Abstract
Ethnomedicinal plants are recommended for the prevention, control, and treatment of several diseases. Due to the rapid growth of global interest in use of medicinal plants, their effects and safety evaluation have become substantial. In this study, Bellis perennis L aqueous extract (BP) was extracted to investigate its hematoprotective and nephroprotective activities on renal structural, hematological, and biochemical changes in CCl4-induced nephrotoxicity in male mice. Fifty male mice were divided into five groups (n = 10): Group I served as control and received 1 mL/kg olive oil intraperitoneally and 0.5 mL distilled water through gavage; group II served as untreated group and received 1 mg/kg CCl4 mixed with olive oil in the ratio of 1:1, intraperitoneally and 0.5 mL distilled water orally; and groups III, IV, V, and VI received CCl4 mixed with olive oil in the ratio of 1:1 intraperitoneally and 50, 100, and 200 mg/kg of BP through gavage for 45 consecutive days. At 45th day, the mice were dissected, blood and kidney samples collected for hematological, biochemical, and histological parameters analysis. The data were analyzed using one-way ANOVA and post hoc Duncan’s tests. Histologically, different doses of BP (especially BP200) could significantly (p ≤ 0.05) decrease the volume and length of the renal structures as compared to the untreated group. Hematologically, BP could reduce significantly (p ≤ 0.05) the raised levels of WBC, platelet, and increased RBC parameters as compared to the untreated group. Biochemically, BP at all dose (especially BP200) could significantly (p ≤ 0.05) reduce the raised levels of urea and creatinine and increased SOD and CAT levels as compared to the untreated group. BP has protective properties on the kidney and blood, thereby reducing the causation of hematotoxicity and nephrotoxicity in experimental mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The kidneys as one of the most important organs in the body are constantly exposed to poisonous contaminants. Renal failure is a main complication of the kidney, encountered globally (Palm et al. 2004; Tiong et al. 2014). Sodium and water retention, hyperkalemia, metabolic acidosis, and reduction in glomerular filtration rate are other features of renal failure (Kitabchi et al. 2009; Brosius et al. 2009; Le-Devehat et al. 2001; Dhodi et al. 2014; Schetz et al. 2005). Chemical materials and drug-induced nephrotoxicity are one of the leading causes of renal failure (Dhodi et al. 2014; Schetz et al. 2005). A list of chemical materials and drugs that cause nephrotoxicity include warfarin, risperidone, mannitol, paracetamol, hydrochlorothiazide, losartan, furosemide, hydralazine, acetazolamide, captopril, metronidazole, nitrofurantoin, oxacillin, pyrazinamide, vancomycin, benzylpenicillin, ceftazidime, ceftriaxone, ciprofloxacin, clarithromycin, erythromycin, gentamicin, amikacin, amoxicillin, amphotericin B, streptozotocin, and carbon tetrachloride (CCl4) (Brosius et al. 2009; Bicalho et al. 2015).
Carbon tetrachloride (CCl4) is a toxic substance for most organs of the body such as the kidneys, liver, heart, testis, brain, and blood (Breyer et al. 2005; Alia et al. 2003). Furthermore, different documented case studies have established that CCl4 produces diseases with a changed antioxidant status in humans (Alia et al. 2003). Findings from the screening of several ethnomedicinal plants describe their antioxidant effect and demonstrate that they could protect kidney against CCl4 by increasing the levels of antioxidant enzymes (Goodarzi et al. 2017; Sherkatolabbasieh et al. 2017). Some medicinal plants have the high content of alkaloids, flavonoids, naphthaquinone, saponinsand, tannins, and triterpenes, so they can increase the quality and rate of nephrotoxicity (Moradi et al. 2017; Goodarzi et al. 2017; Sherkatolabbasieh et al. 2017; Tahvilian et al. 2017; Foroughi et al. 2016). A list of medicinal plants consumed for their nephroprotective effect include Aerva javanica (fresh roots), Boerhaavia diffusa (root), Rubia cordifolia Linn (root), Curcuma longa (rhizome), Ficus religiosa L (latex), Tectona grandis (bark), Carica papaya (seed), Strychnos potatorum (seed), Tamarindus indica (fruit pulp), Crataeva nurvula (fruit), Punica granatum L (fruit peel), Euphorbia neriifolia (leaf), Acorus calamus (aerial parts), Vernonia cinerea (aerial parts), Orthosiphon stamineus (whole plant), and Aerva lanata (whole plant) (Mohana-Lakshmi et al. 2012). Research on nephroprotective agents is one of the advanced fields in recent biomedical sciences. The expensive costs of modern medicines indicate that alternative strategies are needed for better management of diseases and their related problems.
Bellis perennis L (BP) is an endemic plant of Iran that grows widely in the western parts of the country. The Bellis genus is placed in the Asteraceae family and Asterales order (Nazaruk and Gudej 2001). The consumption and cooking of parts of BP are due to the large variety of flavors and textures of the species. BP have been cultivated from the earliest times, and it is economically important as garden vegetable (Karakaş et al. 2012). In traditional medicine, several extracts of this plant are traditionally used in treating different inflammatory, gastric ulcer, bacterial, parasitic, viral, and fungal diseases (Karakaş et al. 2012; Li et al. 2005; Morikawa et al. 2008). In this study, we checked the ameliorative effect of the BP by studying the microscopic structural changes in mice kidney after CCl4-induced nephrotoxicity using modern design-based stereological methods. Renal functions were also evaluated out by examining hematological and biochemical biomarkers.
Materials and methods
Plant extraction
BP at maturity were collected from around of Kermanshah city during August 2017. Leaves of the plant were shade dried for 1 week. About 200 g of the obtained dried powder was extracted with 2000 mL of distilled water for 2 h at 40 °C with continuous shaking. In rotary evaporator, the extract was concentrated and then lyophilized.
Animals
In this study, 50 male Balb/c mice weighing between 38 and 40 g were fed with standard pellet diet (metabolism energy 2860 kcal/kg, crude protein 21.5%, crude fiber 3.55%, calcium 1.05%, phosphor 0.5%, sodium 0.17%, chlorine 0.23%, methionine (digestible) 0.59%, methionine + cysteine (digestible) 0.92%, lysine (digestible) 1.2%, arginine (digestible) 1.33%, threonine (digestible) 0.82%, linoleic acid 1.5%, dry matter 88%) and water ad libitum conditions (standard environmental and nutritional) during the study.
Experimental design
The mice were divided into five groups of 10 mice each. Group I served as control and received 1 mL/kg olive oil intraperitoneally and 0.5 mL distilled water through gavage. Group II served as untreated group and received 1 mg/kg CCl4 mixed with olive oil in the ratio of 1:1, intraperitoneally and 0.5 mL distilled water orally. Groups III, IV, and V received CCl4 mixed with olive oil in the ratio of 1:1 intraperitoneally and 50, 100, and 200 mg/kg of BP (BP50, BP100, and BP200) through gavage, respectively. The animals treated twice a week for 45 consecutive days. At the end of the 45-day treatment, the mice were euthanized by ketamine HCl (40 mg/kg; Goodarzi et al. 2017; Sherkatolabbasieh et al. 2017). Immediately, blood samples were drawn from animals’ heart and inserted in plasma (for determination of hematological parameters) and serum (for determination of urea and creatinine) tubes.
The ability of the extracts to boost the capacity of antioxidant enzymes was evaluated by determining the activity of two endogenous antioxidant enzymes (superoxide dismutase (SOD) and catalase (CAT)) as follows:
Superoxide dismutase
SOD activity was evaluated according to the method described by Martin et al. (1987). Exactly 920 μL of assay buffer was added into clean test tube containing 40 μL of sample, mixed and incubated for 2 min at 25 °C, following which 40 μL of hematoxylin solution was added, mixed quickly, and the absorbance was measured immediately at 560 nm.
Catalase
CAT activity was measured using the procedure reported by Abei (1974). Briefly, a total of 10 μl of sample was added to the test tube containing 2.8 ml of 50 mmol/L phosphate buffer (pH 7.0). The reaction was initiated by adding 0.1 mL of fresh 30 mmol/L H2O2, and the decomposition rate of H2O2 was measured at 240 nm for 5 min on a spectrophotometer. A molar extinction coefficient of 0.0411 mmol/L/cm was used to calculate CAT activity.
For histological studding, the left kidney was weighed and then fixed in 10% neutral buffered formalin solution for 1 week. Total kidney volume is obtained from kidney dipping method in water. For assessment of volume and length kidney subcomponents, we used the Mandarim-de-Lacerda (2003) protocol:
In this protocol, immersion method was then used to determine the primary volume of the kidney. To estimate the final volume of the organs, the amount of tissue shrinkage must be specified. Isotropic Uniform Random (IUR) sections must be obtained to estimate tissue shrinkage and tubular length. These sections were achieved using orientator method. Totally, 7–10 slabs were obtained from each kidney through orientator method. A circular piece was sampled from a kidney slab, and the area of this piece was calculated. The slabs and circular piece were processed, sectioned (5 μm thicknesses), and stained by Periodic Acid Schiff (PAS) method. The area of the circular piece was calculated again, and tissue shrinkage was estimated by the following relation:
where AA and AB are the areas of the circular piece after and before tissue processing. The total volume of the organ was then estimated using:
Tissue sections were examined using a video microscopy system composed of a microscope (Olympus CX2, Japan) connected to a video camera (Dinocapture ver.5, dino-lit.com 30.5 mm) and a P4 PC, and the stereological parameters were estimated. The fractional volume of the renal structures was estimated using a point probe (with an area of 100 cm2 and containing 25 points) and the following formula (Fig. 1):
Body weight levels in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
- P structure :
-
sum of points hitting the interested structures
- P reference :
-
sum of points hitting the reference space
Length density
The length density of the tubules and vessels was estimated using an unbiased counting probe (740 × 740 μm). The tubule structures were considered in such a manner that they were lying completely or partly inside the counting probe and did not touch the down and left lines. Otherwise, they were not considered (Fig. 2). The length density was estimated by the following formula:
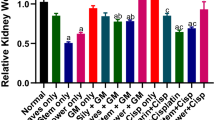
Urea and creatinine levels in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
∑Q = sum of the tubules counted, a (frame) = probe area, 547600 μm2, and ∑frame = total number of the counted frames.
Statistical analysis
The results were analyzed by SPSS-18 software-Duncan’s post hoc test.
Results
Effect of BP on body weight
Body weight decreased significantly (p ≤ 0.05) in untreated mice compared to the control group (Fig. 1). Administration of BP at all doses could significantly (p ≤ 0.05) increase body weight in comparison with the untreated group.
Effect of BP on levels of kidney biochemical parameters
The estimated values of the kidney biochemical parameters are presented in Figs. 2 and 3. CCl4-induced toxicity increased urea and creatinine but decreased SOD and CAT levels significantly (p ≤ 0.05) as compared to the control group. Several doses of BP could significantly (p ≤ 0.05) ameliorate above parameters. There is no significant difference (p ≤ 0.05) between BP200 and control groups in creatinine, SOD, and CAT levels.
The levels of SOD and CAT in kidney in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract, SOD superoxide dismutase, CAT catalase. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
Effect of BP on hematological parameters
The number of white blood cell (WBC) and platelet was increased, but the levels of red blood cell (RBC), packed cell volume (PCV), mean corpuscular volume (MCV), hemoglobin (Hb), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and monocyte decreased significantly (p ≤ 0.05) in untreated mice. Treatment with several doses of BP significantly (p ≤ 0.05) reduced WBC and platelet number and increased the levels of RBC, PCV, MCV, Hb, MCH, MCHC, and monocyte in comparison of untreated group. There is no difference significant among all groups in percent of lymphocyte, neutrophil and eosinophil (p ≤ 0.05). Also, administration of BP at all doses could significantly (p ≤ 0.05) increase percent of monocyte similar to the control group (Figs. 4, 5, 6, 7, 8, 9, 10, and 11).
RBC numbers in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract, RBC red blood cell. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
PCV levels in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract, PCV packed cell volume. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
MCV levels in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract, MCV mean corpuscular volume. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
The levels of MCHC and Hb in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract, Hb hemoglobin, MCHC mean corpuscular hemoglobin concentration. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
The levels of MCH in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract, MCH mean corpuscular hemoglobin. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
Platelet numbers in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
WBC numbers in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract, WBC white blood cell. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
Lymphocytes, monocytes, neutrophils, eosinophils, and basophils percent in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
Effect of BP on stereological parameters
Administration of BP could significantly (p ≤ 0.05) ameliorate the kidney, cortical, and medullary volumes compared to the untreated group. In addition, the difference of medulla volume between BP200 and control groups was not significant (p ≤ 0.05, Figs. 12 and 13).
Weights of kidney in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
Absolute volume of the kidney (mm3), and absolute volume (mm3) of cortex and medulla in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
The volume of proximal convoluted tubule, distal convoluted tubule, collecting duct, loop of Henle, vessels, and interstitial tissue were enhanced significantly (p ≤ 0.05) in untreated mice compared to the control ones (Figs. 14 and 15). Administration of BP at all doses could significantly (p ≤ 0.05) decrease the volume of the above structures in comparison with the untreated group. Also, several doses of BP significantly (p ≤ 0.05) reduced the volumes of distal convoluted tubule and collecting duct similar to control group. The difference of loop of Henle volume between BP200 and control groups was not significant (p ≤ 0.05).
Absolute volume (mm3) of proximal, collecting ducts and distal convoluted tubules in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
Absolute volume (mm3) of interstitial tissues, vessels and loop of Henle in all of the experimental groups. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
The data of the mean absolute length of kidney subcomponents in treated and untreated groups are shown in Fig. 16. Length of the proximal convoluted tubule, distal convoluted tubule, collecting duct, loop of Henle, and vessels were significantly (p ≤ 0.05) increased in untreated mice compared to the control ones. BP at all doses could significantly (p ≤ 0.05) reduce the length of the above parameters compared to the untreated groups (p ≤ 0.05). There is no significant difference (p ≤ 0.05) between BP200 and control groups in length of vessel.
Absolute length (m) of the vessels, collecting ducts, proximal and distal convoluted tubules, and loop of Henle. C control, UT untreated, BP50 treated with 50 mg/kg of Bellis perennis L aqueous extract, BP100 treated with 100 mg/kg of Bellis perennis L aqueous extract, BP200 treated with 200 mg/kg of Bellis perennis L aqueous extract. Non-identical letters indicate a significant difference between the groups (p ≤ 0.05)
Discussion
Ethnomedicinal plants are popular remedies used by people (Zangeneh et al. 2017). The impression of medicinal plants in prevention and treatment of diseases is irrecusable (Foroughi et al. 2017; Najafi et al. 2017; Poorshamohammad et al. 2017). They have the immense potential for the management and remedy of every disease such as nephrotoxicity (Goodarzi et al. 2018; Hagh-Nazari et al. 2017; Ghashghaii et al. 2017). In this experimental study, the nephroprotective effect of BP at different doses was determined in CCl4-induced nephrotoxicity in mice model. But, to our knowledge, this is the first time BP with these doses has been used from experimentally induced nephrotoxicity in mice, and there is no information about other beneficial effects of BP.
Nephroprotective effect of BP
Renal inconveniences are assessed by the elevated histological examination as well as by serum levels of cytoplasmic parameters (Tay et al. 2005; Mishra et al. 2014). During the short-term study, the administration of BP ameliorate the renal morphological changes at all doses especially 200 dose. Untreated mice indicated some degree of renal hypertrophy which was mainly due to the enlargement of the cortex, medullary, and its subcomponents. The pathogenesis of renal hypertrophy can be attributed to the overproduction of oxygen-free radicals following administration of toxins such as CCl4, which is expressed in response to cytokines (Mishra et al. 2014). Thus, this study suggested that BP could be used to ameliorate renal structural changes due to CCl4-induced toxicity. Agreeing with this experiment, studies indicated that ethnomedicinal plants decrease weight and volumes of kidney and its subcomponents in CCl4-induced nephrotoxicity in mice (Sherkatolabbasieh et al. 2017; Zangeneh et al. 2018; Farzaei et al. 2018).
The enhanced serum parameter levels such as creatinine and urea have been attributed to the blemished structural integrity of the kidney, because these are cytoplasmic in location and are released into the circulation after cellular injury (Mishra et al. 2014). Also, nephrotoxicity reduces the concentration of antioxidants, ascorbic acid, catalase, superoxide dismutase, glutathione, and vitamin-E which is the protective tissue that reacts and removes reactive oxygen species (Mishra et al. 2014). In our study, we observed acute renal damage in toxic group mice following CCl4 administration manifested by normal shifts in renal antioxidant enzymes activities (CAT ↓, SOD ↓) and renal function tests (urea ↑, creatinine ↑) in renal tissue with altered histopathological signs as compared to the control mice. But, BP at all dose could significantly (p ≤ 0.05) ameliorate above parameters.
Hematoprotective activity of BP
Alteration in the various hematological parameters and the immune system during the course of toxicity have been reported (Saba et al. 2010). Previous studies indicated that administration of toxins such as CCl4 yielded microcytic hypochromic anemia and enhanced white blood cell and platelet count (Saba et al. 2010; Swenson 1993; Huff et al. 2005; Larson et al. 1985). Agreeing with the above reports, in our present study, the number of WBC and platelet were increased and levels of RBC parameters reduced significantly (p ≤ 0.05) in untreated mice. But, treatment with BP at all doses significantly (p ≤ 0.05) reduced WBC and platelet numbers and enhanced RBC parameters levels in comparison of untreated mice.
Conclusion
The new study has revealed the nephroprotective and hematoprotective activity of the BP, offering its possible use as a therapeutics supplement or drug. Additional clinical trial studies would be needed to justify and further assess the potential of the plant as a nephroprotective and hematoprotective agent in human.
References
Abei H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 673–684
Alia M, Horcajo C, Bravo L, Goya L (2003) Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr Res 23(9):1251–1267
Bicalho MD, Soares DB, Botoni FA, Reis AMM, Martins MAP (2015) Drug-induced nephrotoxicity and dose adjustment recommendations: agreement among four drug information sources. Int J Environ Res Public Health 12(9):11227–11240
Breyer MD, Bottinger E, Brosius FC, Coffman TM, Harris RC, Heilig CW, Sharma K, AMDCC (2005) Mouse models of diabetic nephropathy. J Am Soc Nephrol 16(1):27–45
Brosius FC, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T (2009) Mouse models of diabetic nephropathy. J Am Soc Nephrol 20(12):2503–2512
Dhodi DK, Bhagat SB, Pathak D, Patel SB (2014) Drug-induced nephrotoxicity. Int J Basic Clin Pharmacol 3(4):591–597
Farzaei MH, Zangeneh MM, Goodarzi N, Zangeneh A (2018) Stereological assessment of nephroprotective effects of Trachyspermum ammi essential oil against carbon tetrachloride-induced nephrotoxicity in mice. Int J Morphol 36(2):750–757
Foroughi A, Pournaghi P, Najafi F, Zangeneh A, Zangeneh MM, Moradi R (2016) Antibacterial effect and phytochemical screening of essential oil of Pimpinella anisum against Escherichia coli O157:H7 and Staphylococcus aureus. Int J Current Med Pharm Res 7(6):367–371
Foroughi A, Pournaghi P, Najafi F, Zangeneh A, Zangeneh MM, Moradi R (2017) Medicinal plants: antibacterial effects and chemical composition of essential oil of Foeniculum vulgare. Int J Curr Pharm Rew Res 8(1):13–17
Ghashghaii A, Hashemnia M, Nikousefat Z, Zangeneh MM, Zangeneh A (2017) Wound healing potential of methanolic extract of Scrophularia striata in rats. Pharm Sci 23(4):256–263
Goodarzi N, Zangeneh MM, Zangeneh A, Najafi F, Tahvilian R (2017) Protective effects of ethanolic extract of Allium Saralicum R.M. Fritsch on CCl4-induced hepatotoxicity in mice. J Rafsanjan Univ Med Sci 16(3):227–238
Goodarzi N, Zangeneh MM, Zangeneh A (2018) The effect of ethanolic extract of Allium Saralicum R.M. Fritsch on diabetic hepatopathy in male mice. Sci Res J Shahed Univ 25:21–30
Hagh-Nazari L, Goodarzi N, Zangeneh MM, Zangeneh A, Tahvilian R, Moradi R (2017) Stereological study of kidney in streptozotocin-induced diabetic mice treated with ethanolic extract of Stevia rebaudiana (bitter fraction). Comp Clin Pathol 26(2):455–463
Huff GR, Huff WE, Balog JM, Rath NC, Anthony NB, Nestor KE (2005) Stress response differences and disease susceptibility reflected by heterophil to lymphocyte ratio in turkeys selected for increased body weight. Poult Sci 84(5):709–717
Karakaş FP, Karakaş A, Boran Ç, Türker AU, Yalçin FN, Bilensoy E (2012) The evaluation of topical administration of Bellis perennis fraction on circular excision wound healing in Wistar albino rats. Pharm Biol 50(8):1031–1037
Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN (2009) Hyperglycemic crises in adult patients with diabetes. Diabetes Care 32(7):1335–1343
Larson CT, Gross WB, Davis JW (1985) Social stress and resistance of chicken and swine to Staphylococcus aureus challenge infections. Can J Comp Med 49(2):208–210
Le-Devehat C, Khodabandehlou T, Vimeux M (2001) Impaired hemorheological properties in diabetic patients with lower limb arterial ischaemia. Clin Hemorheol Microcirc 25(2):43–48
Li W, Aaada Y, Koike K, Nikaido T, Furuya T, Yoshikawa T, Bellisosides AF (2005) Six novel acylatedtriterpenoidsaponins from Bellis perennis (Compositae). Tetrahedron 61(11):2921–2929
Mandarim-de-Lacerda CA (2003) Stereological tools in biomedical research. An Acad Bras Cienc 75(4):469–486
Martin JP Jr, Dailey M, Sugarman E (1987) Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys 255(2):329–336
Mishra S, Ranjan-Pani S, Sahoo S (2014) Anti-nephrotoxic activity of some medicinal plants from tribal rich pockets of Odisha. Pharm Res 6(3):210–217
Mohana-Lakshmi S, Usha-Kiran-Reddy T, Sandhya-Rani KS (2012) A review on medicinal plants for nephroprotective activity. Asian J Pharm Clin Res 5(4):8–14
Moradi R, Hajialiani M, Zangeneh MM, Zangeneh A, Faizi S, Zoalfaghari M, Marabi A (2017) Study a plant extract as an antibacterial agent. Int J Curr Med Pharm Res 3(2):1360–1362
Morikawa T, Li X, Nishida E, Nakamura S, Ninomiya K, Matsuda H, Ody Y, Muraoka O, Yoshikawa M (2008) Perennisosides I-VII, acylatedtriterpenesaponins with antihyperlipidemic activities from the flowers of Bellis perennis. J Nat Prod 71(5):828–835
Najafi F, Goodarzi N, Zangeneh MM, Zangeneh A, Hagh-Nazari L (2017) Antidiabtic and hepatoprotective effects of bitter fraction of Stevia rebaudiana alcoholic extract on streptozotocin-induced diabetic male mice. J Rafsanjan Univ Med Sci 16(6):493–504
Nazaruk J, Gudej J (2001) Qualitative and quantitative chromatographic investigation of flavonoids in Bellis perennis L. Acta Pol Pharm 58(5):401–404
Palm F, Ortster H, Hansell P, Liss P, Carlsson PO (2004) Differentiating between effects of streptozotocin per se and subsequent hyperglycemia on renal function and metabolism in the streptozotocin diabetic rat model. Diabetes Metab Res Rev 20(6):452–459
Poorshamohammad C, Souri N, Amini Z, Kosari F, Jamshidpour R, Zangeneh MM, Zangeneh A (2017) Cucurbita moschata: a plant with antibacterial properties. Int J Current Med Pharm Res 3(2):1356–1359
Saba AB, Oyagbemi AA, Azeez OI (2010) Amelioration of carbon tetrachloride-induced hepatotoxicity and haemotoxicity by aqueous leaf extract of Cnidoscolus aconitifolius in rats. Nig J Physiol Sci 25(2):139–147
Schetz M, Dasta J, Goldstein S, Golper T (2005) Drug-induced acute kidney injury. Curr Opin Crit Care 11(6):555–565
Sherkatolabbasieh H, Hagh-Nazari L, Shafiezadeh S, Goodarzi N, Zangeneh MM, Zangeneh A (2017) Ameliorative effects of the ethanolic extract of Allium saralicum R.M. Fritsch on CCl4-induced nephrotoxicity in mice: a stereological examination. Arch Biol Sci 69(3):535–543
Swenson MJ (1993) Physiological properties and cellular and chemical constituent of blood. In: Dukes’ Physiology of domestic Animals Comstock Publishing Associates, Ithaca. 29–32
Tahvilian R, Moradi R, Zhaleh H, Zangeneh MM, Zangeneh A, Yazdani H, Hajialiani M (2017) Chemical composition and screening of antibacterial activity of essential oil of Pistacia khinjuk against two selected pathogenic bacteria. Ann Trop Med Public Health 10(5):1159–1164
Tay YC, Wang Y, Kairaitis L, Rangan GK, Zhang C, Harris DCH (2005) Can murine diabetic nephropathy be separated from superimposed acute renal failure? Kidney Int 68(1):391–398
Tiong HY, Huang P, Xiong S, Li Y, Vathsala A, Zink D (2014) Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Mol Pharm 11(7):1933–1948
Zangeneh MM, Najafi F, Tahvilian R, Salmani S, Haghnazari L, Zangeneh A, Moradi R (2017) Ethnomedicinal plants: in vitro antibacterial effects of ethanolic extract of Stevia rebaudiana. Int J Ayu Pharm Chem 6(1):251–259
Zangeneh MM, Goodarzi N, Zangeneh A, Tahvilian R, Najafi F (2018) Amelioration of renal structural changes in STZ-induced diabetic mice with ethanolic extract of Allium saralicum R.M. Fritsch. Comp Clin Pathol. https://doi.org/10.1007/s00580-018-2674-9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zangeneh, M.M., Zangeneh, A., Tahvilian, R. et al. Preclinical evaluation of hematoprotective and nephroprotective activities of Bellis perennis L aqueous extract on CCl4-induced renal injury in mice. Comp Clin Pathol 27, 1557–1566 (2018). https://doi.org/10.1007/s00580-018-2773-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2773-7