in adults: intermediate and long-term results in 51 patients. Neurosurgery 25:855–859. doi:10.1097/00006123-198912000-00001
Cristante L, Herrmann HD (1994) Surgical management of intramedullary spinal cord tumors: functional outcome and sources of morbidity. Neurosurgery 35:69–74. doi:10.1097/00006123-199407000-00011, discussion 74–66
Doireau V, Grill J, Zerah M, Lellouch-Tubiana A, Couanet D, Chastagner P, Marchal JC, Grignon Y, Chouffai Z, Kalifa C (1999) Chemotherapy for unresectable and recurrent intramedullary glial tumours in children. Brain Tumours Subcommittee of the French Society of Paediatric Oncology (SFOP). Br J Cancer 81:835–840. doi:10.1038/sj.bjc.6690772
Epstein F (1986) Spinal cord astrocytomas of childhood. Adv Tech Stand Neurosurg 13:135–169
Epstein F, Epstein N (1982) Surgical treatment of spinal cord astrocytomas of childhood. A series of 19 patients. J Neurosurg 57:685–689
Epstein FJ, Farmer JP, Freed D (1992) Adult intramedullary astrocytomas of the spinal cord. J Neurosurg 77:355–359
Epstein FJ, Farmer JP, Freed D (1993) Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg 79:204–209
Farwell JR, Dohrmann GJ (1977) Intraspinal neoplasms in children. Paraplegia 15:262–273
Garcia DM (1985) Primary spinal cord tumors treated with surgery and postoperative irradiation. Int J Radiat Oncol Biol Phys 11:1933–1939
Goh KY, Velasquez L, Epstein FJ (1997) Pediatric intramedullary spinal cord tumors: is surgery alone enough? Pediatr Neurosurg 27:34–39. doi:10.1159/000121222
Guidetti B, Mercuri S, Vagnozzi R (1981) Long-term results of the surgical treatment of 129 intramedullary spinal gliomas. J Neurosurg 54:323–330
Hanggi D, Steiger HJ (2008) Spontaneous intracerebral haemorrhage in adults: a literature overview. Acta Neurochir (Wien) 150:371–379. doi:10.1007/s00701-007-1484-7, discussion 379
Hardison HH, Packer RJ, Rorke LB, Schut L, Sutton LN, Bruce DA (1987) Outcome of children with primary intramedullary spinal cord tumors. Childs Nerv Syst 3:89–92. doi:10.1007/BF00271131
Helseth A, Mork SJ (1989) Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg 71:842–845
Huddart R, Traish D, Ashley S, Moore A, Brada M (1993) Management of spinal astrocytoma with conservative surgery and radiotherapy. Br J Neurosurg 7:473–481. doi:10.3109/02688699308995069
Hulshof MC, Menten J, Dito JJ, Dreissen JJ, van den Bergh R, Gonzalez D (1993) Treatment results in primary intraspinal gliomas. Radiother Oncol 29:294–300. doi:10.1016/0167-8140(93)90147-Z
Innocenzi G, Raco A, Cantore G, Raimondi AJ (1996) Intramedullary astrocytomas and ependymomas in the pediatric age group: a retrospective study. Childs Nerv Syst 12:776–780. doi:10.1007/BF00261597
Innocenzi G, Salvati M, Cervoni L, Delfini R, Cantore G (1997) Prognostic factors in intramedullary astrocytomas. Clin Neurol Neurosurg 99:1–5. doi:10.1016/S0303-8467(96)00555-0
Jallo GI, Danish S, Velasquez L, Epstein F (2001) Intramedullary low-grade astrocytomas: long-term outcome following radical surgery. J Neurooncol 53:61–66. doi:10.1023/A:1011886516506
Johnson DL, Schwarz S (1987) Intracranial metastases from malignant spinal-cord astrocytoma. Case report. J Neurosurg 66:621–625
Jyothirmayi R, Madhavan J, Nair MK, Rajan B (1997) Conservative surgery and radiotherapy in the treatment of spinal cord astrocytoma. J Neurooncol 33:205–211. doi:10.1023/A:1005758313700
Kalichman L, Hunter DJ (2008) Diagnosis and conservative management of degenerative lumbar spondylolisthesis. Eur Spine J 17:327–335. doi:10.1007/s00586-007-0543-3
Karim AB, Afra D, Cornu P, Bleehan N, Schraub S, De Witte O, Darcel F, Stenning S, Pierart M, Van Glabbeke M (2002) Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis. Int J Radiat Oncol Biol Phys 52:316–324. doi:10.1016/S0360-3016(01)02692-X
Karnofsky D, Abelman W, Craver L (1948) The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer 1:634–656. doi:10.1002/1097-0142(194811)1:4<634::AID-CNCR2820010410>3.0.CO;2-L
Kim MS, Chung CK, Choe G, Kim IH, Kim HJ (2001) Intramedullary spinal cord astrocytoma in adults: postoperative outcome. J Neurooncol 52:85–94. doi:10.1023/A:1010680924975
Kopelson G (1982) Radiation tolerance of the spinal cord previously-damaged by tumor and operation: long term neurological improvement and time-dose-volume relationships after irradiation of intraspinal gliomas. Int J Radiat Oncol Biol Phys 8:925–929
Kopelson G, Linggood R (1982) Infratentorial glioblastoma: the role of neuraxis irradiation. Int J Radiat Oncol Biol Phys 8:999–1003
Kopelson G, Linggood RM (1982) Intramedullary spinal cord astrocytoma versus glioblastoma: the prognostic importance of histologic grade. Cancer 50:732–735. doi:10.1002/1097-0142(19820815)50:4<732::AID-CNCR2820500418>3.0.CO;2-0
Kopelson G, Linggood RM, Kleinman GM, Doucette J, Wang CC (1980) Management of intramedullary spinal cord tumors. Radiology 135:473–479
Kothbauer K, Deletis V, Epstein FJ (1997) Intraoperative spinal cord monitoring for intramedullary surgery: an essential adjunct. Pediatr Neurosurg 26:247–254. doi:10.1159/000121199
Lambert PM (1978) Radiation myelopathy of the thoracic spinal cord in long term survivors treated with radical radiotherapy using conventional fractionation. Cancer 41:1751–1760. doi:10.1002/1097-0142(197805)41:5<1751::AID-CNCR2820410516>3.0.CO;2-N
Lee HK, Chang EL, Fuller GN, Aldape KD, Atkinson GJ, Levy LB, McCutcheon IE, Maor MH (2003) The prognostic value of neurologic function in astrocytic spinal cord glioma. Neuro-oncology 5:208–213. doi:10.1215/S1152851702000595
Linstadt DE, Wara WM, Leibel SA, Gutin PH, Wilson CB, Sheline GE (1989) Postoperative radiotherapy of primary spinal cord tumors. Int J Radiat Oncol Biol Phys 16:1397–1403
Lowis SP, Pizer BL, Coakham H, Nelson RJ, Bouffet E (1998) Chemotherapy for spinal cord astrocytoma: can natural history be modified? Childs Nerv Syst 14:317–321. doi:10.1007/s003810050233
Marcus RB Jr, Million RR (1990) The incidence of myelitis after irradiation of the cervical spinal cord. Int J Radiat Oncol Biol Phys 19:3–8
McCormick PC, Torres R, Post KD, Stein BM (1990) Intramedullary ependymoma of the spinal cord. J Neurosurg 72:523–532
McGirt MJ, Goldstein IM, Chaichana KL, Tobias ME, Kothbauer KF, Jallo GI (2008) Extent of surgical resection of malignant astrocytomas of the spinal cord: outcome analysis of 35 patients. Neurosurgery 63:55–61. doi:10.1227/01.NEU.0000335070.37943.09
McLaughlin MP, Buatti JM, Marcus RB Jr, Maria BL, Mickle PJ, Kedar A (1998) Outcome after radiotherapy of primary spinal cord glial tumors. Radiat Oncol Invest 6:276–280. doi:10.1002/(SICI)1520-6823(1998)6:6<276::AID-ROI5>3.0.CO;2-6
Merchant TE, Nguyen D, Thompson SJ, Reardon DA, Kun LE, Sanford RA (1999) High-grade pediatric spinal cord tumors. Pediatr Neurosurg 30:1–5. doi:10.1159/000028751
Minehan KJ, Shaw EG, Scheithauer BW, Davis DL, Onofrio BM (1995) Spinal cord astrocytoma: pathological and treatment considerations. J Neurosurg 83:590–595
Nadkarni TD, Rekate HL (1999) Pediatric intramedullary spinal cord tumors. Critical review of the literature. Childs Nerv Syst 15:17–28. doi:10.1007/s003810050321
Nakamura M, Chiba K, Ishii K, Ogawa Y, Takaishi H, Matsumoto M, Toyama Y (2006) Surgical outcomes of spinal cord astrocytomas. Spinal Cord 44:740–745. doi:10.1038/sj.sc.3101932
Nakamura M, Ishii K, Watanabe K, Tsuji T, Takaishi H, Matsumoto M, Toyama Y, Chiba K (2008) Surgical treatment of intramedullary spinal cord tumors: prognosis and complications. Spinal Cord 46:282–286. doi:10.1038/sj.sc.3102130
Nishio S, Morioka T, Fujii K, Inamura T, Fukui M (2000) Spinal cord gliomas: management and outcome with reference to adjuvant therapy. J Clin Neurosci 7:20–23. doi:10.1054/jocn.1999.0128
O’Sullivan C, Jenkin RD, Doherty MA, Hoffman HJ, Greenberg ML (1994) Spinal cord tumors in children: long-term results of combined surgical and radiation treatment. J Neurosurg 81:507–512
Phillips TL, Buschke F (1969) Radiation tolerance of the thoracic spinal cord. Am J Roentgenol 105:659–665
Przybylski GJ, Albright AL, Martinez AJ (1997) Spinal cord astrocytomas: long-term results comparing treatments in children. Childs Nerv Syst 13:375–382. doi:10.1007/s003810050103
Raco A, Esposito V, Lenzi J, Piccirilli M, Delfini R, Cantore G (2005) Long-term follow-up of intramedullary spinal cord tumors: a series of 202 cases. Neurosurgery 56:972–981, discussion 972–981
Reimer R, Onofrio BM (1985) Astrocytomas of the spinal cord in children and adolescents. J Neurosurg 63:669–675
Robinson CG, Prayson RA, Hahn JF, Kalfas IH, Whitfield MD, Lee SY, Suh JH (2005) Long-term survival and functional status of patients with low-grade astrocytoma of spinal cord. Int J Radiat Oncol Biol Phys 63:91–100. doi:10.1016/j.ijrobp.2005.01.009
Rodrigues GB, Waldron JN, Wong CS, Laperriere NJ (2000) A retrospective analysis of 52 cases of spinal cord glioma managed with radiation therapy. Int J Radiat Oncol Biol Phys 48:837–842. doi:10.1016/S0360-3016(00)00690-8
Rossitch E Jr, Zeidman SM, Burger PC, Curnes JT, Harsh C, Anscher M, Oakes WJ (1990) Clinical and pathological analysis of spinal cord astrocytomas in children. Neurosurgery 27:193–196. doi:10.1097/00006123-199008000-00003
Samii M, Klekamp J (1994) Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery 35:865–873. doi:10.1097/00006123-199411000-00010, discussion 873
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62:753–766. doi:10.1227/01.neu.0000318159.21731.cf
Sandler HM, Papadopoulos SM, Thornton AF Jr, Ross DA (1992) Spinal cord astrocytomas: results of therapy. Neurosurgery 30:490–493. doi:10.1097/00006123-199204000-00003
Santi M, Mena H, Wong K, Koeller K, Olsen C, Rushing EJ (2003) Spinal cord malignant astrocytomas. Clinicopathologic features in 36 cases. Cancer 98:554–561. doi:10.1002/cncr.11514
Shirato H, Kamada T, Hida K, Koyanagi I, Iwasaki Y, Miyasaka K, Abe H (1995) The role of radiotherapy in the management of spinal cord glioma. Int J Radiat Oncol Biol Phys 33:323–328. doi:10.1016/0360-3016(95)00179-3
Shrivastava RK, Epstein FJ, Perin NI, Post KD, Jallo GI (2005) Intramedullary spinal cord tumors in patients older than 50 years of age: management and outcome analysis. J Neurosurg Spine 2:249–255
Slooff JL, Kernohan JW, MacCarty CS (1964) Primary intramedullary tumors of the spinal cord and filum terminale. WB Saunders Company, Philadelphia
Stein BM, McCormick PC (1992) Intramedullary neoplasms and vascular malformations. Clin Neurosurg 39:361–387
van den Bent MJ, Afra D, de Witte O, Ben Hassel M, Schraub S, Hoang-Xuan K, Malmstrom PO, Collette L, Pierart M, Mirimanoff R, Karim AB (2005) Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 366:985–990. doi:10.1016/S0140-6736(05)67070-5
Wara WM, Phillips TL, Sheline GE, Schwade JG (1975) Radiation tolerance of the spinal cord. Cancer 35:1558–1562. doi:10.1002/1097-0142(197506)35:6<1558::AID-CNCR2820350613>3.0.CO;2-7
Weiner HL, Freed D, Woo H, Rezai AR, Kim R, Epstein FJ (1997) Intra-axial tumors of the cervicomedullary junction: surgical results and long-term outcome. Pediatr Neurosurg 27:12–18. doi:10.1159/000121219
Weiss E, Klingebiel T, Kortmann RD, Hess CF, Bamberg M (1997) Intraspinal high-grade astrocytoma in a child–rationale for chemotherapy and more intensive radiotherapy? Childs Nerv Syst 13:108–112. doi:10.1007/s003810050055
Zakrzewska JM, Lopez BC (2003) Quality of reporting in evaluations of surgical treatment of trigeminal neuralgia: recommendations for future reports. Neurosurgery 53:110–122. doi:10.1227/01.NEU.0000068862.78930.EE
Zileli M, Coskun E, Ozdamar N, Ovul I, Tuncbay E, Oner K, Oktar N (1996) Surgery of intramedullary spinal cord tumors. Eur Spine J 5:243–250. doi:10.1007/BF00301327
Acknowledgments
The authors would like to express their gratitude to the library service of Regional Hospital Liberec, particulary to E. Machonská and J. Klímová, for their help in obtaining full text versions of numerous articles cited in this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Note added in proof
Concurrently with revision submission (and subsequent acceptance) of this article, the largest series of intramedullary astrocytomas to date appeared on Medline (Minehan KJ, Brown PD, Scheithauer BW, Krauss WE, Wright MP (2009) Prognosis and treatment of spinal cord astrocytoma. Int J Radiat Oncol Biol Phys 73: 727–733). Although the authors are aware of this study, due to this unfortunate timing, this series was not included in the review and readers are kindly asked to turn their attention to it.
Rights and permissions
About this article
Cite this article
Beneš, V., Barsa, P., Beneš, V. et al. Prognostic factors in intramedullary astrocytomas: a literature review. Eur Spine J 18, 1397–1422 (2009). https://doi.org/10.1007/s00586-009-1076-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-009-1076-8