Abstract
Medicinal plants are considered as modern resources for producing agents that could act as alternatives to chemical drugs. Falcaria vulgaris (FV) has been used in medicine as an antioxidant, antifungal, anti-inflammatory, and antibacterial agent. The aim of the present study was to investigate the hepatoprotective and hematoprotective effects of FV aqueous extract against CCl4-induced hepatotoxicity in mice. Sixty male mice were divided into six groups (n = 10); group I served as control, received 1 mL/kg olive oil intraperitoneally and 0.5 mL distilled water through gavage. Group II served as untreated group, received 1 mg/kg CCl4 mixed with olive oil in the ratio of 5:5 intraperitoneally and 0.5 mL distilled water orally. Groups III, IV, V, and VI received CCl4 mixed with olive oil in the ratio of 5:5 intraperitoneally and 20, 40, 80, and 160 mg/kg of FV aqueous extract through gavage for 45 consecutive days. Different doses of FV (especially FV160) could significantly (p ≤ 0.05) decrease the raised levels of alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, cholesterol, low-density lipoprotein, white blood cell, and platelet and increase high-density lipoprotein, superoxide dismutase, catalase, and red blood cell as compared to the untreated group. The weight and volume of the hepatic structures were decreased significantly (p ≤ 0.05) in different doses of FV (especially FV160) compared to the untreated group. FV aqueous extract can protect hepatic tissue and regulate liver enzymes in CCl4-induced hepatotoxicity. FV has hepatoprotective and hematoprotective properties, thereby reducing the causation of diabetes in experimental mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon tetrachloride (CCl4) is a toxic substance for most organs of the body such as the liver, kidneys, heart, testes, brain, and blood (Alia et al. 2003). Furthermore, different documented case studies have established that CCl4 produces hepatic disease with a changed antioxidant status in humans (Alia et al. 2003). Findings from the screening of several ethno medicinal plants describe their antioxidant effect and demonstrate that they could protect the liver against CCl4 by increasing the levels of antioxidant enzymes (Goodarzi et al. 2017; Sherkatolabbasieh et al. 2017). Research on hepatoprotective agents is one of the advanced fields in recent biomedical sciences. The expensive costs of modern medicines indicate that alternative strategies are need for better management of diseases and their related problems.
Ethno medicinal plants are popular remedies used by most people (Faramarzi et al. 2017; Foroughi et al. 2016; Tahvilian et al. 2017). The impression of ethno medicinal plants in prevention and treatment of diseases is irrecusable (Najafi et al. 2017). Aqueous extract is a kind of fraction of plants that includes aromatic and nonaromatic compounds (Moradi et al. 2017). This fraction could be extracted from several parts such as fruits, flowers, leaves, stems, and roots (Poorshamohammad et al. 2017). In recent years, interest in aqueous extract has been enhanced for pharmacological studies which claim that the aqueous extract with decreasing free radicals has useful effect for prevention and treatment of liver disease (Pooyanmehr et al. 2017; Zangeneh et al. 2017).
Falcaria vulgaris (FV) (Paghazou and Ghaziaghi in Persian) grows widely in the western parts of Iran (Ahvazi et al. 2012). The Falcaria genus is placed in the Apiales order and Apiaceae family (Ahvazi et al. 2012). FV have been cultivated from the earliest times and it is economically important as garden vegetable (Jaberian et al. 2013). The consumption and cooking of parts of FV are due to the large variety of flavors and textures of the species (Khazaei and Salehi 2006). FV as an eatable plant has generated a lot of interest throughout human history as a medicinal plant (Ahvazi et al. 2012). In traditional medicine, several extracts of this plant are traditionally used in treating different inflammatory, bacterial, parasitic, viral, and fungal diseases; gastric ulcer; and diabetes (Jaberian et al. 2013; Khazaei and Salehi 2006).
In the present study, we investigated the ameliorative property of the FV aqueous extract by studying the microscopic structural changes in mouse liver after CCl4-induced hepatotoxicity using modern design-based stereological methods. Hepatic functions were also investigated by examining hematological and biochemical biomarkers.
Materials and methods
Plant collection
FV at maturity were collected from around of Kermanshah city during May 2017. The plant was identified for the first time at the herbarium of research center of Faculty of Agriculture, Razi University, Kermanshah, Iran.
Plant extraction
Leaves of the plant were shade dried for 1 week. Dried aerial parts of the plants were ground and about 150 g of the obtained powder was extracted with 450 mL of distilled water for 2 h at 40 °C with continuous shaking. The extract was left for 24 h at room temperature; then, it was filtered through Whatman paper no. 2. In a rotary evaporator, the extract was concentrated, then lyophilized.
Animals
Male Balb/c mice weighing between 38 and 40 g were procured from laboratory animal center of Kermanshah University of Medical Sciences. The animals were housed in an air-conditioned room (22 ± 2 °C) with 12-h light/dark cycle and has free access to standard pellet diet (metabolism energy 2860 kcal/kg, crude protein 21.5%, crude fiber 3.55%, calcium 1.05%, phosphorous 0.5%, sodium 0.17%, chlorine 0.23%, methionine (digestible) 0.59%, methionine + cysteine (digestible) 0.92%, lysine (digestible) 1.2%, arginine (digestible) 1.33%, threonine (digestible) 0.82%, linoleic acid: 1.5%, dry matter 88%) and water ad libitum conditions (standard environmental and nutritional) during the study.
Experimental design
In the present study, a total of 60 mice were used. The mice were divided into six groups of ten mice each. Group I served as control, received 1 mL/kg olive oil intraperitoneally and 0.5 mL distilled water through gavage. Group II served as untreated group, received 1 mg/kg CCl4 mixed with olive oil in the ratio of 5:5 intraperitoneally and 0.5 mL distilled water orally. Groups III, IV V, and VI received CCl4 mixed with olive oil in the ratio of 5:5 intraperitoneally and 20, 40, 80, and 160 mg/kg of FV (FV20, FV40, FV80, and FV160) through gavage, respectively. The animals treated twice a week for 45 consecutive days. At the end of the 45-day treatment, the animals of all the groups were euthanized by xylazine (5 mg/kg) and ketamine HCl (40 mg/kg). Immediately after euthanizing, blood samples were drawn from animals’ heart and inserted in serum (for determination of alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL)) and plasma (for determination of red blood cell (RBC), white blood cell (WBC), and platelet) bottles. All above parameters were measured by available commercial kits (Pars Azmun Co., Iran) according to its procedures. Also, the capacity of antioxidant enzymes was assessed by determining the activity of superoxide dismutase (SOD) and catalase (CAT) in the liver using the procedures reported by Abei (1974) and Martin et al. (1987).
Histological study
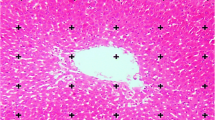
The whole liver of each group (n = 10) weighed and immersed in 10% neutral buffered formaldehyde. Liver volume is obtained from liver dipping method in water. After 72-h fixation, the livers were cut using orientator method. Totally, 7–10 slabs were collected from each liver. The slabs were embedded in paraffin and sections (5 μm thicknesses) were prepared and stained by hematoxylin and eosin stain.
Volume density of liver structures including hepatocytes, sinusoids, central veins, portal veins, hepatic arteries, and bile ducts was estimated with point counting rule briefly as follows: one section from each liver was used. The images of microscopical fields from each section were projected on point probe (frame 15 cm × 15 cm) by a video projector via a microscope equipped with a camera (Dinocapture ver.5, dino-lit.com 30.5 mm) attached to a computer.
At total magnification of × 2000, points that hit desired structures were counted and volume density was estimated using the following formula:
where “Pstructure” and “Preference” were the number of points falling on the structure’s profile and on the reference space, respectively. Ten to 14 microscopic fields were examined in each liver (Mandarim-de-Lacerda 2003; Nyengaard 1999).
Statistical analysis
Descriptive statistics including the mean, standard error, median, minimum, and maximum were calculated for all variables. The one-way ANOVA followed by Duncan’s post hoc test was used for comparison of different parameters. The data were analyzed by SPSS software, version 22.0 (SPSS Inc., Chicago, IL, USA) and p ≤ 0.05 was accepted as statistically significant.
Results
Effect of CCl4 and FV on levels of liver biochemical parameters
The estimated values of the liver enzymes are presented in Figs. 1, 2, and 3. CCl4-induced toxicity increased ALP, AST, ALT, cholesterol, and LDL and decreased HDL, SOD, and CAT significantly (p ≤ 0.05) as compared to the control group. Different doses of FV could significantly (p ≤ 0.05) decrease the raised levels of ALP, AST, ALT, cholesterol, and LDL and increase HDL, SOD, and CAT significantly (p ≤ 0.05) as compared to the untreated group. There is no difference significant between FV160 and control in ALP, LDL, and HDL levels (p ≤ 0.05).
Effect of FV on hematological parameters
The numbers of WBC and platelet were increased and the number of RBC decreased significantly (p ≤ 0.05) in untreated mice. Treatment with FV significantly (p ≤ 0.05) reduced WBC and platelet number and increased RBC number in comparison with the untreated group. Also, there is no difference significant between FV160 and control in RBC number (p ≤ 0.05) (Figs. 4, 5, and 6).
Effect of FV on stereological parameters
Administration of FV at all doses could significantly (p ≤ 0.05) improve the liver volume compared to the untreated group (Figs. 7 and 8). The volumes of hepatocytes, central veins, sinusoids, portal veins, hepatic arteries, and bile ducts were increased significantly (p ≤ 0.05) in untreated diabetic mice compared to the control ones (Figs. 9, 10, and 11). Administration of FV at all doses could significantly (p ≤ 0.05) decrease the volume of the above structures in comparison with the untreated group. Administration of FV160 could significantly (p ≤ 0.05) reduce volume of central veins, portal veins, and hepatic arteries similar to the control group. FV80 could significantly (p ≤ 0.05) decrease volumes of central veins and hepatic arteries similar to the FV160 and control groups. Also, there was no significant difference in volume of hepatic arteries (p ≤ 0.05) among FV20, FV40, and control groups.
Discussion
Medicinal plants are popular remedies used by people. The impression of medicinal plants in prevention and treatment of diseases is irrecusable (Foroughi et al. 2017; Najafi et al. 2016). Plants have the immense potential for the management and remedy of every disease such as hepatotoxicity (Hagh-Nazari et al. 2017; Ghashghaii et al. 2017). A list of medicinal plants that are consumed for their hepatoprotective property includes Fagonia schweinfurthii (whole plant), Vitex glabrata (whole plant), Astragalus kahiricus (root), Zingiber officinale Roscoe (rhizome), Cissus quadrangularis (stem), Feronia limonia (stem bark), Terminalia paniculata (bark), Melastoma malabathricum L (leaf), Ficus religiosa (leaf), Feijoa sellowiana (fruit and peel), Garcinia indica (fruit rind), Daucus carota (seed), Moringa oleifera Lam (seed oil), Abelmoschus manihot (flower), and Acacia nilotica Linn (aerial parts) (Roy et al. 2014).
In this experimental study, the hepatoprotective property of FV at various doses was determined in CCl4-induced hepatotoxicity in mouse model. But, to our knowledge, this is the first time FV with these doses has been used from experimentally induced hepatotoxicity in mice.
Hepatoprotective effect of FV
The extension of hepatic damages is assessed by the elevated serum levels of cytoplasmic enzymes as well as by histological examination (Recknagel et al. 1991; Zangar et al. 2000). The increased serum parameter levels such as ALP, AST, ALT, cholesterol, and LDL have been attributed to the damaged structural integrity of the liver (Recknagel et al. 1991; Zangar et al. 2000). Administration of CCl4 produced liver damage in mice as manifested by the rise in serum parameter levels of ALP, AST, ALT, cholesterol, and LDL. The raised levels of above parameters were significantly decreased following administration of different doses of FV. Also, FV at all doses (especially 160 mg/kg) with ameliorating of liver function increased levels of HDL, SOD, and CAT. The hepatoprotective effect of FV in the present study may be partly related to its anti-inflammatory and antioxidant constituents. Previous studies indicated that the FV is rich of spathulenol, carvacrol, alpha-pinene, and limonene (anti-inflammatory and antioxidant constituents). So FV have strong anti-inflammatory and antioxidant properties (Khanahmadi and Shahrezaei 2008; Shafaghat 2011; Jaberian et al. 2013).
Other results of this study indicated that the liver of CCl4-treated mice demonstrated a considerable hypertrophy which leads to increase in weight, volume, and length of the hepatic structures. These changes were alleviated significantly in all doses of FV (especially 160 mg/kg). Thus, this study suggested that FV could be used to meliorate hepatic structural changes due to CCl4-induced toxicity.
Hematoprotective effect of FV
Alteration in the various hematological parameters and the immune system during the course of hepatotoxicity has been reported (Saba et al. 2010). Previous studies indicated that CCl4 administration yielded microcytic hypochromic anemia by the reduction in the RBC (Saba et al. 2010; Swenson 1993). Acute stress associated with CCl4 has been widely reported to be associated with increased white blood cell and platelet count occasioned by significant increase in the neutrophil count and the neutrophil/lymphocyte ratio (Huff et al. 2005; Larson et al. 1985). Agreeing with above reports, in our present study, the numbers of WBC and platelet were increased and number of RBC reduced significantly (p ≤ 0.05) in untreated mice. Treatment with FV at all doses (especially FV160) significantly (p ≤ 0.05) reduced WBC and platelet numbers and enhanced RBC numbers in comparison with untreated mice.
Conclusion
Finally, it can be concluded that FV can protect the liver against CCl4-induced hepatocellular injuries at all dose (especially 160 mg/kg). Clinical trial studies would be needed to justify and further assess the potential of the plant as a hepatoprotective and hematoprotective agent in human.
References
Abei H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 673–684
Ahvazi M, Khalighi-Sigaroodi F, Charkhchiyan MM, Mojab F, Mozaffarian VA, Zakeri H (2012) Introduction of medicinal plants species with the most traditional usage in Alamut region. Iran J Pharm Res 11(1):185–194
Alia M, Horcajo C, Bravo L, Goya L (2003) Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr Res 23(9):1251–1267
Faramarzi E, Zangeneh MM, Zangeneh A, Moradi R (2017) Effect of Cinnamomum zelanicumon oil on hyponeophagia anxiety test in Balb C male mice. Onl J Vet Res 21(2):77–80
Foroughi A, Pournaghi P, Najafi F, Zangeneh A, Zangeneh MM, Moradi R (2016) Antibacterial effect and phytochemical screening of essential oil of Pimpinella anisum against Escherichia coli O157:H7 and Staphylococcus aureus. Int J Current Med Pharm Res 7(6):367–371
Foroughi A, Pournaghi P, Najafi F, Zangeneh A, Zangeneh MM, Moradi R (2017) Medicinal plants: antibacterial effects and chemical composition of essential oil of Foeniculum vulgare. Int J Curr Pharm Rew Res 8(1):13–17
Ghashghaii A, Hashemnia M, Nikousefat Z, Zangeneh MM, Zangeneh A (2017) Wound healing potential of methanolic extract of Scrophularia striata in rats. Pharm Sci 23(4):256–263
Goodarzi N, Zangeneh MM, Zangeneh A, Najafi F, Tahvilian R (2017) Protective effects of ethanolic extract of Allium Saralicum R.M. Fritsch on CCl4-induced hepatotoxicity in mice. J Rafsanjan Univ Med Sci 16(3):227–238
Hagh-Nazari L, Goodarzi N, Zangeneh MM, Zangeneh A, Tahvilian R, Moradi R (2017) Stereological study of kidney in streptozotocin-induced diabetic mice treated with ethanolic extract of Stevia rebaudiana (bitter fraction). Comp Clin Pathol 26(2):455–463
Huff GR, Huff WE, Balog JM, Rath NC, Anthony NB, Nestor KE (2005) Stress response differences and disease susceptibility reflected by heterophil to lymphocyte ratio in turkeys selected for increased body weight. Poult Sci 84(5):709–717
Jaberian H, Piri K, Nazari J (2013) Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem 136(1):237–244
Khanahmadi M, Shahrezaei F (2008) Review and identify the chemical constituents of volatile oils of Falcaria vulgaris Bernh. J Med Plants 6(3):52–57
Khazaei M, Salehi H (2006) Protective effect of Falcaria vulgaris extract on ethanol induced gastric ulcer in rat. IJPT 5(1):43–46
Larson CT, Gross WB, Davis JW (1985) Social stress and resistance of chicken and swine to Staphylococcus aureus challenge infections. Can J Comp Med 49(2):208–210
Mandarim-de-Lacerda CA (2003) Stereological tools in biomedical research. An Acad Bras Cienc 5(4):469–486
Martin JP Jr, Dailey M, Sugarman E (1987) Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys 255(2):329–336
Moradi R, Hajialiani M, Zangeneh MM, Zangeneh A, Tahvilian R, Hidaryan H, Rezaeeasl N, Kohneshin A (2017) Antibacterial properties of an Iranian ethnomedicinal plant. Int J Ayu Pharm Chem 6(3):128–137
Najafi F, Goodarzi N, Zangeneh MM, Zangeneh A, Hagh-Nazari L (2017) Antidiabtic and hepatoprotective effects of bitter fraction of Stevia rebaudiana alcoholic extract on streptozotocin-induced diabetic male mice. J Rafsanjan Univ Med Sci 16(6):493–504
Najafi F, Tahvilian R, Zangeneh MM, Zangeneh A, Moradi R (2016) Screening of essential oil of Allium sativum for antibacterial effects against Bacillus subtilis. Int J Rec Sci Res 7(11):14172–14176
Nyengaard JR (1999) Stereologic methods and their application in kidney research. J Am Soc Nephrol 10(5):1100–1123
Poorshamohammad C, Souri N, Amini Z, Kosari F, Jamshidpour R, Zangeneh MM, Zangeneh A (2017) Cucurbita moschata: a plant with antibacterial properties. Int J Current Med Pharm Res 3(2):1356–1359
Pooyanmehr M, Zangeneh MM, Zangeneh A, Almasi M (2017) Effect of Verbascum thapsus aqueous extract on Escherichia coli O157:H7. Onl J Vet Res 21(9):580–583
Recknagel RO, GlendeJr EA, Britton RS (1991) Free radical damage and lipid peroxidation. In: Meeks RG (ed) Hepatotoxicology. CRC Press, Florida, pp 401–436
Roy A, Bhoumik D, Sahu RK, Dwivedi J (2014) Medicinal plants used in liver protection—a review. UK J Pharm Biosci 2(1):23–33
Saba AB, Oyagbemi AA, Azeez OI (2010) Amelioration of carbon tetrachloride-induced hepatotoxicity and haemotoxicity by aqueous leaf extract of Cnidoscolus aconitifolius in rats. Nig J Physiol Sci 25(2):139–147
Shafaghat A (2011) Volatile oil constituents and antibacterial activity of different parts of Falcaria vulgaris Bernh. growing wild in two localities from Iran. Nat Prod Res 25(4):368–373
Sherkatolabbasieh H, Hagh-Nazari L, Shafiezadeh S, Goodarzi N, Zangeneh MM, Zangeneh A (2017) Ameliorative effect of the ethanolic extract of Allium saralicum R.M. Fritsch on CCl4-induced nephrotoxicity in mice: a stereological examination. Arch Biol Sci 69(3):535–543
Swenson MJ (1993) Physiological properties and cellular and chemical constituent of blood. In: Dukes’ physiology of domestic animals. Comstock Publishing Associates, Ithaca and London. 29–32
Tahvilian R, Moradi R, Zhaleh H, Zangeneh MM, Zangeneh A, Yazdani H, Hajialiani M (2017) Chemical composition and screening of antibacterial activity of essential oil of Pistacia khinjuk against two selected pathogenic bacteria. Ann Trop Med Public Health 10(5):1159–1164
Zangar RC, Benson JM, Burnett VL, Springer DL (2000) Cytochrome P4502E1 is the primary enzyme responsible for low-dose carbon tetrachloride metabolism in human liver microsomes. Chem Biol Interact 125(3):233–243
Zangeneh A, Zangeneh MM, Hajialiani M, Moradi R, Nazari F, Sanjabi Shirazi F, Ansari N, Lotfi-Hamadani M, Ekhtiari A (2017) Peruse a plant product as an antimicrobial agent. Int J Current Med Pharm Res 3(2):1348–1351
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethic approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Zangeneh, M.M., Zangeneh, A., Tahvilian, R. et al. Hepatoprotective and hematoprotective effects of Falcaria vulgaris aqueous extract against CCl4-induced hepatic injury in mice. Comp Clin Pathol 27, 1359–1365 (2018). https://doi.org/10.1007/s00580-018-2747-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2747-9