Abstract
Royal jelly is produced by worker bees as nutrition for bee larvae and adult queens and has also been shown to have protective effects against antibiotics. The aim of this investigation was to determine protective effect of royal jelly on the reproductive functions of male rats treated with ofloxacin. In this experiment, 32 mature male albino rats were randomly allocated into four groups (n = 8): control, ofloxacin only, royal jelly only, and ofloxacin with royal jelly. The results revealed that ofloxacin alone caused significant decreases (P < 0.05) in follicle-stimulating hormone, luteinizing hormone, testosterone, sperm count, sperm viability, total thiol molecules, and total antioxidant capacity compared to the control group. However, levels of immature sperm, DNA impaired sperm, malondialdehyde and nitric oxide were significantly increased (P < 0.05) in the ofloxacin group compared to the control. In the ofloxacin with royal jelly group, no significant increases or decreases were observed. Royal jelly has protective effects on reproductive function of male rat treated with ofloxacin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ofloxacin is a synthetic fluorinated carboxyquinolone compound with a slight calcium chelating affect in vitro (Montvale 1995) that is soluble in water and has antimicrobial activity. It is used to treat urinary system infections in animals and humans (Djurdjevic and Jelikic-Stankov 1999). However, Crotty et al. (1995) have shown that ofloxacin can cause disorders in sperm production. In animals, reactive oxygen species (ROS) created by fluoroquinolone caused oxidative stress. Other studies showed that fluoroquinolones excite oxidative metabolism in rabbit’s chondrocytes (Thoung et al. 1996) and influence cell differentiation in humans (Montanari et al. 1998; Majtán and Majtánová 1998). Fluoroquinolones stimulate the production of ROS in neutrophils cells and activate oxidative metabolism of leukocytes cells (Matsumoto et al. 1996).
All worker bees of the genus Apismeliphera produce royal jelly (Nakajima et al. 2009) and Eniseh et al. (2014) showed that royal jelly has protective effects on spermatogenesis and the amount of testosterone and peroxide lipid in mature male mice treated with oxymetholone. Additionally, Silici et al. (2010) showed that in mature, male rats, royal jelly has high anti-oxidative activity. Enzymatic hydrolysates and water and alkaline extracts of royal jelly have also been shown to display anti-oxidant properties (Nagai and Inoue 2004; Nagai et al. 2006). This investigation focused on studying the oxidative stresses produced in male rats by ofloxacin and the anti-oxidant effect of royal jelly.
Materials and methods
Animals and treatment
Thirty-two apparently healthy, mature, male, albino rats (body weight 160–180 g) were allowed to acclimatize in the unit for 7 days: temperature 20–23 °C, 12 h light /12 h dark cycle with tap water ad libitum and a standard pellet diet. Laboratory work was carried out according to international laws for care and use of laboratory animals as approved by the Ethical Committee of Urmia Farhangian University (Ref no = 50,201/509/240).
Animals were randomly allocated into four equal groups (n = 8) and dosed daily for 28 days by oral gavage: Control - distilled water (216 mg/kg.BW); Ofloxacin group (OF) - ofloxacin (216 mg/kg.BW); Royal Jelly group (RJ) - royal jelly (100 mg/kg.BW); RJ+ OF group - 216 mg/kg.BW ofloxacin and 100 mg/kg.BW royal jelly. The conventions used in this study, including doses and duration of treatment for ofloxacin and RJ, were all planned according to former studies (Taymour 2010; Silici et al. 2009).
Tissue preparation
At termination, both testes were taken. One fixed in formalin, processed and embedded in paraffin wax, sections cut at 6 μm, mounted on slides, stained with Weigert’s Iron Haematoxylin and the morphology examined using light microscopy. The other was used for the molecular assays.
Total anti-oxidant capacity (TAOC) assays
To define the effect of ofloxacin and royal jelly on oxidative stress the TAOC for control and experimental groups were calculated. This was achieved using the ferric reducing anti-oxidant power assay of Benzie and Strain (1999). Briefly, in acidic PH, reduction of colourless FeIII-tripyridyltriazine to the blue ferrous form can be measured at 593 nm. The strength of the complex following the addition of the test material is directly correlated to the final reducing influence of the electron gift giving antioxidant. FeII in solution and ascorbic acid were used as positive and negative controls. The content of the protein of the samples was assessed based on the Lowry method (Lowry et al. 1951).
Total thiol molecules (TTM) assay
Assessment of the TTM in blood serum was based on the Hu and Dillared method (Hu and Dillared 1994). Whereby, 0.2cm3 serum was added to 0.6 cm3 EDTA buffer, followed by 40 μl DTNB in a 10cm3 glass test tube and then made up to a final volume of 4.0cm3 by the addition of methanol. The samples were incubated at room temperature for 15 min, centrifuged for 10 min at 3000×g, and the supernatant was assessed spectrophotometrically at 412 nm.
Malondealdehyde (MDA) analyses
To assess the level of testicular lipid peroxidation, the amount of MDA was evaluated using the thiobarbituric acid reaction (Niehaus and Samuelsson 1968). 0.3–0.4 g of testicular tissue was homogenized in icy potassium chloride and centrifuged at 3000×g for 10 min. 0.5 mL of the supernatant was mixed with 3 mL H3PO4 and then, following vortex mixing, 2 mL of 6.7 g/L TBA was added. After heating the samples at 100 °C for 45 min they were chilled on ice. Eventually, 3 mL Nbutanol was added and the samples were again centrifuged at 3000×g for further 10 min. The supernatant was assessed spectrophotometrically at 532 nm and amount of MDA was evaluated by organized calibration curves using MDA values. The MDA concentration was revealed as nanomoles per milligramme of protein which corresponds with findings of Lowry and his colleagues (Lowry et al. 1951).
Evaluating nitric oxide (NO)
To identify the amount of NO, we analysed it based on the Griess reaction (Green et al. 1982). In this method, NO is quickly changed into NO2 then NO2 is transformed into HNO2. and when sulphanilamide is added, N-(1-naphthyl) ethylenediamine 2HCl reacts with HNO2 and forms a diazonium salt which can be assessed spectrophotometrically at a wavelength of 540 nm. The nitric oxide content was measured as nanomoles per milligramme of protein.
Hormonal assays
The hormones including luteinizing hormone (LH) and follicle stimulating hormone (FSH) were assessed in serum and ELISA and electrochemiluminescence techniques were used (Roshd kits: 08200067 271).
Statistical analysis
The data are presented as mean ± SE. Evaluation of the data was done with one-way ANOVA followed by Tukey HSD as post-test. Significance level was regarded as p < 0.05. We used SPSS (version 19) to analyse the data and Excel software to draw histograms and graphs.
Results
Histomorphology
Cross section of testis from the control group revealed that the morphologic appearance of the testis tissue was normal (Fig. 1A). However, in the OF group, modifications in morphologic appearance of the testis tissue, spermatogenic cells, interstitial cells, tunica albuginea and the microarchitecture were evident: curved spermatids were observed, but spermatozoa were not seen in the tubules (Fig. 1B); the epithelia of seminiferous tubules were reduced to below four cell layers (tubular differentiation index negative); considerable decrease in all types of spermatogenic cells, spermatogonia cells, primary spermatocytes, round and elongated spermatids were detected but spermatozoa were completely absent. The population of Leydig’s cell in the interstitial tissue were also reduced (Fig. 1B).
a Cross section of testis from control rat (Weigert’s stain). b Cross section of testis from ofloxacin treated rat with negative tubule differentiation index (TDI) (Weigert’s stain) 1 - spermatogonium type B; 2 - Primary spermatocyte; 3 - secondary spermatocyte; 4 - spermatids; 5 - spermatozoa; 6 - luminal space of seminiferous tubule; 7 - tunica albuginea; 8 - Sertoli cell; 9 - Leydig’s cell; 10 - interstitial tissue
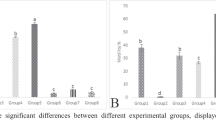
MDA, NO, TTM, TAOC
In comparison to the control group, the OF group showed a significant increase (p < 0.05) in both MDA and NO but a significant decrease (p < 0.05) in TTM and TAOC However, compared to the OF group, the RJ + OF showed a reduced amount of MDA, NO and an increased level of TTM and TAOC (Fig. 2A, B, C, D). There was no significant difference between the RJ and control groups.
Sperm parameters
Evaluation of sperm count (%) and sperm viability (%) revealed a significant (P < 0.05) decrease in the OF group compared to the controls, but RJ + OF showed a significant (P < 0.05) increase in comparison to control and RJ group (Table 1). There is important difference (P < 0.05) in the percentage of immature sperm and DNA damage in sperm, between control and OF groups. RJ + OF showed lower levels of in these parameters compared to OF (Table 1).
Hormonal evaluation
Testosterone, FSH and LH significantly decreased in the OF group compared to the controls (P < 0.05). In other words RJ + OF leads to significant increase (P < 0.05) in FSH, LH and testosterone levels compared to the OF group (Table 2).
Discussion
Ofloxacin, a derivate of fluoroquinolone, has an antibacterial action (Seibert et al. 1983; Nelson et al. 2007; Kawahara 1998) and the toxic reproductive effects of ofloxacin are widely studied (Aresh et al. 2009). According Crotty et al. (1995) ofloxacin can cause abnormalities in sperm formation. However, Tayebeh et al. (2014) reported that royal jelly in male rats treated with bleomycin can have protective influence on reproductive and biological molecules. For this reason, we designed this investigation to find out the protective effects of royal jelly on different parameters of the male rat reproductive system, including, sperm parameters, oxidative and nitrosative stresses and hormonal assays.
According to our results, the OF group, in comparison to controls, showed increased levels of MDA and NO but reduced levels of TTM and TAOC. This is due to a decline in anti-oxidant enzymes through the production of ROS by ofloxacin, but the RJ + OF group showed diminished levels of MDA, NO and improved levels of TTM and TAOC in comparison to the OF group possibly because royal jelly has anti-oxidative activity and conservation against oxidative stress in laboratory animals (Jamnik et al. 2007; El-Nekeety et al. 2007; Kanbur et al. 2009).
OF showed a decrease in sperm count and sperm viability in comparison to the control group, possibly due to disorder in proliferation cells in the tubules and the toxic effect of ofloxacin on sperm cell membranes. However, the RJ + OF group showed a significant increase in compared to the control and RJ groups this may be due to the anti-oxidant effect of royal jelly that has been shown in other studies (Tamura et al. 2009). Previous studies have also demonstrated an increase in sperm number and viability in rats given royal jelly (Hassan 2009; Virro et al. 2004).
Our study showed that the percentage of both immature sperm and DNA damage to sperm increased in the OF group compared to controls probably because ofloxacin causes high level of DNA damage via mechanisms that may be mediated by oxidative stress (Virro et al. 2004; Agarwal et al. 2006; Elnagar 2010). Ofloxacin co-administration with royal jelly decreases this parameter in comparison to ofloxacin alone. Similar to our results, Silici et al. (2009) reported that royal jelly has high anti-oxidative activity in mature male rats and counteracts bucks summer infertility.
In this experimental study amount of testosterone, FSH and LH decreased in the OF group due to increased levels of ROS and reduced TAOC and TTM in rats affected by ofloxacin. Oxidative stress created by ROS acts on the hypothalamus and causes decline in gonadotropin releasing hormone production diminishing the hormones from the pituitary gland. On the other hand, ofloxacin co-administrated with royal jelly leads to an increase in FSH, LH and testosterone levels in comparison to the OF group which is supported by Tayebeh et al. (2014) who reported that royal jelly in male rats increases testosterone levels and Hassan (2009) who states that royal jelly significantly increases luteinizing hormone (LH) levels (Hassan 2009).
Conclusion
According to the results of this study, we conclude that, ofloxacin causes histomorphologic changes in testes, in addition to hormonal alterations and increases in the levels of ROS and NO along with alterations in reproductive functionality in mature rats. On the other hand, administering royal jelly with ofloxacin results in reproductive parameters to close to usual levels.
References
Agarwal A, Sharma RK, Nallella KP, Thomas AJJR, Alvarez JG, Sikka SC (2006) Oxygen species as an independent marker of male factor infertility. Fertil Steril 86:878–885
Aresh K, Marefat G, AmirAfshin K, Fatemeh F, Morteza K, Jafar H, Reza S (2009) Comparative study of the effect of gentamicin, neomycin, streptomycin: and ofloxacin antibiotics on sperm parameters and testis apoptosis in rat. Pak J Biol Sci 11(13):1683–1689
Benzie IF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Crotty KL, May R, Kulvicki A, Kumar D, Neal DE Jr (1995) The effect of antimicrobial therapy on testicular aspirate flow cytometry. J Urol 153:835–838
Djurdjevic PT, Jelikic-Stankov M (1999) Study of solution equilibria between aluminium (III) ion and ofloxacin. J Pharm Biomed Anal 19:501–510
Elnagar SA (2010) Royal jelly counteracts bucks “summer infertility”. Anim Reprod Sic 121:174–180
El-Nekeety AA, El-Kholy W, Abbas NF, Ebaid A, Amra HA, Abdel-Wahhab MA (2007) Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon 50(2):256–269
Eniseh Z, Gholamreza N, Vahid N, Reza H (2014) Protective effect of royal jelly on the sperm parameters and testosterone level and lipid peroxidation in adult mice treated with oxymetholone. AJP 4(1):43–52
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem 126:131–138
Hassan A (2009) Effect of royal jelly on sexual efficiency in adult male rats. Iraqi J Vet Sci 23:155–160
Hu M, Dillared CJ (1994) Plasma SH and GSH measurement. Methods Enzymol 233:385–387
Jamnik P, Goranovič D, Raspor D (2007) Antioxidative action of royal jelly in the yeast cell. Exp Gerontol 42(7):594–600
Kanbur M, Erasian G, Beyaz L, Silici S, Liman BC, Altinordulu S, Atasever A (2009) The effects of royal jelly on liver damage induced by paracetamol in mice. Exp Toxicol Pathol 61(2):123–132
Kawahara S (1998) Chemotherapeutic agents under study. Nippon Rinsho December 56(12):3096–3099
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Majtán V, Majtánová L (1998) Post-antibiotic effect of some antibiotics on the metabolism of Pseudomonas Aeruginosa. J Basic Microbiol 38:221–227
Matsumoto T, Takahashi K, Nagafuji T, Kubo S, Sakumoto M, Mochida O (1996) Fleroxacin enhancement of superoxide production by polymorphonuclear leukocytes: the role of protein kinases. Chemotherapy 42:280–285
Montanari MP, Prenna M, Mingoia M, Ripa S, Varaldo PE (1998) In vitro antibacterial activity of trovafloxacin and five other fluoroquinolones. Chemotherapy 44:85–93
Montvale NJ (1995) Physician’s desk reference 49th edition. Medical Economics Data Production Company 14:57–61
Nagai T, Inoue R (2004) Preparation and the functional properties of water extract and alkaline extract of royal jelly. Food Cham 84:181–186
Nagai T, Inoue R, Suzuki N, Nagashima T (2006) Antioxidant properties of enzymatic hydrolysates from royal jelly. J Med Food 9:363–367
Nakajima Y, Tsuruma K, Shimazawa M, Mishmi S, Hara H (2009) Comparison of bee products based on assays of antioxidant capacities. Complement Altern Med 9:1–4
Nelson JM, Chiller TM, Powers JH, Angulo FJ (2007) Fluoroquinolone-resistant campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story. Clin Infect Dis 44(7):977–980
Niehaus WG, Samuelsson JRB (1968) Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6:126–130
Seibert G, Limbert M, Klesel N (1983) Comparison of the antibacterial in vitro and in vivo activity of ofloxacin (HOE 280 DL 8280) and nalidixic acid analogues. Eur J Clin Microbiol 2:548–553
Silici S, Ekmekcioglu O, Eraslan G, Demirtas A (2009) Antioxidative effect of royal jelly in cisplatin-induced testes damage. Urology 74:545–551
Silici S, Ekmekcioglu O, Kanbur M, Deniz K (2010) The protective effect of royal jelly against cisplatin-induced renal oxidative stress in rats. World J Urol 29:127–132
Tamura S, Kono T, Harada C, Yamaguchi K, Moriyama T (2009) Estimation and characterisation of major royal jelly proteins obtained from the honeybee Apismerifera. Food Chem 114:1491–1497
Tayebeh A, Gholamreza N, Vahid N (2014) Protective effect of royal jelly on fertility and biochemical parameters in bleomycin- induced male rats. Iran J Reprod Med March 12(3):209–216
Taymour M (2010) Long-term ofloxacin testicular toxicity: an experimental study. Andrology 42(2):92–96
Thoung GM, Domarle O, Pocidalo J, Hayem G (1996) Effects of fluoroquinolones on cultured articular chondrocytes flow cytometric analysis of free radical production. J Pharmacol Exp Therap 271:1544–1549
Virro MR, Larson-Cook KL, Evenson DP (2004) Sperm chromatin structure assay related to blastocyst rate, pregnancy rate and spontaneous abortion in IVF and ICSI cycles. Fertil Steril 8:1289–1295
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state that they have no conflict of interest. All investigation processes in this work were based on laws of the Research Ethics Committee for Research on Laboratory Animals Farhangain University of Urmia.
Rights and permissions
About this article
Cite this article
Manas, G.E., Najafi, G. Protective effects of royal jelly on the histomorphologic, oxidative stress and sperm parameters in Ofloxacin treated rat. Comp Clin Pathol 26, 1111–1115 (2017). https://doi.org/10.1007/s00580-017-2494-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-017-2494-3