Abstract
The vertebral endplates are critical for maintaining disc function yet like other components of the disc are vulnerable to degeneration. This paper provides an overview of the development and normal function of the endplates as well as an impression of what happens when they undergo progressive degeneration. Recent research suggests that the degenerative process can be retarded or reversed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Manuscript
Despite all that is known from decades of research the intervertebral disc remains an enigma. It is a unique and remarkable entity and perhaps the one aspect that is responsible for much of its mystery is that such a large structure is able to survive and function under the most difficult physiological conditions. The discs of the human spine are the largest non-vascularised structures in the body, and in the largest of them (in the lumbar spine) some cells can be 20 mm from the nearest direct blood supply. Despite their apparent resilience however, the resident cells are not immortal. It is not unusual for discs to show signs of deterioration by the age of 25 years and it is in this regard that the vertebral endplate plays an important role.
Discs are roughly cylindrical structures that vary in size and shape progressively from the cervical to the lumbar region. They all comprise a well-hydrated central nucleus pulposus that is surrounded by the firm but flexible collagenous lamellae of the annulus fibrosus [40]. At the cranial and caudal ends of each disc are the endplates that separate the vertebral bone from the disc itself and prevent the highly hydrated nucleus from bulging into the adjacent vertebrae. The endplates also absorb the considerable hydrostatic pressure that results from mechanical loading of the spine [10]. The endplates are typically less than 1 mm thick, and while this varies considerably across the width of any single disc, they tend to be thinnest in the central region adjacent to the nucleus [16, 44].
The endplates are identifiable from an early embryological stage and have an osseous as well as a hyaline cartilage component [50]. The cartilaginous component appears to generate great interest since it persists throughout normal maturation while the adjacent vertebrae undergo ossification. It comprises a gel of hydrated proteoglycan molecules reinforced by a network of collagen fibrils. Unlike the articular cartilage of the synovial joints the collagen fibrils do not connect the endplate directly to the vertebral bone [22], although the endplate does have intimate contact with the disc through the lamellae of the inner annulus [21]. A network of microscopic blood vessels penetrates the endplates during development of the growing spine, principally to provide nutrition for the disc, before disappearing around the time of skeletal maturity [50]. Apart from a sparse vascular supply in the outer lamellae of the annulus mature discs are almost totally reliant on diffusion of essential solutes across the endplates for nutrition and metabolic exchange [20].
The biochemical composition of the endplates, from normality through the spectrum of degenerative conditions, has been documented extensively [4, 6]. Of the several species of collagen present in the disc Type X is thought to be the most important in the endplate since it is a marker of hypertrophic chondrocytes and is involved with calcification [1]. Further, inactivation of one Collagen II gene allele in young mice has been shown to lead to lower glyscosaminogloycan levels in the endplates and thicker, more irregular endplates that become calcified prematurely [47].
Proteoglycan molecules within the matrix are critical for the control of solute transport and maintenance of water content in particular throughout the disc, and depletion of proteoglycans from the endplate cartilage is associated with loss of proteoglycans from the nucleus [45]. It follows therefore that proteoglycan loss would ultimately lead to degeneration of the disc [37]. Alterations in disc biochemistry, particularly in the endplate, during the skeletal growth phase also may be involved in the development of scoliosis [3, 38, 43].
Much attention has been focussed on understanding aspects of disc nutrition and the general processes associated with disc metabolism. In vitro studies using small dye molecules have demonstrated that the lateral margins of the endplate near the vertebral rim are relatively impermeable compared with the central portion or the entire annulus [32]. Quantitative studies with human autopsy specimens have shown that the endplate permeability is due to microscopic blood vessels in the central endplate that are more numerous than in the margins of the disc [15, 28]. This vascular network has been demonstrated using simple injection techniques [15] and shows that diffusion of small solutes from these vessels is the principal mechanism for transfer of nutrients into the disc [51, 52]. The process however is selective based entirely on molecular size and ionic charge of the molecules involved. The net negative charge of the nucleus conferred by the high concentration of proteoglycans in the nucleus permits passage of positive ions such as sodium and calcium and uncharged molecules such as glucose and oxygen, while impeding movement of negatively charged ions such as sulphate and chloride and macromolecules such as immunoglobulins and enzymes. The significance of the endplate in the metabolism of the disc has been confirmed by a variety of laboratory techniques [14, 20, 35].
Upon reaching skeletal maturity the cartilage of the endplate undergoes substantial remodelling, resulting in extensive mineralisation which is eventually resorbed and replaced by true bone [8, 34]. Importantly this new tissue most likely impedes the hitherto critical diffusion and nutrient exchange between the vertebral marrow and the disc [42]. The small blood vessels within the endplate likewise become obliterated by this calcification, further limiting the exchange of vital nutrients.
Perhaps surprisingly the endplate can become revascularised after maturity in some species under normal [36] and pathological [30] conditions. In the latter study the revascularisation, presumed to be an attempt at tissue repair, was not able to reverse the inevitable cascade of degeneration caused by annular disruption. The creation of blood vessels in the endplate occurs by activation of the matrix degrading metalloproteinase (MMP) enzymes which are normally maintained in a latent form by tissue inhibitors [13, 18, 23, 41, 57].
Blood flow in the region of the endplates is not entirely passive as there are muscarinic receptors present that can influence disc nutrition under altered physiological conditions [55]. Additional studies have identified nerve fibres and blood vessels in the endplates and subchondral bone in degenerate discs suggesting that tissue repair may be associated with back pain [11, 17].
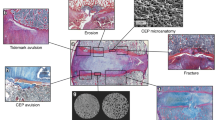
Morphological changes to the endplates are usually seen with advancing age but are also evident in association with pathological changes to the nucleus and annulus in advanced stages of degenerative disc disease [53]. The earliest microscopic changes seen are fissures and clefts along the length of the endplate in the horizontal plane with occasional chondrocyte death. It is not unusual to see invading blood vessels with adjacent bony endplate ossification. Eventually the cartilage is overcome by ossification. If it is still reasonably healthy the nucleus fills the voids created as blood vessels perforate the endplate, although these defects do not breach the bony endplate. By the fifth decade nuclear material is seen to protrude into the vertebral marrow with focal bony sclerosis resulting from the active remodelling. Often the cartilage is completely lost. In an animal model of spondylolysis disc degeneration, including loss of the endplate, was seen and was accompanied by increased apoptosis of endplate chondrocytes, indicating possible involvement of programmed cell death in age-related disc degeneration [5].
Theoretical finite element modelling [33] agrees with detailed microscopic observations [53] that the endplate is susceptible to mechanical failure, almost without exception at the point of attachment to the subchondral bone and presumably due to the poor attachment of the collagen fibrils to the bone as mentioned earlier [22]. Autopsy studies also show that portions of the endplate can become separated from the vertebral body and herniate from the disc along with attached annular fibres [31, 49]. It appears that the point at which the annular fibres insert into the vertebral body in the vicinity of the epiphyseal ring is inherently weak, and seems more than coincidental that this is a common site for fracture in adolescents [7]. Experimental studies with the spines of adolescent pigs have reproduced similar findings after mechanical compression [25]. It should be noted that this injury pattern is quite different from that seen in the adult spine, where the endplate and adjacent trabecular bone are involved [26, 46].
The most common endplate defect observed is probably the Schmorl’s node, which is a vertical protrusion of the contents of the nucleus into the adjacent vertebral body [48]. Schmorl’s nodes are seen in more than 70% of spines at autopsy with equal frequency above and below the age of 50 years, suggesting that they appear relatively early in life [19]. That they should be twice as common in men up to the age of 59 years suggests that they occur as a result of occupational trauma. Curiously however there is a gender switch after 60 years of age and they are twice as common in women! This occurs at a time when the disc is more likely to rupture due to changes, such as osteoporosis, that are generally associated with advanced age. In any event, discs with Schmorls’ nodes are more degenerate than other discs at an early age [54].
Exactly what causes Schmorl’s nodes to form remains a mystery. There seems little doubt that they begin as small defects and are therefore not always seen as often on clinical radiographs as they are at autopsy [39]. They become more apparent radiologically as nuclear prolapse results in reduced disc height and a cartilaginous cap and eventually new bone form around the prolapse. Although most endplates do not show any evidence of natural perforations, Schmorl suggested that these lesions arise from focal weak spots caused by degenerate cartilage [48]. In the absence of direct trauma or destruction resulting from neoplastic involvement the endplates are intact and it is generally assumed that scar tissue that remains after closure of the small vascular channels in the developing spine [12] allows protrusion through these weak spots [27]. It may be significant that specimens with Schmorl’s nodes have significantly more marrow contacts in the endplates, suggesting that these lesions may contribute to additional pathology such as Scheuermann disease, in which they feature prominently.
Although it would be completely erroneous to suggest that disc degeneration per se is the sole cause of back pain, it would nonetheless be naive to ignore the strong correlation that exists between the two entities. As a result of exciting developments in the field of spinal research we are now more aware than ever of the cellular processes that occur in disc degeneration, and as we move into the exciting era of “regenerative medicine” or “biological treatments” there is increasing interest and even an expectation that degenerative diseases can be treated by a “magic bullet”.
The treatments that could potentially be available for regeneration of the endplates in particular are numerous and diverse, and in fact most are being considered in the context of the disc as a whole because of the complex interactions between the individual components of the disc. Such approaches include the use of recombinant proteins, cytokines or growth factors [29], molecular therapy [58], gene transfer techniques [24, 56], cell therapy [2, 9]. These separate topics are so detailed that any attempt to summarise them in a few paragraphs would not do them justice. The reader is referred instead to the comprehensive literature (including the reviews referenced above) that contains many excellent papers on each topic.
Most of these concepts have barely progressed from in vitro testing, and as such are unlikely to have practical clinical application in the near future. This is not a criticism of these works. On the contrary it is a cautious warning that it may be many years before we see the results of appropriately conducted trials that evaluate their clinical efficacy. Realistically these treatments will not completely reverse the degenerative process but they may offer the potential to halt or at least, delay the inevitable consequences. The key to this approach will be to identify appropriate targets, whether they are genes, bioactive molecules, particular cell types or most importantly, the patient. Recipients of these treatments will need to be selected carefully as there is compelling evidence that factors as diverse as genetics, cigarette smoking, occupation and immobilisation, influence disc cell metabolism through endplate diffusion and hence nutrition of cells. It will be equally important to ensure that the cells in the disc survive and function appropriately to obtain the maximum benefit of such treatments.
Disc degeneration is a complex issue that involves myriad factors, of which the endplate is only one example. Careful incremental research is slowly unravelling its mysteries and there is reason to be optimistic that one day there will be treatments available to address the universal problems associated with back pain.
References
Aigner T, Gresk-Otter KR, Fairbank JC et al (1998) Variation with age in the pattern of type X collagen expression in normal and scoliotic human intervertebral discs. CalcifTissue Int 63:263–268
Anderson DG, Risbud MV, Shapiro IM, Vaccaro AR, Albert TJ (2005) Cell-based therapy for disc repair. Spine J 5:297S–303S
Antoniou J, Arlet V, Goswami T et al (2001) Elevated synthetic activity in the convex side of scoliotic intervertebral discs and endplates compared with normal tissues. Spine 26:198–206
Antoniou J, Goudsouzian M, Heathfield TF et al (1996) The human lumbar endplate. Evidence of changes in biosynthesis and denaturation of the extracellular matrix with growth, maturation, aging, and degeneration. Spine 21:1153–1161
Ariga K, Miyamoto S, Nakase T et al (2001) The relationship between apoptosis of endplate chondrocytes and aging and degeneration of the intervertebral disc. Spine 26:2414–2420
Bayliss MT, Johnstone B (1992) Biochemistry of the intervertebral disc. In: Jayson MIV (ed) The lumbar spine and back pain, 4th edn. Edinburgh, Churchill Livingstone, pp 111–131
Beggs I, Addison J (1998) Posterior vertebral rim fractures. Br J Radiol 71:567–572
Bernick S, Caillet R (1982) Vertebral end-plate changes with aging of human vertebrae. Spine 7:97–102
Brisby H, Tao H, Ma DDF, Diwan AD (2004) Cell therapy for disc degeneration—potentials and pitfalls. Orthop Clin North Am 35:1–9
Broberg KB (1983) On the mechanical behaviour of intervertebral discs. Spine 8:151–165
Brown MF, Hukkanen MVJ, McCarthy ID, Redfern DRM, Batten JJ, Crock HV, Hughes SPF, Polak JM (1997) Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg 79:147–153
Chandraraj S, Briggs CA, Opeskin K (1998) Disc herniation in the young and end-plate vascularity. Clin Anat 11:171–176
Crean JK, Roberts S, Jaffray DC et al (1997) Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine 22:2877–2884
Crock HV, Goldwasser M (1984) Anatomic studies of the circulation in the region of the vertebral endplate in adult greyhound dogs. Spine 9:702–706
Crock HV, Yoshizawa H (1976) The blood supply of the lumbar vertebral column. Clin Orthop Rel Res 115:6–21
Edwards WT, Zheng Y, Ferrara LA, Yuan HA (2001) Structural features and thickness of the vertebral cortex in the thoracolumbar spine. Spine 26:218–225
Fagan AB, Moore RJ, Vernon-Roberts B et al (2003) The innervation of the intervertebral disc: a quantitative analysis. Spine 28:2570–2576
Goupille P, Jayson MI, Valat JP, Freemont AJ (1998) Matrix metalloproteinases: the clue to intervertebral disc degeneration?. Spine 23:612–626
Hilton RC, Ball J, Benn RT (1976) Vertebral end-plate lesions (Schmorl’s nodes) in the dorso-lumbar spine. Ann Rheum Dis 35:127–132
Holm S, Maroudas A, Urban JPG et al (1981) Nutrition of the intervertebral disc. Solute transport and metabolism. Connect Tiss Res 8:101–119
Hukins DWL (1988) Disc structure and function. In: Ghosh P (ed) The biology of the intervertebral disc. CRC Press, Boca Raton, pp 1–37
Inoue H (1981) Three-dimensional architecture of lumbar intervertebral discs. Spine 6:139–146
Kang JD, Stefanovic-Racic M, McIntyre LA et al (1997) Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2 and matrix metalloproteinases. Spine 22:1065–1073
Levicoff EA, Gilbertson LG, Kang JD (2005) Gene therapy for disc repair. Spine J 5:287S–296S
Lundin O, Ekstrom L, Hellstrom M et al (1998) Injuries in the adolescent porcine spine exposed to mechanical compression. Spine 23:2574–2579
Lundin O, Ekstrom L, Hellstrom M et al (2000) Exposure of the porcine spine to mechanical compression: differences in injury pattern between adolescents and adults. Eur Spine J 9:466–471
McFadden KD, Taylor JR (1989) End-plate lesions of the lumbar spine. Spine 14:867–469
Maroudas A, Stockwell RA, Nachemson A, Urban J (1975) Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat 120:113–130
Masuda K, An HS (2004) Growth factors and the intervertebral disc. Spine J 4:330S–340S
Moore RJ, Osti OL, Vernon-Roberts B, Fraser RD (1992) Changes in endplate vascularity after an outer anulus tear in the sheep. Spine 17:874–878
Moore RJ, Vernon-Roberts B, Fraser RD et al (1996) The origin and fate of herniated lumbar intervertebral disc tissue. Spine 21:2149–2155
Nachemson A, Lewin T, Maroudas A, Freeman MAR (1970) In vitro diffusion of dye through the endplate and the annulus fibrosus of human intervertebral discs. Acta Orthop Scand 41:589–607
Natarajan RN, Ke JH, Andersson GB (1994) A model to study the disc degeneration process. Spine 19:259–265
Oda J, Tanaka H, Tsuzuki N (1988) Intervertebral disc changes with aging of human cervical vertebra from the neonate to the eighties. Spine 13:1205–1211
Ogata K, Whiteside LA (1981) Nutritional pathways in the intervertebral disc. An experimental study using hydrogen washout technique. Spine 6:211–216
Oki S, Matsuda Y, Shibata T et al (1996) Morphologic differences of the vascular buds in the vertebral endplate—scanning electron microscopic study. Spine 21:174–177
Pearce RH, Grimmer BJ, Adams ME (1987) Degeneration and the chemical composition of the human intervertebral disc. J Orthop Res 5:198–205
Pedrini-Mille A, Pedrini VA, Tudisio C et al (1983) Proteoglycans of human scoliotic intervertebral disc. J Bone Joint Surg 65A:815–823
Pfirrmann CWA, Resnick D (2001) Schmorl nodes of the thoracic and lumbar spine: radiographic–pathologic study of prevalence, characterization, and correlation with degenerative changes of 1,650 spinal levels in 100 cadavers. Radiol 219:368–374
Pooni JS, Hukins DW, Harris PF, Hilton RC, Davies KE (1986) Comparison of the structure of human intervertebral discs in the cervical, thoracic and lumbar regions of the spine. Surg Radiol Anat 8:175–182
Roberts S, Caterson B, Menage J et al (2000) Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine 25:3005–3013
Roberts S, McCall IW, Menage J et al (1997) Does the thickness of the vertebral subchondral bone reflect the composition of the intervertebral disc?. Eur Spine J 6:385–389
Roberts S, Menage J, Eisenstein SM (1993) The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res 11:747–757
Roberts S, Menage J, Urban JP (1989) Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine 14:166–174
Roberts S, Urban JP, Evans H, Eisenstein SM (1996) Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine 21:415–420
Rolander SD, Blair WE (1975) Deformation and fracture of the lumbar vertebral endplates. Orthop Clin North Am 6:75–81
Sahlman J, Inkinen R, Hirvonen T et al (2001) Premature vertebral endplate ossification and mild disc degeneration in mice after inactivation of one allele belonging to the Col2a1 gene for type II collagen. Spine 26:2558–2565
Schmorl G, Junghanns H (1971) The human spine in health and disease, 2nd edn. Grune and Stratton, New York
Tanaka M, Nakahara S, Inoue H (1993) A pathologic study of discs in the elderly. Separation between the cartilaginous endplate and the vertebral body. Spine 18:1456–1462
Taylor JR, Twomey LT (1988) Growth of human intervertebral discs and vertebral bodies. J Anat 120:49–68
Urban JPG, Holm S, Maroudas A (1978) Diffusion of small solutes into the intervertebral disc. An in vivo study. Biorheology 15:203–221
Urban JPG, Holm S, Maroudas A, Nachemson A (1977) Nutrition of the intervertebral disc. An in vivo study of solute transport. Clin Orthop Rel Res 129:101–114
Vernon-Roberts B (1992) Age-related and degenerative pathology of intervertebral discs and apophyseal joints. In: Jayson MIV (ed) The lumbar spine and back pain, 4th edn. Edinburgh, Churchill Livingstone, pp 17–41
Vernon-Roberts B, Pirie CJ (1977) Degenerative changes in the intervertebral discs and their sequelae. Rheum Rehab 16:13–21
Wallace AL, Wyatt BC, McCarthy ID, Hughes SPF (1994) Humoral regulation of blood flow in the vertebral endplate. Spine 19:1324–1328
Wallach CJ, Gilbertson LG, Kang JD (2003) Gene therapy applications for intervertebral disc degeneration. Spine 28:S93–S98
Weiler C, Nerlich AG, Zipperer J et al (2002) SSE Award Competition in Basic Sciences: expression of major matrix metalloproteinases is associated with intervertebral disc degeneration and resorption. Eur Spine J 11:308–320
Yoon TS (2005) Molecular therapy of the intervertebral disc. Spine J 5:280S–286S
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moore, R.J. The vertebral endplate: disc degeneration, disc regeneration. Eur Spine J 15 (Suppl 3), 333–337 (2006). https://doi.org/10.1007/s00586-006-0170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-006-0170-4