Abstract
Using ethno-medicinal plants is the oldest way of mankind to treat the several diseases. Due to the rapid growth of global interest in use of medicinal plants, their effects and safety evaluation have become substantial. In this study, Alyssum meniocoides (AM) Boiss aqueous extract was extracted to investigate its nephroprotective activity on renal structural and biochemical changes in streptozotocin-induced diabetic nephrotoxicity in male mice. In this study, 80 mice were used. Diabetes was experimentally induced by intraperitoneal injection of streptozotocin (STZ) in 70 mice. Fasting blood glucose (FBG) levels were assessed everyday by glucometer strips. Mice with plasma glucose level > 250 mg/dL were considered diabetic. After 3 days, they were divided randomly into 8 groups. Groups 1 and 2 served as non-diabetic and untreated diabetic controls, respectively. Group 3 received 40 mg/kg glibenclamide orally. Groups 4, 5, 6, 7, and 8 were given 10, 20, 40, 80, and 160 mg/kg, respectively of AM for 20 days orally. At the 20th day, the mice were dissected, blood and kidney samples collected for biochemical and histological analysis. Histologically, several doses of AM could significantly (p ≤ 0.05) decrease the volume and length of the renal structures as compared to the untreated group. Biochemically, AM at all doses could significantly (p ≤ 0.05) reduce the raised levels of urea and creatinine and increased SOD and catalase (CAT) levels as compared to the untreated group. In conclusion, AM has nephroprotective property, thereby reducing the causation of diabetes in experimental mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal failure is a main complication of kidney, encountered globally (Tiong et al. 2014). Sodium and water retention, hyperkalemia, metabolic acidosis, and reduction in glomerular filtration rate are other features of renal failure (Kitabchi et al. 2009; Le-Devehat et al. 2001; Dhodi et al. 2014; Schetz et al. 2005). Chemical material-induced nephrotoxicity is one of the leading causes of renal failure (Dhodi et al. 2014; Schetz et al. 2005).
Streptozotocin (STZ) is the compound that was used as a diabetogenic agent in diabetes experiment (Breyer et al. 2005; Brosius et al. 2009). It is a naturally occurring alkylating antineoplastic agent that is particularly toxic to the insulin-producing beta cells of the pancreas in mammals (Tesch and Allen 2007; Lenzen 2008). STZ also causes kidney damage (Weiss 1982; Rerup 1970; Tay et al. 2005; Kraynak et al. 1995; Palm et al. 2004).
Findings from the screening of various ethno-medicinal plants describe their antioxidant effects and reveal that they could protect the kidney against STZ-induced oxidative stress by altering the levels of antioxidant enzymes (Najafi et al. 2017; Hagh-Nazari et al. 2017). Some medicinal plants have the high content of alkaloids, flavonoids, naphthoquinone, saponins, and tannins and triterpenes, so they can decrease the rate of nephrotoxicity (Goodarzi et al. 2017; Sherkatolabbasieh et al. 2017; Tahvilian et al. 2017; Foroughi et al. 2016).
Iran is rich of ethno-medicinal plants that are used for treatment of different diseases (Moradi et al. 2017; Zangeneh et al. 2017; Ghashghaii et al. 2017). Alyssum meniocoides (AM) Boiss is an endemic plant of Iran that grows widely in the western parts of the country. The Alyssum genus is placed in the Brassicaceae family (Ghaderiana et al. 2007). The consumption and cooking of parts of AM is due to the large variety of flavors and textures of the species. AM has been cultivated from the earliest times and it is economically important as a garden vegetable (Ghaderiana et al. 2007). In traditional medicine, several extracts of this plant are traditionally used in treating parasitic, bacterial, viral, and fungal diseases (Ghaderiana et al. 2007).
In the present study, we checked the ameliorative activity of the AM by studying the microscopic structural changes in mice kidney after STZ-induced diabetic nephrotoxicity using modern design-based stereological methods. Renal functions were also checked out by examining biochemical biomarkers.
Materials and methods
Animals
Eighty male BALB/c mice weighing between 38 and 40 g were housed in an air-conditioned room (22 ± 2 °C) and has free access to standard pellet diet (metabolism energy, 2860 kcal/kg; crude protein, 21.5%; crude fiber, 3.55%; calcium, 1.05%; phosphor, 0.5%; sodium, 0.17%; chlorine, 0.23%; methionine (digestible), 0.59%; methionine + cysteine (digestible), 0.92%; lysine (digestible), 1.2%; arginine (digestible), 1.33%; threonine (digestible), 0.82%; linoleic acid, 1.5%; dry matter, 88%) and water ad libitum conditions (standard environmental and nutritional) during the study.
Plant extraction
AM at maturity were collected from around of Kermanshah city during May 2017. Leaves of the plant were shade-dried for 1 week. Dried leaves of the plants were ground and about 150 g of the obtained powder was extracted with 450 mL of distilled water for 2 h at 40 °C with continuous shaking. The extract was left for 24 h at room temperature, then it was filtered through Watman paper no. 2. In rotary evaporator, the extract was concentrated, then lyophilized.
Experimental design
In this study, 80 mice were used. Diabetes was experimentally induced by intraperitoneal injection of STZ (60 mg/kg) in 70 mice. Fasting blood glucose levels were assessed everyday by glucometer strips. Mice with plasma glucose level > 250 mg/dL was considered diabetic (Goodarzi et al. 2018). After 3 days, the mice were divided into eight following groups (n = 10):
-
1.
Control group (C) which received 200 μL normal saline orally
-
2.
Untreated diabetic group (UD) which received 200 μL normal saline orally
-
3.
Treated group with 40 mg/kg glibenclamide (G40)
-
4.
Treated group with 10 mg/kg of the aqueous extract of AM (AM10)
-
5.
Treated group with 20 mg/kg of the aqueous extract of AM (AM20)
-
6.
Treated group with 40 mg/kg of the aqueous extract of AM (AM40)
-
7.
Treated group with 80 mg/kg of the aqueous extract of AM (AM80)
-
8.
Treated group with 160 mg/kg of the aqueous extract of AM (AM160)
Blood sampling and determination of biochemical parameters
Blood samples were obtained in 0, 4, 7, 10, 13, 16, and 20 days from tail vein to assess the blood glucose level by Easy Gluco glucometer (Ames, Korea). Twenty-three days after diabetes induction and at the end of the 20-day treatment, the animals of all groups were euthanized by xylazine (5 mg/kg) and ketamine HCl (40 mg/kg). Immediately, blood samples were drawn from animals’ heart and inserted in plasma and serum tube. Levels of creatinine and urea were evaluated in serum (Hagh-Nazari et al. 2017). The capacity of antioxidant enzymes was assessed by determining the activity of SOD and catalase (CAT) in the kidney using the procedures reported by Abei (1974) and Martin et al. (1987).
Stereological study
Volume density
After dissection, the left kidney was weighed then fixed in 10% neutral buffered formalin solution for 1 week. Immersion method was used to evaluate the kidney primary volume. For assessment of kidney final volume, the amount of tissue shrinkage must be determined (Braendgaard and Gundersen 1986; Gundersen et al. 1992). The sections of organ were performed using the orientator method. In total, 7–8 slabs were obtained from one kidney. A circular piece was sampled from a kidney slab and the area of this piece was calculated. The slabs and circular piece were processed, sectioned (5-μm thicknesses), and stained by Periodic Acid–Schiff (PAS) method. The area of the circular piece was calculated again and tissue shrinkage was estimated as (Mandarim-de-Lacerda 2003):
AA and AB are the areas of the circular piece after and before tissue processing.
The total volume of the organ was then estimated using:
Tissue sections were examined using a video microscopy system. The fractional volume of the renal structures was estimated using a point probe (with an area of 100 cm2 and containing 25 points) and the following formula:
Length density
The length density of the tubules and vessels was estimated using an unbiased counting probe (740 × 740 μm). The length density was estimated as:
∑Q = sum of the tubules counted, a (frame) = probe area, 547600 μm2, ∑frame = total number of the counted frames.
Statistical analysis
The results were analyzed by SPSS-18 software using one-way analysis of variance (ANOVA) followed by Duncan’s post hoc test. Data were considered statistically significant at p ≤ 0.05.
Results
Effect of AM on fasting blood glucose level
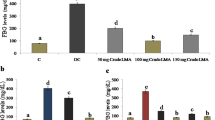
The blood glucose levels of untreated diabetic group enhanced to approximately 500% (p ≤ 0.05) of the normal control group in a time-dependent manner. But, treatment of STZ-diabetic mice with the AM at 80 and 160 doses could significantly (p ≤ 0.05) decrease the blood glucose levels similar to the G40-treated at the end of the experiment. The AM has most effect on days 20 of the experiment (Fig. 1).
Blood glucose levels on different days in all of the experimental groups. C (control), UD (untreated diabetic), G40 (treated diabetics with 40 mg/kg glibenclamide), AM10 (treated diabetics with 10 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM20 (treated diabetics with 20 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM40 (treated diabetics with 40 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM80 (treated diabetics with 80 mg/kg of Alyssum meniocoides Boiss aqueous extract), and AM160 (treated diabetics with 160 mg/kg of Alyssum meniocoides Boiss aqueous extract). Non-identical letters demonstrate a significant difference between the groups (p ≤ 0.05)
Effect of AM on stereological parameters
The results indicated that the kidney volume was increased 106% (p ≤ 0.05) in the untreated mice when compared to the control ones. Cortical and medullary volumes increased 112 and 93% (p ≤ 0.05), respectively in this group (p ≤ 0.05) in comparison with the control group. Administration of AM could significantly (p ≤ 0.05) ameliorate the kidney and cortical and medullary volumes compared to the untreated group. In addition, the difference of kidney and cortical volumes among AM80, AM160, and G40 groups were not significant (p ≤ 0.05, Fig. 2).
Absolute volume of the kidney (mm3), and absolute volumes (mm3) of cortex and medulla in all of the experimental groups. C (control), UD (untreated diabetic), G40 (treated diabetics with 40 mg/kg glibenclamide), AM10 (treated diabetics with 10 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM20 (treated diabetics with 20 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM40 (treated diabetics with 40 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM80 (treated diabetics with 80 mg/kg of Alyssum meniocoides Boiss aqueous extract), and AM160 (treated diabetics with 160 mg/kg of Alyssum meniocoides Boiss aqueous extract). Non-identical letters demonstrate a significant difference between the groups (p ≤ 0.05)
The volumes of proximal convoluted tubule, distal convoluted tubule, collecting duct, loop of Henle, vessels, and interstitial tissue were enhanced significantly (p ≤ 0.05) in untreated mice compared to the control ones (Figs. 3 and 4). Administration of AM at all doses to the mice could significantly (p ≤ 0.05) decrease the volumes of the above structures in comparison with the untreated group. Also, AM80, AM160, and G40 groups significantly (p ≤ 0.05) reduced the volume of vessels similar to the control group.
Absolute volumes (mm3) of proximal and distal convoluted tubules, collecting ducts in all of the experimental groups. C (control), UD (untreated diabetic), G40 (treated diabetics with 40 mg/kg glibenclamide), AM10 (treated diabetics with 10 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM20 (treated diabetics with 20 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM40 (treated diabetics with 40 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM80 (treated diabetics with 80 mg/kg of Alyssum meniocoides Boiss aqueous extract), and AM160 (treated diabetics with 160 mg/kg of Alyssum meniocoides Boiss aqueous extract). Non-identical letters demonstrate a significant difference between the groups (p ≤ 0.05)
Absolute volumes (mm3) of interstitial tissues, vessels, and loop of Henle in all of the experimental groups. C (control), UD (untreated diabetic), G40 (treated diabetics with 40 mg/kg glibenclamide), AM10 (treated diabetics with 10 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM20 (treated diabetics with 20 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM40 (treated diabetics with 40 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM80 (treated diabetics with 80 mg/kg of Alyssum meniocoides Boiss aqueous extract), and AM160 (treated diabetics with 160 mg/kg of Alyssum meniocoides Boiss aqueous extract). Non-identical letters demonstrate a significant difference between the groups (p ≤ 0.05)
The data of the mean absolute lengths of kidney subcomponents in treated and untreated groups are shown in Fig. 5. The lengths of the proximal convoluted tubule, distal convoluted tubule, collecting duct, loop of Henle, and vessels were significantly (p ≤ 0.05) increased in untreated mice compared to the control ones. AM at all doses could significantly (p ≤ 0.05) reduce the lengths of the proximal convoluted tubule, distal convoluted tubule, collecting duct, loop of Henle, and vessels compared to the untreated groups (p ≤ 0.05). There are not significant difference (p ≤ 0.05) among AM80, AM160, and G40 in length of collecting duct.
Absolute lengths (m) of the vessels, collecting ducts, proximal and distal convoluted tubules, and loop of Henle. C (control), UD (untreated diabetic), G40 (treated diabetics with 40 mg/kg glibenclamide), AM10 (treated diabetics with 10 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM20 (treated diabetics with 20 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM40 (treated diabetics with 40 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM80 (treated diabetics with 80 mg/kg of Alyssum meniocoides Boiss aqueous extract), and AM160 (treated diabetics with 160 mg/kg of Alyssum meniocoides Boiss aqueous extract). Non-identical letters demonstrate a significant difference between the groups (p ≤ 0.05)
Effect of AM on levels of kidney biochemical parameters
The estimated values of the kidney biochemical parameters are presented in Figs. 6 and 7. STZ-induced toxicity increased urea and creatinine and decreased SOD and CAT levels significantly (p ≤ 0.05) as compared to the untreated group. Different doses of AM could significantly (p ≤ 0.05) ameliorate the above parameters. There are no significant differences (p ≤ 0.05) among AM80, AM160, and G40 in urea, SOD, and CAT levels.
Urea and creatinine levels in all of the experimental groups. C (control), UD (untreated diabetic), G40 (treated diabetics with 40 mg/kg glibenclamide), AM10 (treated diabetics with 10 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM20 (treated diabetics with 20 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM40 (treated diabetics with 40 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM80 (treated diabetics with 80 mg/kg of Alyssum meniocoides Boiss aqueous extract), and AM160 (treated diabetics with 160 mg/kg of Alyssum meniocoides Boiss aqueous extract). Non-identical letters demonstrate a significant difference between the groups (p ≤ 0.05)
The levels of SOD and CAT in kidney in all of the experimental groups. C (control), UD (untreated diabetic), G40 (treated diabetics with 40 mg/kg glibenclamide), AM10 (treated diabetics with 10 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM20 (treated diabetics with 20 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM40 (treated diabetics with 40 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM80 (treated diabetics with 80 mg/kg of Alyssum meniocoides Boiss aqueous extract), AM160 (treated diabetics with 160 mg/kg of Alyssum meniocoides Boiss aqueous extract), SOD (Superoxide dismutase), and CAT (Catalase). Non-identical letters demonstrate a significant difference between the groups (p ≤ 0.05)
Discussion
Medicinal plants are popular remedies used by people (Foroughi et al. 2017; Moradi et al. 2017). The impression of medicinal plants in prevention and treatment of diseases is irrecusable (Poorshamohammad et al. 2017; Pooyanmehr et al. 2017; Najafi et al. 2016). They have the immense potential for the management and remedy of every disease such as nephrotoxicity (Najafi et al. 2017; Hagh-Nazari et al. 2017). A list of medicinal plants that consumed for their nephroprotective effects included Rubia cordifolia Linn (root), Boerhaavia diffusa (root), Aerva javanica (fresh roots), Curcuma longa (rhizome), Ficus religiosa L. (latex), Tectona grandis (bark), Strychnos potatorum (seed), Carica papaya (Seed), Crataeva nurvala (fruit), Tamarindus indica (fruit pulp), Punica granatum L. (fruit peel), Euphorbia neriifolia (leaf), Vernonia cinerea (aerial parts), Acorus calamus (aerial parts), Aerva lanata (whole plant), and Orthosiphon stamineus (whole plant) (Mohana-Lakshmi et al. 2012). In this experimental study, the nephroprotective effect of AM at various doses was determined in STZ-induced diabetes nephrotoxicity in mice model. But, to our knowledge, this is the first time AM with these doses has been used from experimentally induced diabetic in mice and there is no information about other beneficial effects of AM.
Hypoglycemic effect of AM
In the recent study, diabetes was induced in all mice by single intraperitoneal injection of STZ. STZ partially annihilates the beta cells of islets of Langerhans, hepatocytes, nephron, and RBC resulting in inexpressive insulin secretion causing type 2 diabetes, hepatotoxicity, nephrotoxicity, and hematotoxicity (Rerup 1970; Tay et al. 2005). The results of serum glucose levels showed that AM80 and AM160 in 20 days have significant difference in comparison with untreated diabetic group. But there was no significant difference between the experimental doses of AM and classic antidiabetic drug, G40 in this day. In agreement with the present results, there is a study which have shown the antidiabetic effects of Alyssum genus (Hachi et al. 2016).
Nephroprotective effect of AM
Renal inconveniences is assessed by the elevated histological examination as well as by serum levels of cytoplasmic parameters (Mishra et al. 2014). During the short-term study, the administration of AM ameliorates the renal morphological changes at all doses especially 160 doses. Untreated mice showed some degree of renal hypertrophy which was mainly due to the enlargement of the cortex, medulla, and its subcomponents. These changes was ameliorated significantly with AM.
The enhanced serum parameter levels such as creatinine and urea have been attributed to the blemished structural integrity of the kidney, because these are cytoplasmic in location and are released into the circulation after cellular injury. Also, nephrotoxicity reduces the concentration of antioxidants, ascorbic acid, catalase, superoxide dismutase, glutathione, and vitamin E which are the protective tissues that react and remove reactive oxygen species (Mishra et al. 2014). In our study, we observed acute renal damage in toxic group mice following STZ administration manifested by normal shifts in renal antioxidant enzyme activities (CAT ↓, SOD ↓) and renal function tests (urea ↑, creatinine ↑) in renal tissue with altered histopathological signs as compared to the control mice. But, AM at all doses could significantly (p ≤ 0.05) ameliorate the above parameters. The nephroprotective effect of AM in the present study may be partly related to its anti-inflammatory and antioxidant compounds. Previous studies showed that the most medicinal plants are rich of anti-inflammatory and antioxidant compounds and their nephroprotective effects are related to these compounds (Hagh-Nazari et al. 2017; Sherkatolabbasieh et al. 2017; Zangeneh et al. 2018a; Zangeneh et al. 2018b).
Conclusion
The present observation provides evidence that AM leaves that exhibited hypoglycemic effect on STZ-induced diabetic mice may be due to increasing the peripheral utilization of glucose by correcting the impaired liver or kidney glycolysis and by suppression of its gluconeogenic property similar to G40. Also, the new study has revealed the nephroprotective activity of the AM, offering its possible use as a therapeutic supplement or drug. Additional clinical trials studies would be needed to justify and further assess the potential of the plant as a nephroprotective agent in human.
References
Abei H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 673–684
Braendgaard H, Gundersen HJ (1986) The impact of recent stereological advances on quantitative studies of the nervous system. J Neurosci Methods 18(1–2):39–78
Breyer MD, Bottinger E, Brosius FC, Coffman TM, Harris RC, Heilig CW, Sharma K, AMDCC (2005) Mouse models of diabetic nephropathy. J Am Soc Nephrol 16(1):27–45
Brosius FC, Alpers CE, Bottinger EP, Breyer MD, Coffman TM, Gurley SB, Harris RC, Kakoki M, Kretzler M, Leiter EH, Levi M, McIndoe RA, Sharma K, Smithies O, Susztak K, Takahashi N, Takahashi T (2009) Mouse models of diabetic nephropathy. J Am Soc Nephrol 20(12):2503–2512
Dhodi DK, Bhagat SB, Pathak D, Patel SB (2014) Drug-induced nephrotoxicity. Int J Basic Clin Pharmacol 3(4):591–597
Foroughi A, Pournaghi P, Najafi F, Zangeneh A, Zangeneh MM, Moradi R (2016) Antibacterial effect and phytochemical screening of essential oil of Pimpinella anisum against Escherichia coli O157:H7 and Staphylococcus aureus. Int J Current Med Pharm Res 7(6):367–371
Foroughi A, Pournaghi P, Najafi F, Zangeneh A, Zangeneh MM, Moradi R (2017) Medicinal plants: antibacterial effects and chemical composition of essential oil of Foeniculum vulgare. Int J Curr Pharm Rew Res 8(1):13–17
Ghaderiana SM, Mohtadia A, Rahiminejada MR, Baker AJM (2007) Nickel and other metal uptake and accumulation by species of Alyssum (Brassicaceae) from the ultramafics of Iran. Environ Pollut 145(1):293–298
Ghashghaii A, Hashemnia M, Nikousefat Z, Zangeneh MM, Zangeneh A (2017) Wound healing potential of methanolic extract of Scrophularia striata in rats. Pharm Sci 23(4):256–263
Goodarzi N, Zangeneh MM, Zangeneh A, Najafi F, Tahvilian R (2017) Protective effects of ethanolic extract of Allium Saralicum R.M. Fritsch on CCl4-induced hepatotoxicity in mice. J Rafsanjan Univ Med Sci 16(3):227–238
Goodarzi N, Zangeneh MM, Zangeneh A (2018) The effect of ethanolic extract of Allium Saralicum R.M. Fritsch on diabetic hepatopathy in male mice. Sci Res J Shahed Univ 25(132):21–30
Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A (1992) Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Acta pathologica microbiologica immunologica. Scandinavica 96(5):379–394
Hachi M, Ouafae B, Hachi T, Mohamed EB, Imane B, Atmane R, Zidane L (2016) Contribution to the ethnobotanical study of antidiabetic medicinal plants of the Central Middle Atlas region (Morocco). Lazaroa 37:135–144
Hagh-Nazari L, Goodarzi N, Zangeneh MM, Zangeneh A, Tahvilian R, Moradi R (2017) Stereological study of kidney in streptozotocin-induced diabetic mice treated with ethanolic extract of Stevia rebaudiana (bitter fraction). Comp Clin Pathol 26(2):455–463
Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN (2009) Hyperglycemic crises in adult patients with diabetes. Diabetes Care 32(7):1335–1343
Kraynak AR, Storer RD, Jensen RD, Kloss MW, Soper KA, Clair JH, DeLuca JG, Nichols WW, Eydelloth RS (1995) Extent and persistence of streptozotocin-induced DNAdamage and cell proliferation in rat kidney as determined by in vivo alkaline elution and BrdUrd labeling assays. Toxicol Appl Pharmacol 135(2):279–286
Le-Devehat C, Khodabandehlou T, Vimeux M (2001) Impaired hemorheological properties in diabetic patients with lower limb arterial ischaemia. Clin Hemorheol Microcirc 25(2):43–48
Lenzen S (2008) The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51(2):216–226
Mandarim-de-Lacerda CA (2003) Stereological tools in biomedical research. An Acad Bras Cienc 75(4):469–486
Martin JP Jr, Dailey M, Sugarman E (1987) Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch Biochem Biophys 255(2):329–336
Mishra S, Ranjan-Pani S, Sahoo S (2014) Anti-nephrotoxic activity of some medicinal plants from tribal rich pockets of Odisha. Pharm Res 6(3):210–217
Mohana-Lakshmi S, Usha-Kiran-Reddy T, Sandhya-Rani KS (2012) A review on medicinal plants for nephroprotective activity. Asian J Pharm Clin Res 5(4):8–14
Moradi R, Hajialiani M, Zangeneh MM, Zangeneh A, Faizi S, Zoalfaghari M, Marabi A (2017) Study a plant extract as an antibacterial agent. Int J Curr Med Pharm Res 3(2):1360–1362
Najafi F, Tahvilian R, Zangeneh MM, Zangeneh A, Moradi R (2016) Screening of essential oil of Allium sativum for antibacterial effects against Bacillus subtilis. Int J Rec Sci Res 7(11):14172–14176
Najafi F, Goodarzi N, Zangeneh MM, Zangeneh A, Hagh-Nazari L (2017) Antidiabtic and hepatoprotective effects of bitter fraction of Stevia rebaudiana alcoholic extract on streptozotocin-induced diabetic male mice. J Rafsanjan Univ Med Sci 16(6):493–504
Palm F, Ortster H, Hansell P, Liss P, Carlsson PO (2004) Differentiating between effects of streptozotocin per se and subsequent hyperglycemia on renal function and metabolism in the streptozotocin diabetic rat model. Diabetes Metab Res Rev 20(6):452–459
Poorshamohammad C, Souri N, Amini Z, Kosari F, Jamshidpour R, Zangeneh MM, Zangeneh A (2017) Cucurbita moschata: a plant with antibacterial properties. Int J Current Med Pharm Res 3(2):1356–1359
Pooyanmehr M, Zangeneh MM, Zangeneh A, Almasi M (2017) Effect of Verbascum thapsus aqueous extract on Escherichia coli O157:H7. Online J Vet Res 21(9):580–583
Rerup CC (1970) Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev 22(4):485–518
Schetz M, Dasta J, Goldstein S, Golper T (2005) Drug-induced acute kidney injury. Curr Opin Crit Care 11(6):555–565
Sherkatolabbasieh H, Hagh-Nazari L, Shafiezadeh S, Goodarzi N, Zangeneh MM, Zangeneh A (2017) Ameliorative effects of the ethanolic extract of Allium saralicum R.M. Fritsch on CCl4-induced nephrotoxicity in mice: a stereological examination. Arch Biol Sci 69(3):535–543
Tahvilian R, Moradi R, Zhaleh H, Zangeneh MM, Zangeneh A, Yazdani H, Hajialiani M (2017) Chemical composition and screening of antibacterial activity of essential oil of Pistacia khinjuk against two selected pathogenic bacteria. Ann Trop Med Public Health 10(5):1159–1164
Tay YC, Wang Y, Kairaitis L, Rangan GK, Zhang C, Harris DCH (2005) Can murine diabetic nephropathy be separated from superimposed acute renal failure? Kidney Int 68(1):391–398
Tesch GH, Allen TJ (2007) Rodent models of streptozotocin induced diabetic nephropathy. Nephrology (Carlton) 12(3):261–266
Tiong HY, Huang P, Xiong S, Li Y, Vathsala A, Zink D (2014) Drug-induced nephrotoxicity: clinical impact and preclinical in vitro models. Mol Pharm 11(7):1933–1948
Weiss RB (1982) Streptozocin: a review of its pharmacology, efficacy, and toxicity. Cancer Treat Rep 66(3):427–438
Zangeneh MM, Najafi F, Tahvilian R, Salmani S, Haghnazari L, Zangeneh A, Moradi R (2017) Ethnomedicinal plants: in vitro antibacterial effects of ethanolic extract of Stevia rebaudiana. Int J Ayu Pharm Chem 6(1):251–259
Zangeneh MM, Goodarzi N, Zangeneh A, Tahvilian R, Najafi F (2018a) Amelioration of renal structural changes in STZ-induced diabetic mice with ethanolic extract of Allium saralicum R.M. Fritsch. Comp Clin Pathol. https://doi.org/10.1007/s00580-018-2674-9
Zangeneh MM, Zangeneh AA, Tahvilian R, Moradi R (2018b) Evaluation of the nephroprotective effect of Glycyrrhiza glabra L aqueous extract on CCl4-induced nephrotoxicity in mice. https://doi.org/10.1007/s00580-018-2707-4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethic approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Zangeneh, M.M., Zangeneh, A., Amiri, H. et al. Nephroprotective activity of Alyssum meniocoides Boiss aqueous extract on streptozotocin-induced diabetic nephrotoxicity in male mice. Comp Clin Pathol 27, 1147–1154 (2018). https://doi.org/10.1007/s00580-018-2712-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-018-2712-7