Abstract
The fatty liver as a metabolic disorder has involved human beings globally and is usually followed by extreme obesity, increased blood lipid, and diabetes type II. Appropriate strategies for treating this disease are prioritized by each country. The aim of the recent research was to survey the remedial potential of aqueous extract of Allium saralicum R.M. Fritsch on the high-fat diet-induced fatty liver disease in Wistar male rats. In this study, 60 rats were used. A total of 10 rats were selected as the negative control, and the rest of them were treated with a high-fat diet for 4 months. Then, the animals were randomly divided into six subgroups, including negative healthy control, untreated negative control, and four groups receiving the aqueous extract of A. saralicum at 20, 40, 80, and 160 mg/kg concentrations. After 2 months, the rats were sacrificed and blood and liver samples of them were collected to analyze the biochemical and histopathological parameters. The data were analyzed by SPSS-21 software. All doses of A. saralicum (especially A160) could significantly (p ≤ 0.05) decrease the raised levels of ALP, AST, ALT, GGT, cholesterol, LDL, triglyceride, total and conjugated bilirubin, glucose, and GR and increased HDL, total protein, albumin, SOD, CAT, and GPx as compared to the untreated group. Also, aqueous extract of A. saralicum (especially A160) decreased the degree of hepatic steatosis as compared to the untreated group. In conclusion, the acquired results showed the hepatoprotective potential of aqueous extract of A. saralicum, so that it can use for the treatment of fatty liver disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fatty liver disease is one of the most usual metabolic diseases among people around the world. In this disease, triglycerides accumulated in liver cells due to stratification of glycerol and free fatty acids (Fan and Farrell 2009; Ganz et al. 2014; Jacobs et al. 2002; Day 2006, 2011). Fat accumulation in the liver can occur due to the raised synthesis of fat and decreased disposal or oxidation of lipid (Ganz et al. 2014; Jacobs et al. 2002; Day 2011). The fatty liver disease is amalgamated by a series of histopathologic changes varying from steatosis to cirrhosis (Haga et al. 2015; Flora et al. 1998; Tamayo and Diamond 2007; Shaker et al. 2010).
The possible pharmacologic treatments include antioxidants, insulin sensitizers, hepatic protectors, or lipid reducing factors (Comar and Sterling 2005). Since there are numerous pharmaceutical plants with antioxidant and anti-inflammatory potentials, their administrations can be impressive in the treatment of fatty liver disease.
In traditional medicine, plant medicines have been the basis of prevention, control, and treatment of several diseases (Ghashghaii et al. 2017; Hagh-Nazari et al. 2017; Farzaei et al. 2018; Zhaleh et al. 2018; Hamelian et al. 2018). One of the most important herbal medicines which are widely used is Allium saralicum R.M. Fritsch. The plant is widely distributed in Iraq, Iran, and Turkey. A. saralicum is a good source of low-cost food and is a perfect part of Iranian diet (Sherkatolabbasieh et al. 2017; Zangeneh et al. 2018). It applied as a medicinal plant has been used for its antibacterial, antifungal, anti-inflammatory, immunostimulatory, nephroprotective, and hepatoprotective properties (Sherkatolabbasieh et al. 2017; Zangeneh et al. 2018; Goodarzi et al. 2017; Goodarzi et al. 2018).
In this research, we attempted to survey the remedial potential of aqueous extract of A. saralicum on the high-fat diet-induced fatty liver disease in rats.
Materials and methods
Plant collection and extraction
A. saralicum was collected from Kermanshah city in the west of Iran. The leaves of the plant were dried in shadow, and after grinding, each time 100 g of the obtained powder was dissolved in 1000 cc of distilled water and put in Soxhlet extractor for 8 h. The collected extract was filtered by Whatman filter paper no. 1 and steamed into a glass container at the solvent temperature. The remaining dried extract was poured into a glass container and weighed. The powder of the obtained extract was weighed as required depending on the dose and dissolved in normal saline.
Experimental design
This experimental study was conducted on 60 Wistar male rats with the weight of 200 ± 5 g that were kept in individual cages for 10 days to adapt to the environment. During the experiments, the temperature of the animal house was adjusted at 22 ± 3 °C under a 12-h dark/light cycle. A total of 10 rats were selected as the negative control, and the rest of them were treated with a high-fat diet for 4 months. The rats with fatty liver were then divided into five groups, 10 rats in each group: I. Fatty diet, II. Fatty diet plus 20 mg/kg of A. saralicum, III. Fatty diet plus 40 mg/kg of A. saralicum, IV. Fatty diet plus 80 mg/kg of A. saralicum, and V. Fatty diet and 160 mg/kg of A. saralicum. All concentrations of extract were administered via gavage for 2 months. To consider gavage stress, distilled water was administered to the control group every day. After 2 months of gavage, the rats were sacrificed. Blood samples were taken from the rats’ heart to analyze biochemical parameters. The capacity of antioxidant enzymes was evaluated by determining the activity of superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPx) in livers of each group (n = 5). Also, the rats’ liver (n = 5) was subjected to microscopic analysis. The histopathological changes were rated based on fat accumulation in the liver: 0 = No steatosis, 1 = Steatosis in less than 25% of hepatocytes, 2 = Steatosis in 26–50% of hepatocytes, 3 = Steatosis in 51–75% of hepatocytes, and 4 = Steatosis in more than 75% of hepatocytes (Mohammadifar et al. 2018).
Fatty diet preparation
Rats diet powder (28%), butter, (28%), egg yolk (19%), sucrose (14%), and egg white (11%) were mixed to prepare the fatty diet. The obtained powder was dried in a 100 °C oven for 30 min and was given to the rats as a pellet. The fatty diet was prepared weekly and stored in the refrigerator (Mohammadifar et al. 2018).
Statistical analysis
The quantitative data were analyzed by SPSS-21 software using one-way ANOVA followed by Duncan’s test. To determine the normality of data, the Kolmogorov-Smirnov test was applied. To analyze the histopathological data, the Kruskal-Wallis test was run. p ≤ 0.05 was considered significant.
Results
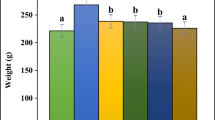
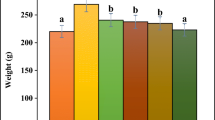
Effect of aqueous extract of A. saralicum on the body and liver weights
The body and liver weights enhanced significantly (p ≤ 0.05) in untreated rats as compared to the control ones (Figs. 1 and 2). Consumption of aqueous extract of A. saralicum at all doses could significantly (p ≤ 0.05) reduce above weights in comparison with the untreated group. There were no significant differences (p ≤ 0.05) among A80, A160, and control groups in the weight of the liver. Also, administration of A160 could significantly (p ≤ 0.05) reduce the body weight similar to the control group. No significant differences (p ≤ 0.05) were found between A20 and A40.
Effect of aqueous extract of A. saralicum on the degree of hepatic steatosis
As indicated in Table 1, the degree of hepatic steatosis increased in untreated rats compared to the control ones. All groups of aqueous extract of A. saralicum could decrease it. There were no significant differences in the degree of hepatic steatosis between A160 and control groups. No significant differences were found between A20 and A40.
Effect of aqueous extract of A. saralicum on the concentrations of antioxidant enzymes
The concentrations of SOD, CAT, and GPx enzymes were significantly (p ≤ 0.05) reduced and the concentration of GR was significantly (p ≤ 0.05) increased in the untreated group. The treatment with aqueous extract of A. saralicum significantly (p ≤ 0.05) improved them. There were no significant differences (p ≤ 0.05) in the level of GPx among several doses of A. saralicum and control group. The concentration of GR was significantly (p ≤ 0.05) increased in A80 and A160 and was similar to the control group. No significant differences (p ≤ 0.05) were found between A20 and A40 in the concentrations of antioxidant enzymes (Figs. 3 and 4).
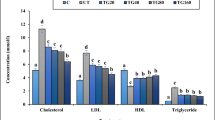
Effect of aqueous extract of A. saralicum on the concentrations of biochemical parameters
High-fat diet-induced fatty liver disease, decreased significantly (p ≤ 0.05) the concentrations of HDL, total protein, and albumin and enhanced significantly (p ≤ 0.05) the concentrations of ALP, AST, ALT, GGT, cholesterol, LDL, triglyceride, total and conjugated bilirubin, and glucose, as compared to the control group. All doses of aqueous extract of A. saralicum could significantly (p ≤ 0.05) improve the above parameters. There were no significant differences (p ≤ 0.05) among all doses of A. saralicum and control group in concentrations of ALT, GGT, triglyceride, total protein, albumin, and total and conjugated bilirubin. Also, administration of A80 and A160 could significantly (p ≤ 0.05) decrease the concentration of AST similar to the control group (Figs. 5, 6, 7, 8, and 9).
The level of ALP, AST, ALT, and GGT in various groups. C control, U untreated, A Allium saralicum R.M. Fritsch, ALP alkaline phosphatase, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT gamma-glutamyl transferase. Non-like letters show a significant difference between the various groups (p ≤ 0.05)
Discussion
Medicinal plants are used in traditional medicine to prevent, control, and treat various diseases including anorexia, nasopharyngitis, diabetes, hypertension, nephrotoxicity, hepatotoxicity, hemorrhoid, anemia, rheumatism, atherosclerosis, Alzheimer, cancer, gastroduodenal ulcers, fatty liver disease, etc. (Sharafzadeh and Alizadeh 2012; Sayyedrostami et al. 2018; Ebrahimi-Mameghani et al. 2014). In this regard, a list of medicinal plants used for their remedial potentials on fatty liver disease includes Cinnamomum zeylanicum (Askari et al. 2014; Nikkhajoei et al. 2016), Berberis vulgaris L. (Kashkooli et al. 2015), Cuminum cyminum L. (Shavakhi et al. 2015), Silybum marianum (Hashemi et al. 2009), Phyllanthus urinaria (Wong et al. 2013), Camelia sinensis (Sakata et al. 2013), and Chlorella vulgaris (Ebrahimi-Mameghani et al. 2014; Panahi et al. 2012). One of the herbs consumed in Iranian traditional medicine to treat fatty liver disease is A. saralicum.
The results of antioxidant enzymes of our study indicated that the high-fat diet significantly (p ≤ 0.05) reduced the concentrations of SOD, CAT, and GPx and enhanced the concentration of GR. But, the treatment with several doses of aqueous extract of A. saralicum could significantly (p ≤ 0.05) improve the concentrations of them. In the study of Ogunlade et al. (2012), it was reported that the aqueous extract of Allium cepa (as a species of Allium genus), with enhancing the degradation of free radicals, enhanced the concentrations of SOD, CAT, GPx, and malondialdehyde (MDA) and reduced glutathione (GSH) in rabbits with alcohol-induced hepatotoxicity. In another study, it was revealed that the extract of Allium cepa had good antioxidant activity, because it increased the concentration of antioxidant enzymes including SOD, CAT, and GPx as compared to the tartrazine-received group (untreated group) (Hoseinpouran et al. 2015). Also, in the study of Saravanan and Ponmurugan (2013), it indicated the very strong antioxidant property of Allium sativum Linn (as a species of Allium genus) with improving the levels of SOD, CAT, and GPx in diabetic rats.
The analysis of biochemical approach of the recent study revealed that a high-fat diet increased significantly (p ≤ 0.05) the concentrations of ALP, AST, ALT, GGT, total and conjugated bilirubin, and glucose and reduced significantly (p ≤ 0.05) the concentrations of total protein and albumin as compared to the control group. Therefore, this high-fat diet caused severe hepatic toxicity. In spite of hepatotoxicity potential of the diet, the treatment with aqueous extract of A. saralicum could significantly (p ≤ 0.05) ameliorate the concentrations of the above parameters. In the study of Goodarzi et al. (2017), it was revealed that extract of A. saralicum decreased the raised levels of hepatic biochemical parameters (ALP, AST, and ALT) and also the volume of the liver, hepatocytes, and sinusoids as compared to the CCl4-treated group. In another study, it was reported that extract of A. saralicum reduced the concentrations of ALP, AST, and ALT and also the volumes of the liver, central vein, hepatic artery, and portal vein in diabetic mice (Goodarzi et al. 2018). Also in the study of Ogunlade et al. (2012), aqueous extract of Allium cepa decreased the raised concentration of ALP, AST, AST, and gamma-glutamyl transpeptidase (GGT) as compared to the alcohol-treated group.
In the study, aqueous extract of A. saralicum reduced the concentrations of cholesterol, LDL, triglyceride, and the degree of hepatic steatosis and increased the concentration of the HDL as compared to the untreated group. In the similar study, the hepatoprotective effect of Allium hookeri (as a species of Allium genus) against high-fat diet-induced fatty liver disease in the guinea pig was demonstrated. In the previous experiment, Allium hookeri reduced the concentrations of cholesterol, triglyceride, and LDL (Lee et al. 2017). Also, there was a similar study reported that Allium hookeri lowered the serum cholesterol and LDL (Won et al. 2013). In another study, Augusti et al. (2001) indicated that Allium sativum Linn treated the fatty liver disease in rats with decreasing the concentrations of triglyceride and cholesterol.
It is revealed that antioxidant compounds played a very necessary and important role in the treatment of fatty liver disease (Ferramosca et al. 2017). In Goodarzi et al. (2018) study, they reported that A. saralicum collected in Kermanshah city was rich in antioxidant compounds including linolenic acid-methyl ester, phytol, neophytadiene 2-phenyl-5-methylindole, hexadecanoic acid, vitamin E, ethanol, 2-tetradecyloxy, n-tetracosane, hexatriacontane, γ-tocopherol, eicosane, n-ethyl-1,3-dithioisoindoline, 2-hexadecene, 3,7,11,15-tetramethyl, hexanedioic acid, and 1,4,8,11-tetraazacyclotetradecane. So, it was normal in our study that A. saralicum treated fatty liver disease in rats.
Conclusion
In accordance with the study, it concludes that aqueous extract of A. saralicum at all doses (especially A160) indicated meaningful hepatoprotective potentials. This extract also revealed amelioration in histopathological and biochemical approaches and so might be of value in the treatment of fatty liver disease.
References
Askari F, Rashidkhani B, Hekmatdoost A (2014) Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr Res 34(2):143–148
Augusti KT, Narayanan A, Pillai LS, Ebrahim RS, Sivadasan R, Sindhu KR, Subha I, Abdeen S, Nair SS (2001) Beneficial effects of garlic (Allium sativum Linn) on rats fed with diets containing cholesterol and either of the oil seeds, coconuts or groundnuts. Indian J Exp Biol 39(7):660–667
Comar KM, Sterling RK (2005) Review article: drug therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther 23(2):207–215
Day CP (2006) From fat to inflammation. Gastroenterol 130:207–210
Day CP (2011) Non-alcoholic fatty liver disease: a massive problem. Clin Med 11(2):176–178
Ebrahimi-Mameghani M, Aliashrafi S, Javadzadeh Y, AsghariJafarabadi M (2014) The effect of Chlorella vulgaris supplementation on liver enzymes, serum glucose and lipid profile in patients with non-alcoholic fatty liver disease. Health Promot Perspect 4(1):107–115
Fan JG, Farrell GC (2009) Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol 50(1):204–210
Farzaei MH, Zangeneh MM, Goodarzi N, Zangeneh A (2018) Stereological assessment of nephroprotective effects of Trachyspermum ammi essential oil against carbon tetrachloride-induced nephrotoxicity in mice. Int J Morphol 36(2):750–757
Ferramosca A, Di Giacomo M, Zara V (2017) Antioxidant dietary approach in treatment of fatty liver: new insights and updates. World J Gastroenterol 23(23):4146–4157
Flora K, Hahn M, Rosen H, Benner K (1998) Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol 93(2):139–143
Ganz M, Csak T, Szabo G (2014) High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J Gastroenterol 20:8525–8534
Ghashghaii A, Hashemnia M, Nikousefat Z, Zangeneh MM, Zangeneh A (2017) Wound healing potential of methanolic extract of Scrophularia striata in rats. Pharm Sci 23(4):256–263
Goodarzi N, Zangeneh MM, Zangeneh A, Najafi F, Tahvilian R (2017) Protective effects of ethanolic extract of Allium Saralicum R.M. Fritsch on CCl4-induced hepatotoxicity in mice. J Rafsanjan Univ Med Sci 16(3):227–238
Goodarzi N, Zangeneh MM, Zangeneh A (2018) The effect of ethanolic extract of Allium saralicum R.M. Fritsch on diabetic hepatopathy in male mice. Sci Res J Shahed Uni 25:21–30
Haga Y, Kanda T, Sasaki R, Nakamura M, Nakamoto S, Yokosuka O (2015) Nonalcoholic fatty liver disease and hepatic cirrhosis: comparison with viral hepatitis-associated steatosis. World J Gastroenterol 21:12989–12995
Hagh-Nazari L, Goodarzi N, Zangeneh MM, Zangeneh A, Tahvilian R, Moradi R (2017) Stereological study of kidney in streptozotocin-induced diabetic mice treated with ethanolic extract of Stevia rebaudiana (bitter fraction). Comp Clin Pathol 26(2):455–463
Hamelian M, Zangeneh MM, Amisama A, Varmira K, Veisi H (2018) Green synthesis of silver nanoparticles using Thymus kotschyanus extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Appl Organometal Chem 32. https://doi.org/10.1002/aoc.4458
Hashemi SJ, Hajiani E, Sardabi EH (2009) A placebo-controlled trial of silymarin in patients with nonalcoholic fatty liver disease. Hepat Mon 9(4):265–270
Hoseinpouran M, Khaki A, Nazem H (2015) Assessment of antioxidant properties of Allium cepa on serum antioxidants and spermatogenesis after consuming tartrazine in rat. Crescent J Med Biol Sci 2(4):125–129
Jacobs BP, Dennehy C, Ramirez G, Sapp J, Lawrence VA (2002) Milk thistle for the treatment of liver disease: a systematic review and meta-analysis. Am J Med 113(6):506–515
Kashkooli RI, Najafi SS, Sharif F, Hamedi A, Asl MKH, Kalyani MN, Birjandi M (2015) The effect of Berberis vulgaris extract on transaminase activities in non-alcoholic fatty liver disease. Hepat Mon 15(2):e25067
Lee N, Lee RM, Lee C (2017) Effects of dietary Allium hookeri root powder on the body fat deposition and biochemical parameters in guinea pigs. J Anim Res Nutr 2(2):1–6
Mohammadifar M, Behnam M, Talaei SA, Khamechian T, Mehran M, Taghizadeh M (2018) Evaluation effect of Silybum marianum, Cynara scolymus L. and Ziziphus jujube Mill. Combination extract on nonalcoholic fatty liver in rats. Iranian J Endocrinol Metab 19(6):410–418
Nikkhajoei M, Choopani R, Tansaz M, Heydarirad G, Hashem-Dabaghian F, Sahranavard S, Saberifiroozi M, Pasalar M (2016) Herbal medicines used in treatment of nonalcoholic fatty liver disease: a mini-review. Galen Med J 5(3):107–113
Ogunlade B, Saalu LC, Ogunmodede OS, Akunna GG, Adeeyo OA, Ajayi GO (2012) The salutary role of Allium cepa extract on the liver histology, liver oxidative status and liver marker enzymes of rabbits submitted to alcohol-induced toxicity. Am J Biochem Mol Biol 2(2):67–81
Panahi Y, Ghamarchehreh ME, Beiraghdar F, Zare R, Jalalian HR, Sahebkar A (2012) Investigation of the effects of Chlorella vulgaris supplementation in patients with non-alcoholic fatty liver disease: a randomized clinical trial. Hepatogastroenterol 59:2099–2103
Sakata R, Nakamura T, Torimura T, Ueno T, Sata M (2013) Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: a double-blind placebo-controlled study. Int J Mol Med 32(5):989–994
Saravanan G, Ponmurugan P (2013) S-allylcysteine improves streptozotocin-induced alterations of blood glucose, liver cytochrome P450 2E1, plasma antioxidant system, and adipocytes hormones in diabetic rats. Int J Endocrinol Metab Disord 11(4):e10927
Sayyedrostami T, Pournaghi P, Ebrahimi Vosta-Kalaeea S, Zangeneh MM (2018) Evaluation of the wound healing activity of Chenopodium botrys leaves essential oil in rats (a short-term study). J Essent Oil Bear Pl 21(1):164–174
Shaker E, Mahmoud H, Mnaa S (2010) Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol 48(3):803–806
Sharafzadeh S, Alizadeh O (2012) Some medicinal plants cultivated in Iran. J Appl Pharm Sci 2(1):134–137
Shavakhi A, Torki M, Khodadoostan M, Shavakhi S (2015) Effects of cumin on nonalcoholic steatohepatitis: a double blind, randomised, controlled trial. Adv Biomed Res. https://doi.org/10.4103/2277-9175.166149
Sherkatolabbasieh H, Hagh-Nazari L, Shafiezadeh S, Goodarzi N, Zangeneh MM, Zangeneh A (2017) Ameliorative effects of the ethanolic extract of Allium saralicum R.M. Fritsch on CCl4-induced nephrotoxicity in mice: a stereological examination. Arch Biol Sci 69(3):535–543
Tamayo C, Diamond S (2007) Review of clinical trials evaluating safety and efficacy of milk thistle (Silybum marianum [L.] Gaertn.). Integr Cancer Ther 6(2):146–157
Won JY, Yoo YC, Kang EJ, Yang H, Kim GH, Seon BJ, Kim SL, Han SH, Lee SS, Lee KS (2013) Chemical components, DPPH radical scavenging activity and inhibitory effects on nitric oxide production in Allium hookeri cultivated under open field and greenhouse conditions. J Korean Soc Food Sci Nutr 42(9):1351–1356
Wong VW, Wong GL, Chan AW, Chu WC, Choi PC, Chim AM, Yiu KK, Yu J, Chan FK, Chan HL (2013) Treatment of non-alcoholic steatohepatitis with Phyllanthus urinaria: a randomized trial. J Gastroenterol Hepatol 28(1):57–62
Zangeneh MM, Goodarzi N, Zangeneh A, Tahvilian R, Najafi F (2018) Amelioration of renal structural changes in STZ-induced diabetic mice with ethanolic extract of Allium saralicum R.M. Fritsch. Comp Clin Pathol 27(4):861–867
Zhaleh M, Sohrabi N, Zangeneh MM, Zangeneh A, Moradi R, Zhaleh H (2018) Chemical composition and antibacterial effects of essential oil of Rhus coriaria fruits in the west of Iran (Kermanshah). J Essent Oil Bear Plants 21(2):493–501
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethic approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Moradi, R., Hajialiani, M., Salmani, S. et al. Effect of aqueous extract of Allium saralicum R.M. Fritsch on fatty liver induced by high-fat diet in Wistar rats. Comp Clin Pathol 28, 1205–1211 (2019). https://doi.org/10.1007/s00580-018-2834-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2834-y