Abstract
Although osteoporosis is a systemic disease, vertebral fractures due to spinal bone loss are a frequent, sometimes early and often neglected complication of the disease, generally associated with considerable disability and pain. As osteoporotic vertebral fractures are an important predictor of future fracture risk, including at the hip, medical management is targeted at reducing fracture risk. A literature search for randomized, double-blind, prospective, controlled clinical studies addressing medical treatment possibilities of vertebral fractures in postmenopausal Caucasian women was performed on the leading medical databases. For each publication, the number of patients with at least one new vertebral fracture and the number of randomized patients by treatment arm was retrieved. The relative risk (RR) and the number needed to treat (NNT, i.e. the number of patients to be treated to avoid one radiological vertebral fracture over the duration of the study), together with the respective 95% confidence intervals (95%CI) were calculated for each study. Treatment of steroid-induced osteoporosis and treatment of osteoporosis in men were reviewed separately, based on the low number of publications available. Forty-five publications matched with the search criteria, allowing for analysis of 15 different substances tested regarding their anti-fracture efficacy at the vertebral level. Bisphosphonates, mainly alendronate and risedronate, were reported to have consistently reduced the risk of a vertebral fracture over up to 50 months of treatment in four (alendronate) and two (risedronate) publications. Raloxifene reduced vertebral fracture risk in one study over 36 months, which was confirmed by 48 months' follow-up data. Parathormone (PTH) showed a drastic reduction in vertebral fracture risk in early studies, while calcitonin may also be a treatment option to reduce fracture risk. For other substances published data are conflicting (calcitriol, fluoride) or insufficient to conclude about efficacy (calcium, clodronate, etidronate, hormone replacement therapy, pamidronate, strontium, tiludronate, vitamin D). The low NNTs for the leading substances (ranges: 15–64 for alendronate, 8–26 for risedronate, 23 for calcitonin and 28–31 for raloxifene) confirm that effective and efficient drug interventions for treatment and prevention of osteoporotic vertebral fractures are available. Bisphosphonates have demonstrated similar efficacy in treatment and prevention of steroid-induced and male osteoporosis as in postmenopausal osteoporosis. The selection of the appropriate drug for treatment of vertebral osteoporosis from among a bisphosphonate (alendronate or risedronate), PTH, calcitonin or raloxifene will mainly depend on the efficacy, tolerability and safety profile, together with the patient's willingness to comply with a long-term treatment. Although reduction of vertebral fracture risk is an important criterion for decision making, drugs with proven additional fracture risk reduction at all clinically relevant sites (especially at the hip) should be the preferred options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis was defined at a 1993 consensus conference as "a systemic skeletal disease characterized by low bone mass and micro-architectural deterioration of bone tissue with a resultant increase in fragility and risk of fracture" [24]. As bone quality cannot be evaluated easily in daily practice, the diagnosis of osteoporosis is made on low bone mineral density (BMD), expressed as the number of standard deviations above or below BMD for normal young adults (T-score). The World Health Organization study group's definition of osteoporosis is a T-score below −2.5 SD. Patients with a T-score below –2.5 who also have suffered a fragility fracture have severe osteoporosis [91].
Although a number of risk factors for osteoporotic fractures have been identified [26, 35], a history of previous vertebral fracture is a particularly important risk factor for future fractures. Postmenopausal women with radiographically detected vertebral fractures are at increased risk for new fractures, independently of bone mass [9, 17, 79]. In addition, vertebral fractures are common: 5% of Caucasian women aged 50 and 25% of those aged 80 have one or more fractures [58], and as many as 11–56% of patients on long-term steroids are estimated to have prevalent vertebral fragility fractures [3, 52, 60]. Vertebral fractures, even those not recognized clinically, are associated with substantial increases in back pain, functional limitation, disability, and with an excess mortality risk [44, 63]. However, physicians frequently do not diagnose osteoporosis in primary care patients with vertebral fractures, thereby missing an important preventive opportunity for patients at highest risk for future fractures: in a recently published study from Neuner et al., only 38% of subjects with vertebral compression fractures noted on routine radiographs (46% of women and 19% of men) were diagnosed with osteoporosis, and only 32% received prescription medication for osteoporosis [62].
Effective medical treatments of osteoporosis have increasingly become available over the last decade and their efficacy in reducing fracture risk, including at the spine, has been reviewed thoroughly in several recent publications [39, 45, 65].
The aim of this publication is to review the available data on drug treatment options in women with postmenopausal osteoporosis, with special focus on vertebral fracture risk reduction, and to briefly comment on steroid-induced osteoporosis and osteoporosis in men.
Materials and methods
We searched Medline, Embase and Current Contents from 1980 to 2002 for randomized controlled trials with drug treatment intervention in Caucasian women with postmenopausal osteoporosis (defined as T-score below –2SD at inclusion and/or prevalent anamnestic fracture) and reporting vertebral fracture data (either as a primary or secondary endpoint or as an adverse event). Duplicates, abstracts, and posters were eliminated by manual selection.
All definitions of radiological vertebral fractures (anterior, middle, or posterior vertebral height loss defined as any % loss and/or as any absolute value in millimeters), as chosen by the authors, were accepted for inclusion in the final analysis. Published results on risk reduction of clinically symptomatic vertebral fractures and risk reduction of multiple fractures were recorded separately.
Studies reporting on the number of patients who suffered at least one fracture were retained. Studies reporting on total number of fractures (i.e., fracture rates in patient-years) per treatment group without mentioning the number of patients with fractures were excluded from the analysis. Counting events instead of patients has been criticized as violating basic statistical assumptions and invalidating the use of common statistical tests as well as cross comparisons [93].
Studies of less than 36 months' duration were eliminated. The minimum required duration for a phase III trial for development of anti-osteoporotic drugs is usually specified at 3 years in Europe and in the US, the European CPMP regulations being the most stringent, requiring demonstrated anti-fracture efficacy prior to registration of an osteoporosis drug [14].
For each publication, the number of patients with a at least one new vertebral fracture and the number of randomized patients by treatment arm was recorded. The relative risk (RR) and the number needed to treat (NNT, i.e. the number of patients to be treated to avoid one radiological vertebral fracture over the duration of the study) as well as the respective 95% confidence intervals were calculated. When different dosages were used in different treatment arms, the results were pooled (active vs control) and dosage-specific comments as stated in the original publication were reported if appropriate.
For steroid-induced osteoporosis and osteoporosis in men, an overview is given based on selected publications.
Results
Forty-five publications resulted from the search of the medical databases. Six publications were excluded because they reported on total number of fractures and the number of patients with at least one fracture was not published and could not be derived from published data [34, 46, 73, 76, 80, 85]. Sixteen publications were excluded because the duration of observation was less than 36 months [5, 7, 15, 21, 29, 32, 33, 36, 41, 53, 55, 59, 66, 67, 90, 92]. Twenty-three publications matched all selection criteria: four with alendronate [10, 11, 27, 50], two with calcitriol [31, 86], one with calcium-vitamin D [69], one with calcitonin [20], two with etidronate [37, 54], three with fluoride [68, 71, 77], one with hormone replacement therapy [70], one with ipriflavone [6], one with pamidronate [16], two with parathormone [51, 61], two with raloxifene [28, 30] and three with risedronate [22, 38, 72]. The study of Neer et al. with parathormone [61] had a median duration of only 21 months, but was kept in the final analysis as it was stopped early by the sponsor.
Radiological vertebral fractures
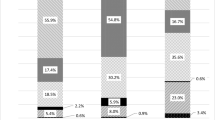
An overview of all calculated RR and NNT values with the respective 95% confidence interval (CI) by drug and by study is given in Table 1. Figure 1 shows the RR and 95%CI for selected drugs in alphabetical order (alendronate, calcitonin, parathormone (teriparatide, PTH), raloxifene and risedronate).
Alendronate
In three long-term endpoint trials [10, 27, 50] and in one published re-analysis of the anti-fracture efficacy in patients with osteoporosis as defined by the WHO [11], alendronate showed a consistent and significant reduction in vertebral fracture risk of between 45 and 49% across all studies. The calculated NNT ranged from 15 (95%CI 10 to 24) to 64 (95%CI 38 to 152), depending on the patient population studied, patients with highest fracture risk having the lowest NNTs.
Calcitonin
Only one published clinical trial of more than 36 months' duration was retrieved [20]. Although the vertebral fracture risk is reported to be significantly reduced, by 33%, at the intranasal dose of 200 IU per day in the original publication, the risk reduction of the pooled dosages (100, 200 and 400 IU/day) reported here is not significant. Accordingly, the NNT is 23, with a 95% CI of 10 to −154.
Parathormone
Two published studies were eligible [51, 61]. Vertebral fracture risk was significantly reduced, by 68% (RR 0.32, 95%CI 0.22 to 0.46) in the endpoint trial [61], with an NNT of 13 (95%CI 9 to 20). In the other smaller trial, the risk reduction was not significant [51].
Raloxifene
Two publications report vertebral fracture data with raloxifene at 36 months [30] and in the 12 months extension [28]. The calculated vertebral fracture risk is significantly reduced, by 41% after 3 years and 38% after 4 years. The calculated NNTs are 28 (95%CI 20 to 42), and 31 (95%CI 21 to 48) respectively.
Risedronate
Risedronate significantly reduced calculated vertebral fracture risk, by 34% and 40% respectively, in two endpoint studies [38, 72]. In a third, smaller, study over 36 months, the risk reduction was not significant (RR 0.7, 95%CI 0.44 to 1.11) [22]. Calculated NNTs ranged from 8 (95%CI 3 to −42) to 26 (95%CI 14 to 83).
Other treatment options
Calcitriol, etidronate, fluoride and pamidronate showed calculated vertebral fracture risk reduction in single studies, while there is no publication demonstrating vertebral fracture risk reduction over 36 months for calcium-vitamin D, hormone replacement therapy or ipriflavone (Table 1).
Clinical (symptomatic) vertebral fractures
Clinical vertebral fractures were defined as clinically diagnosed and radiologically confirmed vertebral fractures, i.e. clinical fractures are usually symptomatic (back pain, height loss, kyphosis). Only two drugs had published data regarding risk reduction of symptomatic vertebral fractures. According to two reports, alendronate reduced the calculated risk for symptomatic vertebral fracture significantly, by 44% and 55% respectively (RR 0.56; 95%CI 0.38 to 0.82 and RR 0.45; 95%CI 0.28 to 0.73 respectively) [10, 11]. Raloxifene reduced the risk of clinical fracture by 47% (RR 0.53; 95%CI 0.4 to 0.72) [30] (Table 1).
Discussion
Postmenopausal osteoporosis
In women with postmenopausal osteoporosis, vertebral fractures can be prevented with efficacious drug treatments. Oral bisphosphonates (specific inhibitors of osteoclastic bone resorption: alendronate and risedronate), oral SERMs (selective estrogen receptor modulators: raloxifene) and subcutaneous PTH (amino-terminal parathyroid hormone 1–34: teriparatide) have demonstrated their clinical efficacy in large-scale trials with fractures as a primary endpoint. Calcium and vitamin D have no long-term clinical data to demonstrate their anti-fracture efficacy in the spine; however, calcium (500–1000 mg/day) and/or vitamin D substitution (400–800 IU/day) were always given to all patients in all treatment groups of all published clinical trials. Therefore, calcium and/or vitamin D substitution has to be considered as the established standard of all drug interventions against osteoporosis, even in the absence of conclusive fracture reduction endpoint data. Hormone replacement therapy (HRT) has not shown documented vertebral fracture risk reduction in large-scale trials to date. However, the effect of HRT on fracture risk (hip fractures and all clinical fractures) has been extensively studied. Hormones have systemic effects, some of which may be expected to be beneficial, others less so. Two recently published studies in 2,763 and 16,608 postmenopausal women respectively have shed a new light on the antifracture efficacy of HRT and its systemic effects. In the HERS trial, a randomized, double-blind, placebo-controlled secondary cardio-vascular prevention trial with combined estrogens and progestin in 2,763 postmenopausal women with documented coronary heart disease, where less than 15% of the women had osteoporosis at inclusion [19, 42, 43], HRT had no significant effect on clinical fractures (RR 0.95, 95%CI 0.75 to 1.21) nor on hip fractures (RR 1.10; 95%CI 0.49 to 2.5) nor on breast cancer incidence, nor on coronary heart disease, nor on stroke. Risk for venous thrombotic disease was significantly greater with HRT (RR 2.89; 95%CI 1.50 to 5.58) [42]. In the WHI trial, a randomized, double-blind, placebo-controlled trial with combined estrogens and progestin designed to assess the major health benefits and risks of combined HRT in 16,608 postmenopausal women who had not undergone hysterectomy, clinical and hip fracture risk was significantly reduced, by 24 and 34% respectively. However, risk for breast cancer, coronary heart disease, venous thrombotic disease and stroke was significantly increased with HRT [95]. The authors concluded that, in this trial, health risks exceeded the benefits from use of combined estrogen plus progestin in healthy postmenopausal women over a 5.2-year period of observation [95]. Therefore, HRT should be reserved for short-term treatment of postmenopausal symptoms and other drug alternatives considered for treatment or prevention of osteoporosis.
Osteoporosis is a systemic disease. Therefore, drugs that have been shown to reduce the risk of fracture at all clinically relevant sites, especially at the hip, should become preferred treatment options. Based on published data to date in postmenopausal women with osteoporosis, alendronate significantly reduced hip fracture risk, by 51% [10, 11], risedronate by 30% [56], while calcitonin [20], raloxifene [28, 30] and PTH [61] had no significant effect. The calculated numbers needed to treat, i.e. the number of patients to be treated to avoid one radiological vertebral fracture over the duration of the study, are comparable with NNTs calculated in other therapeutic fields for interventions usually considered as being good medical practice. The NNT of gemfibrozil in male patients with high cholesterol is 71 over 5 years to avoid one coronary event [40], the NNT to avoid one myocardial infarction with aspirin in healthy males is 111 over 5 years [84], and the NNT to avoid one serious gastrointestinal complication with misoprostol in rheumatoid arthritis patients is 263 over 6 months [83]. If taking additionally the fracture risk reductions achieved at all clinical fracture sites into account, the NNTs for a drug intervention in osteoporosis would be expected to be even lower. This supposes that patients are well diagnosed by DEXA bone mineral density measurement at the hip or the spine, showing a T-score lower than or equal to −2.5 SD with or without anamnestic fractures, before getting drug therapy. An interesting finding was the great disparity in fracture incidences in the control groups of the selected trials (Fig. 2). They reflect the differences in definitions of vertebral fractures on the one hand and the fracture risk of the analysed patient population on the other. The definitions of radiological vertebral fractures used in the different trials range from a 15% reduction in vertebral height, including worsening of pre-existing fractures, to 20% reduction in vertebral height and more than 4 mm. Therefore, an expected finding would be that the most stringent definition will result in fewer fractures being detected than the looser one, independently of the antifracture efficacy of the drug. Some studies have included patients with low BMD (T-score below −2 SD) and no fractures, while others included patients with up to five pre-existent vertebral fractures. Therefore, an expected finding would be that the studies including highest-risk patients would show a greater fracture incidence, including in the control group. However, these studies may fail to be representative of the patients in which the drug will be used later in daily practice. The calculated NNTs should therefore be interpreted in this light, considering that in some cases less efficacious drugs have the best NNTs.
This review has several limitations. Firstly, we excluded from the analysis all studies of less than 36 months' duration. However, osteoporosis is a chronic, slowly debilitating disease, and European CPMP and US American FDA regulations require 36 months' data for registration of an osteoporosis drug [14]. Our results are in line with those of an exhaustive meta-analysis [65] and a recent review [39], which reached similar conclusions. Secondly, we excluded all studies reporting fracture rates only, and considered only studies reporting patients with at least one vertebral fracture. However, the drawback of the loss of data of isolated studies was outweighed by far by the improved quality of the remaining data, especially as the present review focused on vertebral fractures. In fact, for statistical analysis, the basic assumption is that all events can be regarded as independent; a second event in the same patient being as likely as a first event in this or in another patient. Vertebral fractures are not independent events [93]. By considering only patients with fractures (i.e., the true fracture incidence and not the fracture rate), the information about the drug effect on the risk reduction of multiple fractures is lost, and separate analyses would be required to answer this question. One publication addresses the risk reduction for multiple symptomatic fractures with alendronate, showing a significant risk reduction, of 84% (RR 0.16; 95%CI 0.05 to 0.42) [49].
Osteoporosis in men
Osteoporosis in men is more often secondary than primary. Therefore, the underlying cause (drug-induced bone loss, gastro-intestinal diseases, hypercalciuria, endocrine disorders, etc) must be identified and treated first. The best documented drug intervention is with alendronate, which showed similar efficacy in men and in postmenopausal women with regard to achieved increases in BMD. The studies were not statistically powered to evaluate the efficacy on vertebral fracture risk reduction; however, both showed a trend in favor of alendronate [64, 78]. Pooled results of two studies with risedronate in 184 men receiving chronic steroid therapy showed a significant reduction in the risk of vertebral fracture over 1 year of treatment [75]. As is the case in women, calcium and vitamin D deficiency have been prevented by systematic calcium substitution.
Glucocorticosteroid-induced osteoporosis
Glucocorticosteroid-induced osteoporosis (GIO) is by far the most frequent cause of secondary osteoporosis [4, 89], and fracture incidence under corticosteroids may be as high as 50% [3]. The pathogenesis of GIO is complex: proposed mechanisms include decreased osteoblast proliferation and biosynthetic activity as well as increased bone resorption [18], sex-steroid deficiency, decreased intestinal calcium absorption and secondary hyperparathyroidism [47]. Fracture risk is dose dependent, rises within the first months under glucocorticoid treatment, and remains elevated over the entire duration of therapy [87]. However, even short courses of oral corticosteroids or inhaled corticosteroids may be deleterious to bone [87, 94].
The comparative efficacy with respect to bone mineral density of several therapeutic agents for the management of GIO has been recently determined using meta-regression models [8]. Bisphosphonates were the most effective of the evaluated agents, whereas calcitonin and vitamin D were more effective than no therapy or calcium. Promising data with respect to BMD have furthermore been obtained with PTH, which had not yet been included in that meta-analysis of 2002 [48]. However, for all mentioned therapeutic strategies in GIO, fracture data are scarce, since many of the trials had a preventive design and were of short duration (1 or 2 years), including only modest numbers of patients with small numbers of fractures [1, 2, 23, 25, 74, 75, 81, 82, 88].
Using cyclical etidronate in 141 men and women who had recently begun high-dose corticosteroid therapy, Adachi et al. found no significant reduction in vertebral fracture incidence compared with the placebo group overall after 12 months [1]. However, among postmenopausal women 1/31 in the etidronate group versus 7/32 women receiving placebo experienced new vertebral fractures, demonstrating an anti-fracture effect of marginal significance (P=0.05). The combined results of two parallel 12-months trials (one conducted in the US, one in 15 other countries) using alendronate in a total of 477 men and women who had been under glucocorticoid therapy for a varying duration (34% for less than 4 months, 21% for 4–12 months, 45% for more than 12 months) were quite similar compared with those of the etidronate trial. Again they showed no significant difference in overall incidence between the bisphosphonate and placebo groups (P=0.18), but there was a borderline significance, of P=0.05, in postmenopausal women, when a post-hoc binary semiquantitative fracture assessment was used (7/54 patients with new vertebral fractures in the placebo versus 6/135 in the alendronate group) [81]. Although patients had a relatively low background prevalence of vertebral fractures (12–15%) the reduction in the incidence of vertebral fractures under alendronate became significant in a sample of patients (144 women, 66 men) in which that combined trial was extended to 24 months (overall 4/59 patients of the placebo group and 1/143 patients in the alendronate group experienced new morphometric fractures over 2 years, P=0.026) [2]. A recent comparative 2-years trial between calcitriol, vitamin D plus calcium and alendronate plus calcium in 195 subjects (134 women, 61 men) commencing or already taking glucocorticoids showed that alendronate was superior to the other two treatment regimens for glucocorticoid-induced bone loss, especially in the spine [82]. Six of 66 subjects treated with calcitriol, 1 of 61 treated with ergocalciferol, and 0 of 64 treated with alendronate sustained new vertebral fractures. That study was not powered for a fracture endpoint; however, it is interesting to note that, as in all the above-mentioned studies, no vertebral fractures occurred in premenopausal women.
The efficacy of risedronate was evaluated in two 1-year studies for prevention [23] and treatment [74]. The prevention trial included 224 men and women who had begun to take glucocorticoids within the previous 3 months. The treatment study included 285 men and women who had been under glucocorticoids for at least 6 months. Risedronate reduced the risk of new vertebral fractures by 71% (P=0.072) in the prevention trial and by 70% (P=0.042) in the treatment trial. When data from these two studies were combined, risedronate led to a 70% (P=0.01) reduction in the risk of vertebral fracture relative to placebo [88]: after 1 year, 18/111 patients (16%) under placebo and 12/195 patients (6%) under risedronate experienced new morphometric vertebral fractures. The significant antifracture effect in that combined study was reached for all patients together and for postmenopausal women, only. A separate (post hoc) analysis of male data from these two parallel risedronate trials on an intent to treat basis revealed a significant antifracture efficacy also for men under glucocorticoid treatment (P=0.008) [75].
Although more effective than calcium alone in maintaining lumbar BMD [8], calcitonin failed to reduce fracture risk in the spine or femoral neck in GIO [25].
The antifracture efficacy of PTH in that special condition remains to be proven.
Management of acute and chronic pain
Most osteoporotic vertebral fractures are asymptomatic. In the clinical trials that analyzed radiological and clinical vertebral fractures, symptomatic fractures represented 35% of all radiological fractures [10, 11, 30]. However, even asymptomatic fractures lead to spine deformity with chronic back pain and progressive disability. The management of chronic back pain relies on analgesics (paracetamol), non steroidal anti-inflammatory drugs (NSAIDs), and, more recently, on selective COX-II inhibitors (coxibs), which have demonstrated equal efficacy in pain relief and an improved gastrointestinal safety profile as compared to NSAIDs [13, 57]. Calcitonin, administered subcutaneously or intranasally, has demonstrated excellent analgesic efficacy in some patients [12]. Additional non-pharmacologic interventions include physiotherapy, physical activity and fall prevention programs.
Conclusion
The selection of the appropriate drug for treatment of vertebral osteoporosis among a bisphosphonate (alendronate or risedronate), PTH, calcitonin or raloxifene will mainly depend on its efficacy, tolerability and safety profile together with the patient's willingness to comply with a long-term treatment. Although reduction of vertebral fracture risk is an important criterion for decision-making, drugs with proven additional fracture risk reduction at all clinically relevant sites (especially at the hip) should be the preferred options.
References
Adachi JD, Bensen WG, Brown J, et al (1997) Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med 337:382–387
Adachi JD, Saag KG, Delmas PD, et al (2001) Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids. Arthritis Rheum 44:202–211
Adinoff AD, Hollister JR (1983) Steroid-induced fractures and bone loss in patients with asthma. New Engl J Med 309:265–268
Adler RA, Rosen CJ (1994) Glucocorticoids and osteoporosis. Endocrinol Metab Clin North Am 23:641–654
Alexandersen P, Riis BJ, Christiansen C (1999) Monofluorophosphate combined with hormone replacement therapy induces a synergistic effect on bone mass by dissociating bone formation and resorption in postmenopausal women: a randomized study. J Clin Endocrinol Metab 84:3013–3020
Alexandersen P, Toussaint A, Christiansen C, Devogelaer P, et al (2001) Ipriflavone in the treatment of postmenopausal osteoporosis: a randomized controlled trial. JAMA 285:1482–1488
Aloia JF, Vaswani A, Yeh JK, Ellis K, et al (1988) Calcitriol in the treatment of postmenopausal osteoporosis. Am J Med 84:401–408
Amin S, Lavalley M, Simms RW, Felson DT (2002) The comparative efficacy of drug therapies used for the management of corticosteroid-induced osteoporosis: a meta-regression. J Bone Miner Res 17:1512–1526
Black DM, Palermo L, Nevitt MC, et al (1995) Comparison of methods for defining prevalent vertebral deformities: the study of osteoporotic fractures. J Bone Miner Res 10:890–902
Black DM, Cummings SR, Karpf DB, Cauley JA, et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541
Black DM, Thompson D, Bauer DC, Ensrud K, et al (2000) Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. J Clin Endocrinol Metab 85:4118–4124
Blau LA, Hoehns JD (2003) Analgesic efficacy of calcitonin for vertebral fracture pain. Ann Pharmacother 37:564–570
Bombardier C, Laine L, Reicin A, et al — VIGOR Study Group (2000) Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med 343:1520–1528
Bone HG (2001) Development and evaluation of new drugs for osteoporosis. Osteoporosis (Second Edition) 2:533–538
Bone HG, Downs RW, Tucci JR Harris ST, et al (1997) Dose-response relationships for alendronate treatment in osteoporotic elderly women. J Clin Endocrinol Metab 82:265–274
Brumsen C, Papapoulos SE, Lips P, Geelhoed-Duijvestijn P, et al (2002) Daily oral pamidronate in women and men with osteoporosis: a 3-year randomized placebo-controlled clinical trial with a 2-year open extension. J Bone Miner Res 17:1057−1064
Burger H, Van Daele PL, Algra D, et al (1994) Vertebral deformities as predictors of non-vertebral fractures. BMJ 309:991–992
Canalis E (2003) Mechanisms of glucocorticoid-induced osteoporosis. Curr Opin Rheumatol 15:454–457
Cauley JA, Black DM, Barrett-Connor E, Harris F, et al (2001) Effects of hormone replacement therapy on clinical fractures and height loss: the Heart and Estrogen/Progestin Replacement Study (HERS). Am J Med 110:442–450
Chesnut CH, Silverman S, Andriano K, Genant H, et al (2000) A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the Prevent Recurrence Of Osteoporotic Fractures study. Am J Med 109:267–276
Chevalley T, Rizzoli R, Nydegger V, Slosman D, et al (1994) Effects of calcium supplements on femoral bone mineral density and vertebral fracture rate in vitamin-D-replete elderly patients. Osteoporos Int 4:245–252
Clemmesen B, Ravn P, Zegels B, Taquet AN, et al (1997) A 2-year phase II study with 1-year of follow-up of risedronate (NE-58095) in postmenopausal osteoporosis. Osteoporos Int 7:488–495
Cohen S, Levy RM, Keller M, Boling E, et al (1999) Risedronate therapy prevents corticosteroid-induced bone loss: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 42:2309–2318
Consensus Development Conference (1993) Diagnosis, prophylaxis and treatment of osteoporosis. Am J Med 94:646–650
Cranney A, Welch V, Adachi JD, Homik J, et al (2000) Calcitonin for the treatment and prevention of corticosteroid-induced osteoporosis. Cochrane Database Syst Rev 2:1983
Cummings SR, Nevitt MC, Browning WR, et al (1995) Risk factors for hip fracture in white women. N Engl J Med 322:767–773
Cummings SR, Black DM, Thompson DE, Applegate WB, et al (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280:2077–2082
Delmas PD, Ensrud KE, Adachi JD, Harper KD, et al (2002) Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab 87:3609–3617
Dursun N, Dursun E, Yalcin S (2001) Comparison of alendronate, calcitonin and calcium treatments in postmenopausal osteoporosis. Int J Clin Pract 55:505–509
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 282:637–645
Falch JA, Odegaard OR, Finnanger AM, Matheson I (1987) Postmenopausal osteoporosis: no effect of three years treatment with 1, 25-dihydroxycholecalciferol. Acta Med Scand 221:199–204
Fogelman I, Ribot C, Smith R, Ethgen D, et al (2000) Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. J Clin Endocrinol Metab 85:1895–1900
Gallagher JC, Goldgar D (1990) Treatment of postmenopausal osteoporosis with high doses of synthetic calcitriol. A randomized controlled study. Ann Intern Med 113:649–655
Gallagher JC, Riggs BL, Recker RR, Goldgar D (1989) The effect of calcitriol on patients with postmenopausal osteoporosis with special reference to fracture frequency. Proc Soc Exp Biol Med 191:287–292
Greenspan S, Myers E, Maitland L, et aI (1994) Fall severity and bone mineral density as risk factors for hip fracture in ambulatory elderly. JAMA 271:128–133
Gutteridge DH, Stewart GO, Prince RL, Price RI et al. (2002) A randomized trial of sodium fluoride (60 mg) ± estrogen in postmenopausal osteoporotic vertebral fractures: increased vertebral fractures and peripheral bone loss with sodium fluoride; concurrent estrogen prevents peripheral loss, but not vertebral fractures. Osteoporos Int 13:158–170
Harris ST, Watts NB, Jackson RD, Genant HK, et al (1993) Four-year study of intermittent cyclic etidronate treatment of postmenopausal osteoporosis: three years of blinded therapy followed by one year of open therapy. Am J Med 95:557–567
Harris ST, Watts NB, Genant HK, McKeever CD, et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA 282:1344–1352
Häuselmann HJ, Rizzoli R (2003) A comprehensive review of treatments for postmenopausal osteoporosis. Osteoporos Int 14:2–12
Heikki Frick M, Elo O, Haapa A, et al (1987) Helsinki Heart Study: primary prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors and incidence of coronary heart disease. New Engl J Med 317:1237–1245
Hizmetli S, Elden H, Kaptanoglu E, Nacitarhan V, et al (1998) The effect of different doses of calcitonin on bone mineral density and fracture risk in postmenopausal osteoporosis. Int J Clin Pract 52:453–455
Hulley S, Grady D, Bush T, Furberg C, et al (1998) Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 280:605–613
Hulley S, Furberg C, Barrett-Connor E, Cauley J, et al (2002) Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II). JAMA 288:58–66
Jalava T, Sarna S, Pylkkanen L, et al (2003) Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 18:1254–1260
Kanis JA, Brazier JE, Stevenson M (2002) Treatment of established osteoporosis: a systematic review and cost-utility analysis. Health Technology Assessment 6(29)
Kleerekoper M, Peterson EL, Nelson DA, Phillips E, et al (1991) A randomized trial of sodium fluoride as a treatment for postmenopausal osteoporosis. Osteoporos Int 1:155–161
Lafague-Proust MH, Boudignon B, Thierry T (2003) Glucocorticoid-induced osteoporosis: pathophysiological data and recent treatments. Joint Bone Spine 70:109–118
Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD (1998) Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. J Clin Invest 102:1627–1633
Levis S, Quandt SA, Thompson D, Scott J, et al (2002) Alendronate reduces the risk of multiple symptomatic fractures: results from the fracture intervention trial. J Am Geriatr Soc 50:409–415
Liberman UA, Weiss SR, Bröll J, Minne HW, et al (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 333:1437–1443
Lindsay R, Nieves J, Formica C, Henneman E, et al (1997) Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 350:550–555
Luengo M, Picado C, Dei Rio L, et al (1991) Vertebral fractures in patients with rheumatoid arthritis treated with corticosteroids. Thorax 46:803–806
Lufkin EG, Wahner HW, O'Fallon WM, Hodgson SF, et al (1992) Treatment of postmenopausal osteoporosis with transdermal estrogen. Ann Intern Med 117:1–9
Lyritis GP, Tsakalakos N, Papspati I, Skarantavos G, et al (1997) The effect of a modified etidronate cyclical regimen on postmenopausal osteoporosis: a four-year study. Clin Rheumatol 16:354–360
McCloskey E, Selby P, De Takats D, Bernard J, et al (2001) Effects of clodronate on vertebral fracture risk in osteoporosis: a 1-year interim analysis. Bone 28:310–315
McClung MR, Geusens P, Miller PD, Zippel H, et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 344:333–340
McKeogh DF (2003) Celecoxib versus diclofenac and omeprazole to prevent recurrent ulcer bleeding. N Engl J Med 348:2464–2466
Melton LJ, Kan SH, Frye MA, et al (1989) Epidemiology of vertebral fractures in women. Am J EpidemioI 129:1000–1011
Meunier PJ, Slosman DO, Delmas PD, Sebert JL, et al (2002) Strontium ranelate: dose-dependent effects in established postmenopausal vertebral osteoporosis—a 2-year randomized placebo-controlled trial. J Clin Endocrinol Metab 87:2060–2066
Naganathan V, Jones G, Nash P, et al (2000) Vertebral fracture risk with long-term corticosteroid therapy. Arch Intern Med 160:2917–2922
Neer RM, Arnaud CD, Zanchetta JR, Prince R, et al (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Neuner JM, Zimmer, JK, Hamel MB (2003) Diagnosis and treatment of osteoporosis in patients with vertebral compression fractures. J Am Geriatr Soc 51:483–491
Nevitt MC, Ettinger B, Black DM (1998) The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med 128:793–800
Orwoll E, Ettinger M, Weiss S, et al (2000) Alendronate for the treatment of osteoporosis in men. N Engl J Med 343:604–610
Osteoporosis Methodology Group and Osteoporosis Research Advisory Group (2002) Meta-analyses of therapies for postmenopausal osteoporosis. Endocrine Rev 23:495–578
Ott SM, Chesnut CH (1989) Calcitriol treatment is not effective in postmenopausal osteoporosis. Ann Intern Med 110:267–274
Overgaard K, Hansen MA, Jensen SB, Christiansen C (1992) Effect of salcatonin given intranasally on bone mass and fracture rates in established osteoporosis: a dose-response study. BMJ 305:556–561
Pak CY, Sakhaee K, Adams-Huet B, Piziak V, et al (1995) Treatment of postmenopausal osteoporosis with slow-release sodium fluoride. Final report of a randomized controlled trial. Ann Intern Med 123:401–408
Recker RR, Hinders S, Davies KM, Heaney RP, et al (1996) Correcting calcium nutritional deficiency prevents spine fractures in elderly women. J Bone Miner Res 11:1961–1966
Recker RR, Davies KM, Dowd RM, Heaney RP (1999) The effect of low-dose continuous estrogen and progesterone therapy with calcium and vitamin D on bone in elderly women. A randomized controlled trial. Ann Intern Med 130:897–904
Reginster JY, Meurmans L, Zegels B, Rovati LC, et al (1998) The effect of sodium monofluorophosphate plus calcium on vertebral fracture rate in postmenopausal women with moderate osteoporosis. A randomized, controlled trial. Ann Intern Med 129:1–8
Reginster JY, Minne HW, Sorensen OH, Hooper M, et al (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int 11:83–91
Reginster JY, Christiansen C, Roux C, Fechtenbaum J, et al (2001) Intermittent cyclic tiludronate in the treatment of osteoporosis. Osteoporos Int 12:169–177
Reid DM, Hughes RA, Laan RF, et al (2000) Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res 15:1006–1013
Reid DM, Adami S, Devogelaer JP, Chines AA (2001) Risedronate increases bone density and reduces vertebral fracture risk within one year in men on corticosteroid therapy, Calcif Tiss Int 69:242–247
Riggs BL, Hodgson SF, O'Fallon WM, Chao EYS, et al (1990) Effect of fluoride treatment on the fracture rate in postmenopausal women with osteoporosis. N Engl J Med 322:802–809
Ringe JD, Kipshoven C, Cöster A, Umbach R (1999) Therapy of established postmenopausal osteoporosis with monofluorophosphate plus calcium: dose-related effects on bone density and fracture rate. Osteoporos Int 9:171–178
Ringe JD, Faber H, Dorst A (2001) Alendronate treatment of established primary osteoporosis in men: results of a 2-year prospective study. J Clin Endocrinol Metab 86:5252–5255
Ross PD, Davis JW, Epstein RS, et al (1991) Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 114:919–923
Ryan PJ, Blake GM, Davie M, Haddaway M, et al (2000) Intermittent oral disodium pamidronate in established osteoporosis: A 2-year double-masked placebo-controlled study of efficacy and safety. Osteoporos lnt 11:171–176
Saag KG, Emekey R, Schnitzer TJ, et al (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med 339:292–299
Sambrook PN, Kotowicz M, Hash P, et al (2003) Prevention and treatment of glucocorticoid-induced osteoporosis: a comparison of calcitriol, vitamin D plus calcium and alendronate plus calcium. J Bone Miner Res 18:919–924
Silverstein FE, Graham DY, Senior JR, et al (1995) Misoprostol reduces serious gastro-intestinal complications in patients with rheumatoid arthritis receiving non steroidal anti-inflammatory drugs. Ann Int Med 123:241–249
Steering Committee of the Physicians' Health Study Group (1989). Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med 321:129–135
Storm T, Thamsborg G, Steiniche T, Genant HK, et al (1990) Effect of intermittent cyclical etidronate therapy on bone mass and fracture rate in women with postmenopausal osteoporosis. N Engl J Med 322:1265–1271
Tilyard MW, Spears GFS, Thomson J, Dovey S, et al (1992) Treatment of postmenopausal osteoporosis with calcitriol or calcium. N Engl J Med 326:357–362
van Staa TP, Leufkens HG, Cooper C (2001) Use of inhaled corticosteroids and risk of fractures. J Bone Miner Res 16:581–588
Wallach S, Cohen S, Reid DM, et al (2000) Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int 67:277–285
Walsh LJ, Wong CA, Pringle M, Tattersfield AE (1996) Use of oral corticosteroids in the community and the prevention of secondary osteoporosis: a cross-sectional study. BMJ 313:344–346
Watts NB, Harris ST, Genant HK, Wasnich RD, et al (1990) Intermittent cyclical etidronate treatment of postmenopausal osteoporosis. N Engl J Med 323:73–79
WHO study group (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO Technical Report series. WHO, Geneva
Wimalawansa SJ (1998) A four-year randomized controlled trial of hormone replacement and bisphosphonate, alone or in combination, in women with postmenopausal osteoporosis. Am J Med 104:219–226
Windeler J, Lange S (1995) Events per person year, a dubious concept. BMJ 310:454–456
Wong CA, Walsh LJ, Smith CJP, et al (2000) Inhaled corticosteroid use and bone mineral density in patients with asthma. Lancet 355:1399–1403
Writing Group for the Women's Health Initiative Investigators (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288:321–333
Author information
Authors and Affiliations
Corresponding author
Additional information
The author has no conflict of interest
Rights and permissions
About this article
Cite this article
Lippuner, K. Medical treatment of vertebral osteoporosis. Eur Spine J 12 (Suppl 2), S132–S141 (2003). https://doi.org/10.1007/s00586-003-0608-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-003-0608-x