Abstract
Spontaneous spinal epidural hematoma (SSEH) is a rare disease entity; its causative factors and the factors determining the outcome are still controversial. We reviewed our clinical experiences and analyzed the various factors related to the outcome for SSEH. We investigated 14 patients (11 men and 3 women) who underwent hematoma removal for SSEH from April 1998 to August 2004. We reviewed age, gender, hypertension, anticoagulant use and the preoperative neurological status using the Japanese Orthopaedics Association score by examining medical records, operative records, pathology reports, and radiographies, retrospectively. We were checking for factors such as the degree of cord compression owing to hematoma and the extent and location of the hematoma. Most patients included in the study were in their twenties or fifties. Four hematoma were located in the cervical region (29%), three were cervicothoracic (21%), four were thoracic (29%) and three were in the lumbar (21%) region and also 12 were located at the dorsal aspect of the spinal cord. In all cases, the neurological outcome improved after the surgical operation. There was a statistically significant difference between the incomplete and complete neurological injury for the preoperative status (P<0.05). The neurological outcome was good in those cases that had their hematoma removed within 24 h (P<0.05). The patients with incomplete neurological injury who had a surgical operation performed within 12 h had an excellent surgical outcome (P<0.01). Spontaneous spinal epidural hematoma was favorably treated by the means of a surgical operation. The favorable factors for SSEH operations were incomplete neurological injury at the time of the preoperative status and the short operative time interval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous spinal epidural hematoma (SSEH) is a rare disease that results in permanent neurological deficit or death when it is not treated properly. A total of 470 cases have been reported world wide since Jackson reported it for the first time in 1869 [14]. This disease entity accounts for 0.3–0.9 % of the epidural space-occupying lesion [9, 12, 26, 36]. Although the cause of disease has not exactly been made clear, most researchers have reported that it is the result of anticoagulant therapy, vascular malformation, venous epidural plexus defects, arterial problems, and inherited or acquired bleeding disorders [1, 3, 5, 7, 9, 12, 16, 33, 37].
Tsai named it as idiopathic spinal hematoma when there is no known etiologic factor and as spontaneous spinal hematoma when there is no bony injury after minor trauma or after normal daily activity [24, 39]. Lonjon defined idiopathic spinal hematoma and secondarily arisen spinal hematoma in the result of coagulopathy or vascular malformation or tumor bleeding [18, 30]. The standard management for SSEH is to evacuate the hematoma and perform spinal cord decompression by an emergency operation [13, 32]. However, some researchers have reported that conservative treatment is also a treatment option [4, 17, 18, 21, 27, 38].
The purpose of this research is to analyze relationship among preoperative neurological status, the operative time interval, and neurological outcome after surgery for SSEH.
Methods and materials
We investigated 14 patients (11 men and 3 women) who underwent the hematoma removal for SSEH from April 1998 to August 2004. The average age of the patients was 42.3 years and the average follow-up period was 25.1 months.
We reviewed medical records, histories, physical examinations, operative records, pathology reports and radiographies retrospectively. We also examined for age, gender, hypertension, anticoagulant use, preoperative neurological status, the degree of cord compression, and the extent and location of the hematoma. We performed spinal MRI studies on all the patients (Table 1).
The patients received laminectomy via posterior approach and hematoma removal in all cases. The hematoma was assessed at the vertebral level on the sagittal MRI image. The degree of cord compression was measured as the maximal diameter of hematoma in relation to the diameter of spinal canal. The location of hematoma was classified as cervical, cervicothoracic, thoracic, thoracolumbar and lumbar regions on the sagittal MRI image. The position of the hematoma was also classified as dorsal, ventral and posterolateral on the axial MRI image.
Only one case was confirmed pathologically as hematoma due to bleeding from a venous malformation and the other cases were confirmed by pathological examination as simple hematoma. The operative time interval was defined as the time from symptom onset to the time of surgery. We excluded the cases of spinal trauma, such as spinal dislocation or fracture, postoperative hematoma after spine operation, and subdural and subarachnoid hematoma.
Neurological function of the patients was assessed by the Japanese Orthopaedics Association (JOA) score before and after the surgical operation.
The neurological outcome was investigated by the recovery scale of JOA score, which was calculated by the following equation [19]:
Correlation of variables was statistically analyzed using regression analysis with SAS Version 8.2. P values <0.05 were considered significant.
Results
Only one patient had acute back pain as the main complaint, and 13 patients had motor weakness that was accompanied with pain.
There were four cases with bleeding tendencies. One patient had been medicated with heparin because of their cerebrovascular accident, one patient had been given coumadin after cardiac surgery, one patient had been given aspirin because of myocardial infarction, and one patient had been taking warfarin after thrombolectomy for deep vein thrombosis. Four patients were diagnosed as hypertension and so they took antihypertensive agents before the surgical operations.
The hematomas of 13 patients invaded more than two vertebral levels, whereas one case was found to have hematoma only at one vertebral level. The extent of hematoma was distributed from one to nine vertebral segments, its average was 3.6 vertebral levels. There were four cervical hematomas, three cervicothoracic hematomas, four thoracic hematomas and three lumbar hematomas, and most of them were dorsal to the spinal cord. That is to say, 12 cases were dorsal hematomas, 1 case was a ventral hematoma and 1 case was a posterolateral hemtoma. The degree of compression by the hematoma was from 35.4% to 80.3%, and the average compression was 58.1%. The hematomas revealed as mixed intensity on the T1 and T2 weighted MRI (Figs. 1, 2). Most hematomas were spindle- or fusiform-shaped. There were some cases that displayed intramedullary edema that was thought to be cord edema.
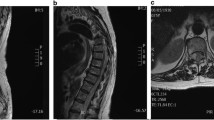
A 21 year-old man suffered from a sudden-onset of posterior neck pain and then he developed paraplegia and anesthesia below T4. a, b On sagittal T1 and T2-weighted images, the hematoma was located on the portion dorsal to the dural sac. It was a spindle-shaped lesion on the cervicothoracic spine (C4-T2). c On the axial T1-weighted image, the hematoma was located on dorsal portion around to dural sac, demonstrating a lentiform-shape, and the dural sac was severely compressed
A 51 year-old man had paraparesis and back pain, and he had been previously treated with warfarin for deep vein thrombosis of the lower extremities. a, b On sagittal T1 and T2-weighted images, the hematoma was located on the portion dorsal to the dural sac on L2–L5, and the lesion revealed with mixed density. c On the axial T1-weighted image, the hematoma was located on dorsal portion to the dural sac and the dural sac was severely compressed
There was no complication related to the surgical operations. In all cases, the recovery scale was improved after surgical operation. The average of the initial JOA (preoperative JOA) was 2.9, but the average of postoperative JOA improved up to a score of 7.9 after surgery. The average recovery scale was 66.1% (18.2–100%). Seven cases had incomplete injury, but another seven cases had complete injury on the preoperative neurological status. The average of the recovery scale in cases of incomplete injury after the operation was 81.7%, and in cases of complete injury the average of the recovery scale was 50.5%. There was a statistically significant difference between the complete and incomplete neurological injury on the preoperative neurological status (P<0.05) (Table 2). Nine cases of 14 cases improved the neurological deficit more than 50% when the scores for before and after surgery were compared.
For the operative time interval, the period from symptom onset to the operation was from 3 h to 312 h, and the average was 60.3 h. There was an inverse correlation between the operative time interval and recovery scale (P<0.05) (Fig. 3).
We performed operation within 24 h for eight cases, and the average of recovery scale for these cases was 80.7%. Seven of these cases improved more than 50% on the recovery scale. There were six cases that we performed operations on that were beyond 24 h, and the average of the recovery scale was 46.7%. Two of them improved more than 50%. Particularly for the operations within 24 h, the scores for the recovery scale of the surgical operation were more obviously improved rather than the operations that were performed after 24 h (P<0.05) (Table 3).
After the symptoms developed, it took an average of 59.6 h for us to begin the operation for the patients with complete injury, and it took 60.9 h for those patients with incomplete injury. There was no statistical difference between complete and incomplete injury patients before surgical operation. Among the patients with incomplete injury (seven cases), there was a statistically significant difference between the patients who were operated on within 12 h (four cases) and those patients (three cases) who were operated on after 12 h (P<0.005). All the incomplete injury patients who were operated within 12 h showed full recovery. None of complete injury patients achieved a full recovery.
However, among the patients with complete injury (seven cases), there was a statistically significant difference between the patients who were operated within 24 h and those who were operated on after 24 h (P<0.05). There was no correlation between postoperative outcome and age, gender, hematoma location, the extent of the hematoma and the degree of cord compression owing to hematoma.
Discussion
Spontaneous spinal epidural hematoma develops only rarely, but it can leave serious sequelae when it does occur.
The causes for spinal epidural hematoma are various and multifactorial. Some reports have asserted that the cause is hypertension or coagulopathy [1, 6, 16, 25]. Beatty and Winston advocated that small disrupted epidural arteries caused SSEH [3]. Cooper and Markham postulated that intra-abdominal or thoracic pressure may be transmitted to the epidural plexus and so cause SSEH [5, 32]. Gundry and Heithoff supposed the idea that a herniated disc tore the fragile epidural veins of Batson and this caused epidural bleeding [15]. Other writers have reported that SSEH is caused by an extradural spinal vascular anomaly, and this was proven by the pathologic diagnosis and histology [7, 12, 37]. Another report has stated that 40% of the underlying causes of this disease are unknown [9]. In our experience, we had four cases that had high blood pressure and four cases that had bleeding tendencies. We found that only one case had a pathological vascular anomaly. It seems that further research may be needed to find whether these elements are the causes of SSEH.
The characteristic clinical symptoms of SSEH are an acute onset of local pain and sometimes there is radicular paresthesia. Within minutes or hours, the signs of spinal cord compression appear as progressive paraplegia and the loss of sensory function. The range of the time until the signs and symptoms present is various, from several minutes to several days [20, 32].
MRI is the neuroimaging tool of choice. We can see the correct position of the hematoma, the extent and time frame of the bleeding, cord compression and spinal cord edema. The MRI signal for the hematoma shows as isointense to the spinal cord on the T1WI within the first 24 h, and as a hyperintense or mixed signal to the cord on the T2WI after 36 h [10]. Holtas et al. [20] said that the signal shift from early-stage isointensity to intermediate-stage hyperintensity on T1WI was a pathognomic finding. We realized that MRI showed a heterogenous mixed signal intensity on the T1 and T2 weighted images (Figs. 1, 2).
Groen et al. have claimed that SSEH occurred mainly in the cervicothoracic or thoracolumbar region [8, 11, 12]. Foo et al. [9] and Major et al. [31] described that surgical outcomes were more favorable in the lumbosacral region than in the thoracic region, and Fukui et al. [10] reported that if the hematoma has a cervical or cervicothoracic localization, spontaneous recovery is possible without a surgical operation. They also reported that the neurological outcome is better when the SSEH is limited to one vertebral level. But other authors reported that there was no interrelation between the hematoma’s extent and the neurological outcome [13, 28]. Our cases were mainly dorsal to the spinal cord, and the hematomas were mostly in the cervical and thoracic region. There was no particular relation between the surgical outcome and hematoma’s location or its extent.
The degree of spinal cord compression and spinal canal compromise might be useful to decide the neurological outcome, but our results found no interrelation for these factors, the same as Fukui et al. [10]. In addition, age and gender did not show any correlation with the postoperative neurological outcomes, which was the same as other authors‘ results [7, 11].
Some authors [4, 17, 27, 35, 38] have reported that the conservative treatment is effective, but the standard management is a quick diagnosis and evacuation of hematoma by a surgical operation [2, 6, 13, 18, 21, 24, 28, 32, 34].
The authors who insisted on the conservative treatment for SSEH have postulated that coagulopathy induced SSEH, and the hematomas did not need a surgical operation because they were not clotted, but that the pooled blood remained in a liquid state. There have been several case reports where the clinical state and the radiological findings improved within a few hours of the symptom onset [4, 17, 18, 21, 27, 38]. However, it was mostly mild or benign cases were treated conservatively. As most of our cases were serious with progressive neurological deterioration, we treated them by surgical operation. The recovery scale increased for all the cases after the surgery. In other words, all the cases had an improved neurological outcome after the surgical operation. Surgical decompression ultimately resulted in functional improvement. Recent report of Little et al. [29] shows that minimally invasive surgery using thrombolytic agents can lower the risk of spinal instability and postoperative pain in the patients who are in poor general condition.
Initial neurological deficit and neurological outcome
Many authors have reported that the preoperative neurological status was the most important factor that decided neurological recovery [9, 10, 26, 28]. Lonjon et al. reported that the preoperative neurological status is the essential prognostic factor [18, 30]. Foo et al. [9] reported the neurological status returned to normal in only 45.3% of the patients among the early complete deficit patients, but the neurological status returned to normal in 95% of the patients among the incomplete sensorimotor deficit ones. In our cases, the neurological status returned to normal in 57.1% among the early incomplete deficit patients, but none of the complete deficit cases returned to normal. We think that the preoperative neurological status mainly affects the neurological outcome after the surgical operation. Nevertheless, three of seven complete deficit cases improved more than 50% after their surgical operation. The neurological outcome was better for those cases with incomplete injury status before the surgical operation than in the cases with complete injury status (P<0.05). Moreover, there was a meaningful statistic difference between the JOA score before the operation and the recovery scale after the operation (P<0.05). The better preoperative JOA score leads to the better postoperative JOA score (Fig. 4). In summary, there was good neurological recovery for the patients who had early incomplete motor and sensory losses.
The surgical time and neurological outcome
Most authors also have asserted that the neurological outcome was strongly related with the time interval, that is, from symptom onset to surgical decompression [2, 6, 13, 24, 28, 32, 34]. However, Foo et al. [9] reported that there was no correlation between the time of surgical decompression and neurological outcome. Up to now, the time interval of spinal cord decompression for patients with SSEH has long been a controversial topic.
Grollmus et al. have reported that the prognosis is favorable if the operation is carried out within 8 h [14, 24, 31]. Alexiadou-Rudolf [2] reported that favorable postoperative functional results were noted for patient whose symptoms had lasted for less than 12 h. Markham et al. [32] have suggested that for patients operated on in less than 24 h, 50% will recover from paralysis. McQuarrie et al. [34] have reported that decompressive surgery within 36 h was critical and Liao et al. [28] showed that within 48 h was critical. Groen and Van Alphen [13] asserted that recovery was better when decompression was performed within 36 h for patients with complete sensorimotor loss and within 48 h for patients with an incomplete sensorimotor deficit. As for our experience, good outcomes were found when the patients were operated on within 24 h. Especially when the patients with incomplete injury were operated within 12 h, all of them returned to a normal condition. In contrast, failure to fully recover was observed with complete injury even if surgical decompression done within 12 h. That is, the patient made a complete recovery by means of the rapid surgical decompression that was done less than 12 h before the neurological status completely deteriorated. Although the preoperative neurological status strongly predicts the neurological outcome, surgery has to be performed within at least 24 h for the patients with a poor neurological status because the patients did improve in those cases of surgical decompression that were done in less than 24 h.
Improvement of the neurological outcome can be understood from the animal models of spinal cord injury. Jarmundovicz et al. [22, 23] have explained that spinal cord compression in rabbits allowed the development of great changes in the nerve fibers, vascular endothelium and microcirculation. The compression resulted in the formation of hemorrhage, central necrosis and edema around the axons as well as at the myelin sheaths. The longer the spinal cord compression time was, then the greater the extent of changes became, which are called secondary damages.
We speculated that early decompression at less than 12 h for incomplete neurological deficits constrains the ischemic change, and the recovery is full. However, if compression of the spinal cord continues for over 24 h, the secondary changes then occur and the function of the spinal cord does not return to normal. We should make an in depth analysis of the assumption in the future. In closing, it is evident that a surgical operation is warranted in those patients with neurological deficit due to SSEH, and those cases that had earlier surgical intervention performed before the onset of complete neurological deficit attained good results. We suggest that the final outcome was related with two factors: one was the preoperative neurological status and the other was the time from the initial onset to the operation.
Conclusion
Spontaneous spinal epidural hematoma has a tendency to progress to neurological aggravation. We treated our patients with surgical operation and we achieved favorable outcomes. The neurological outcome is related with the preoperative status of the neurological deficit and the operative time interval. The better the preoperative neurological status is, the better will be the neurological outcome after the surgical operation. If the time for the surgical operation is shorter, then the neurological outcome is better. Particularly, the outcome is good when patients with incomplete injury were operated on within 12 h. Even patients with complete neurological deficits can improve if the surgical operation is performed within less than 24 h.
References
Ainslie JP (1958) Paraplegia due to spontaneous extradural or subdural haemorrhage. Br J Surg 45:565–567
Alexiadou-Rudolf C, Ernestus RI, Nanassis K, Lanfermann H, Klug N (1998) Acute nontraumatic spinal epidural hematomas. An important differential diagnosis in spinal emergencies. Spine 23:1810–1813
Beatty RM, Winston KR (1984) Spontaneous cervical epidural hematoma. A consideration of etiology. J Neurosurg 61:143–148
Connolly ES Jr, Winfree CJ, McCormick PC (1996) Management of spinal epidural hematoma after tissue plasminogen activator. A case report. Spine 21:1694–1698
Cooper DW (1967) Spontaneous spinal epidural hematoma. Case report. J Neurosurg 26:343–345
Cultrera F, Passanisi M, Giliberto O, Giuffrida M, Mancuso P, Ventura F (2004) Spinal epidural hematoma following coronary thrombolysis. A case report. J Neurosurg Sci 48:43–47
D′Angelo V, Bizzozero L, Talamonti G, Ferrara M, Colombo N (1990) Value of magnetic resonance imaging in spontaneous extradural spinal hematoma due to vascular malformation: case report. Surg Neurol 34:343–344
Flaschka G, Sutter B, Ebner F, Klein GE, Tilz G (1990) Spinal epidural hematoma. Long-term results of four cases. Nervenarzt 61:629–633
Foo D, Rossier AB (1981) Preoperative neurological status in predicting surgical outcome of spinal epidural hematomas. Surg Neurol 15:389–401
Fukui MB, Swarnkar AS, Williams RL (1999) Acute spontaneous spinal epidural hematomas. AJNR Am J Neuroradiol 20:1365–1372
Groen RJ (2004) Non-operative treatment of spontaneous spinal epidural hematomas: a review of the literature and a comparison with operative cases. Acta Neurochir Wien 146:103–110
Groen RJ, Ponssen H (1990) The spontaneous spinal epidural hematoma. A study of the etiology. J Neurol Sci 98:121–138
Groen RJ, van Alphen HA (1996) Operative treatment of spontaneous spinal epidural hematomas: a study of the factors determining postoperative outcome. Neurosurgery 39:494–508 (Discussion 508–499)
Grollmus J, Hoff J (1975) Spontaneous spinal epidural haemorrhage: good results after early treatment. J Neurol Neurosurg Psychiatry 38:89–90
Gundry CR, Heithoff KB (1993) Epidural hematoma of the lumbar spine: 18 surgically confirmed cases. Radiology 187:427–431
Harik SI, Raichle ME, Reis DJ (1971) Spontaneously remitting spinal epidural hematoma in a patient on anticoagulants. N Engl J Med 284:1355–1357
Hentschel SJ, Woolfenden AR, Fairholm DJ (2001) Resolution of spontaneous spinal epidural hematoma without surgery: report of two cases. Spine 26:E525–E527
Hernandez D, Vinuela F, Feasby TE (1982) Recurrent paraplegia with total recovery from spontaneous spinal epidural hematoma. Ann Neurol 11:623–624
Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K (1981) Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine 6:354–364
Holtas S, Heiling M, Lonntoft M (1996) Spontaneous spinal epidural hematoma: findings at MR imaging and clinical correlation. Radiology 199:409–413
Inamasu J, Hori S, Aoki K, Aikawa N, Maruiwa H, Toyama Y (2000) Spontaneous spinal epidural hematoma. Am J Emerg Med 18:837–839
Jarmundowicz W, Lawicki B, Orkisz S (1997) The effect of prolonged spinal cord compression on the extent of morphological changes in experimental spinal cord injury in rabbits. Neurol Neurochir Pol 31:1177–1188
Jarmundowicz W, Tosik D, Chlebinski J, Gorkiewicz Z (1997) The effect of early decompression on the extent of changes in spinal cord microcirculation in experimental traumatic injury to the cord in rabbits. Neurol Neurochir Pol 31:1167–1175
Klossek H, Huller E (1984) Spontaneous spinal epidural hematomas. Zentralbl Neurochir 45:116–123
Krolick MA, Cintron GB (1991) Spinal epidural hematoma causing cord compression after tissue plasminogen activator and heparin therapy. South Med J 84:670–671
Lawton MT, Porter RW, Heiserman JE, Jacobowitz R, Sonntag VK, Dickman CA (1995) Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg 83:1–7
Le Coz P, Helias A, Woimant F, Haguenau M (1997) Transient neurological manifestations disclosing spontaneous acute cervical epidural hematoma. Rev Neurol (Paris) 153:325–330
Liao CC, Lee ST, Hsu WC, Chen LR, Lui TN, Lee SC (2004) Experience in the surgical management of spontaneous spinal epidural hematoma. J Neurosurg Spine 100:38–45
Little CP, Patel N, Nagaria J, Kumar R, Nanra J, Bolger CM (2004) Use of topically applied rt-PA in the evacuation of extensive acute spinal subdural haematoma. Eur Spine J 13:380–383
Lonjon MM, Paquis P, Chanalet S, Grellier P (1997) Nontraumatic spinal epidural hematoma: report of four cases and review of the literature. Neurosurgery 41:483–486 (Discussion 486–487)
Major O, Sipos L, Czirjak S, Benoist G, Horvath M, Pasztor E (1991) Spontaneous spinal epidural haematomas. Acta Neurochir Wien 111:40–42
Markham JW, Lynge HN, Stahlman GE (1967) The syndrome of spontaneous spinal epidural hematoma. Report of three cases. J Neurosurg 26:334–342
Mattle H, Sieb JP, Rohner M, Mumenthaler M (1987) Nontraumatic spinal epidural and subdural hematomas. Neurology 37:1351–1356
McQuarrie IG (1978) Recovery from paraplegia caused by spontaneous spinal epidural hematoma. Neurology 28:224–228
Muthukumar N (2003) Chronic spontaneous spinal epidural hematoma—a rare cause of cervical myelopathy. Eur Spine J 12:100–103
Oldenkott P, Preger R, Todorow S (1981) Spinal epidural hematoma and anticoagulation treatment. Med Welt 32:46–49
Olivero WC, Hanigan WC, McCluney KW (1993) Angiographic demonstration of a spinal epidural arteriovenous malformation. Case report. J Neurosurg 79:119–120
Serizawa Y, Ohshiro K, Tanaka K, Tamaki S, Matsuura K, Uchihara T (1995) Spontaneous resolution of an acute spontaneous spinal epidural hematoma without neurological deficits. Intern Med 34:992–994
Tsai FY, Popp AJ, Waldman J (1975) Spontaneous spinal epidural hematoma. Neuroradiology 10:15–30
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, JJ., Kuh, SU. & Cho, YE. Surgical management of spontaneous spinal epidural hematoma. Eur Spine J 15, 998–1004 (2006). https://doi.org/10.1007/s00586-005-0965-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-005-0965-8