Abstract
Most plant species naturally associate with arbuscular mycorrhizal fungi (AMF), which are known to promote crop nutrition and health in agroecosystems. However, information on how mycorrhizal associations affect plant biotic interactions that occur aboveground with foliar herbivores is limited and needs to be further addressed for the development of pest management strategies. With the objective to examine the influence of maize mycorrhizas on foliar herbivory caused by larvae of Spodoptera frugiperda, a serious pest in maize agroecosystems, we performed a fully factorial greenhouse pot experiment with three factors: Maize genotype (Puma and Milpal H318), AMF (with and without AMF, and without AMF with mineral P) and Insect herbivory (with and without S. frugiperda). Main results showed that inoculation with AMF improved plant growth and foliar P concentration, which coincided with increased foliar damage from herbivory and higher biomass of S. frugiperda larvae. A significant positive correlation between shoot P concentration and larval biomass was also observed. Finally, foliar herbivory by S. frugiperda slightly increased and decreased AMF root colonization in Puma and H318, respectively. In conclusion, our results show that maize plant benefits from AMF in terms of promotion of growth and nutrition, and may also increase the damage caused from insects by improving the food quality of maize leaves for larval growth, which seems to be linked to increased P uptake by the maize mycorrhizal association.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize is one of the basic cereal crops produced worldwide, used mainly for human consumption, livestock fodder, and bioenergy (Bennetzen and Hake 2009). Insect pests, including the Fall Armyworm Spodoptera frugiperda J. E. Smith (Lepidoptera: Noctuidae), are among the most production-limiting factors in maize agroecosystems (Pedigo and Rice 2009). Pest management of includes chemical and biological control, cultural practices, and crop system diversification (Pedigo and Rice 2009). Independent of the pest control strategy, it is important to consider employing an agroecological approach taking into consideration how other plant biotic interactions influence insect pest management (Altieri and Nicholls 2004).
Manifold direct and indirect plant biotic interactions at different trophic levels take place in agroecosystems (Médiène et al. 2011), which are all important for insect pest management (Altieri and Nicholls 2004). Among such plant biotic interactions, the symbiotic association between roots of most plants and arbuscular mycorrhizal fungi (AMF) is a key one to consider since it can strongly alter host plant phenotype such as primary and secondary metabolism, nutrient concentration and defense (Smith and Read 2008), thereby indirectly modifying plant–herbivore interactions.
Maize naturally associates with AMF, which has been shown to result in improved plant growth and P nutrition with single AMF species (Sarabia et al. 2018) and field communities of AMF (Sarabia et al. 2017; Zitlalpopoca-Hernandez et al. 2017; Alvarado-Herrejón et al. 2019). However, the phenotypic plant response to AMF depends on maize genotype (Sawers et al. 2017) and agricultural practices such as fertilization (Sarabia et al. 2017; López-Carmona et al. 2019) and tillage (Gavito and Miller 1998). Plant stress alleviation from abiotic and biotic stress is another key feature related to arbuscular mycorrhizas (Kumar and Verma 2018). Alleviation of biotic stress from plant pests including microbial plant pathogens (Whipps 2004; St-Arnaud and Vujanovic 2007; Veresoglou and Rillig 2012) and arthropod herbivores (Gange 2007; Gehring and Bennett 2009; Koricheva et al. 2009) by arbuscular mycorrhizas has been linked with both induction of systemic resistance (Pozo and Azcón 2007; Mauch-Mani et al. 2017) and improved tolerance from resource compensation (Garrido et al. 2010; Ramírez Gómez and Rodríguez 2012; Zitlalpopoca-Hernandez et al. 2017).
Results from a meta-analysis showed that the influence of mycorrhizal associations on the performance of arthropod herbivores depends on the type of herbivore, with reduced and increased damage from chewing and piercing-sucking herbivores, respectively (Koricheva et al. 2009). Foliar arthropod pests might also interact with AMF, as herbivory can alter the allocation of plant photosynthates to the roots (Gange 2007; Gehring and Bennett 2009; García-Rodríguez et al. 2012; Machado et al. 2013). However, the general knowledge of interactions between arbuscular mycorrhiza and foliar arthropod herbivores is still limited. Most studies have been performed with single species of AMF, with detached leaves, and have focused on plant defense responses.
Hence, the objective of this work was to investigate the interactions between a field community of AMF associated with maize and the insect herbivore S. frugiperda in vivo focusing on the possible role of P in this multitrophic interaction. We tested the main hypothesis that mycorrhizal maize plants compensate damage from foliar herbivory caused by S. frugiperda by improving plant P uptake.
Materials and methods
Experimental design
A multifactorial completely randomized design was employed with the following three factors: (1) Maize genotype (two levels—H-318 and PUMA), (2) Insect herbivory (two levels—with and without S. frugiperda), and (3) AMF (three levels—with and without AMF and without AMF but with P). Each of the 12 treatments (2 × 2 × 3) had five replicates, for a total of 60 experimental units.
Biological materials and substrate
The maize (Zea mays L.) hybrids PUMA (Asgrow®) and H-318 (Milpal®) were used in the present study as examples of maize genotypes commonly grown in the state Michoacán in Mexico. In a previous study, these hybrids were found to differ in response to herbivory from S. frugiperda with more herbivory and hence damage in H-318 than in PUMA (Real-Santillan O, unpublished data).
Larvae of S. frugiperda were obtained from a laboratory culture reared on a semi-synthetic diet before herbivory. Adults were fed with 15% honey water solution (Poitout and Bues 1974).
Soil was obtained from the experimental field station of the National Agricultural University of Mexico, Campus Morelia, Michoacán, Mexico. Soil texture was clayish (53.2% clay, 27.3% silt, and 19.5% sand) with the following chemical characteristics: organic matter = 2.7%, inorganic nitrogen = 23.2 mg kg−1, plant available phosphorus (Olsen P) = 5.8 mg kg−1, and pH (H2O) = 7.3. Soil was mixed with quartz sand (1:1, w/w) and disinfected in an electric soil sterilizer at 90 °C for 24 h.
Plant nutrients were mixed into the soil (mg kg−1 dry soil): K2SO4 (75.0), CaCl2·2H2O (75.0), CuSO4·5H2O (2.1), ZnSO4·7H2O (5.4), MnSO4·H2O (10.5), MgSO4·7H2O (45), Na2MoO4·2H2O (0.18), and NH3NO4 (30). In the treatment with P, 100 mg pot−1 P as KH2PO4 was mixed into the soil.
The field community of AMF in the maize field where the soil was collected, has been reported to be dominated by Glomus spp., Acaulospora spp., Gigaspora spp., and Intraspora spp. (López-Carmona 2013).
Experimental set-up
Soil sand mix (1:1, w/w) (1200 g) was added to each pot (1.5 L). In both treatments, “without AMF” disinfected soil was used, whereas in treatments “with AMF” 25% of the soil/sand substrate was non-disinfected and hence harbored the native field community of AMF and other microbiota. In the treatments “without AMF” and “without AMF with P,” soil microorganisms other than AMF were reestablished by adding 10 mL soil filtrate prepared by sieving a suspension of 100 g non-disinfected soil in 1 L distilled water through a nylon mesh (20 μm), which retained propagules of AMF, but allowed other microorganisms to pass. Prior to filtration, the soil suspension was mixed on a magnetic mixer for 2 h at 650 rpm.
The soil was watered to 80% of the water holding capacity. Three maize seeds were then sown in each pot with the different maize genotypes in their respective treatments. One week after seedling emergence, seedlings were thinned so that each pot contained only one seedling of similar size to minimize variation in plant development not related to the experimental factors examined. During the entire 8-week plant growth period, pots were watered daily by weight to maintain 80% water holding capacity. Every second week, an additional 30 mg N were applied to each pot as a solution of NH3NO4. Plants were grown under greenhouse conditions with 15–20 °C and 25–30 °C night and day temperatures, respectively.
Six weeks after sowing, three L2 larvae of S. frugiperda were placed on a leaf toward the stem center in the treatments with insect herbivory. Prior to application of S. frugiperda larvae, plants of all experimental units both without and with S. frugiperda were individually covered with insect net (200 μm mesh) to confine larvae to their respective experimental unit. Larvae were left to feed for 2 weeks.
Harvest and analyses
The experiment was ended 8 weeks after sowing. Larvae were collected and weighed. The level of shoot herbivory was determined visually according to damage categories as described by Domínguez and Dirzo (1995) using the following levels of damage: 0 = no damage, 1 = 0–5% damage, 2 = 6–12% damage, 3 = 13–25% damage, 4 = 36–50% damage, and 5 = 51–100% damage. Hereafter, the shoot was separated from the root and the root system was washed free of soil. Roots were then cut into 5–10 mm segments and mixed in water to obtain a 2-g representative subsample from each experimental unit for measurement of AMF root colonization. Root subsamples were stored at −20 °C until processing. The shoot and the remaining roots were oven dried for 72 h at 80 °C. Dried shoots were milled and sieved with a 0.425-mm mesh for further analysis of shoot N and P concentration.
Measurement of AMF root colonization was performed with the root intersection microscopy method using a compound stereo microscope according to Giovannetti and Mosse (1980) after clearing and staining the roots according to Kormanik and McGraw (1982) except that trypan blue was used instead of acid fuchsin. Presence and absence of internal AMF structures including vesicles, arbuscules, and/or mycelium only within the line of intersection was scored for 100 root intersections and data presented as percentage AMF root colonization.
For P and N shoot analyses, shoot samples (0.25 g) were pre-digested at room temperature for 24 h in 75 mL glass tubes with 1 g CuSO4, 10 g K2SO4, 3 mL H2O2 (30% v/v), and 7 mL sulfuric acid (H2SO4). For the final complete digestion, the tubes with the samples were placed in a digestor block where temperature was gradually increased (50 °C every 20 min) to 375 °C for 3 h. The final digested mixture was filtered (filter Whatman No. 1, 125 mm) and measured by colorimetric reading at 660 nm in an autoanalyzer Braun+Luebbe III. Measurements of N and P were performed according to Bremner (1996) and Murphy and Riley (1962), respectively.
Statistical analyses
For the variables shoot and root dry weight, shoot N and P concentration, AMF root colonization, larval weight, and recovery, multifactorial parametric analysis of variance (ANOVA), including the factors “Maize genotype,” “Insect herbivory,” and “AMF,” was employed to test for significant effects of individual factors and their possible interactions. Prior to ANOVA, data were tested for variance homogeneity (Bartlett) and normality (Andersen–Darling). In order to meet variance homogeneity, data for shoot P concentration were Johnson transformed, data for shoot N concentration were square root transformed, and data for AMF root colonization were arcsine transformed. LSD post hoc tests were used for comparisons of treatment or factor means. For the variable herbivory damage, which was based on non-parametric range data, Kruskal–Wallis was employed for each of the factors “Maize genotype” and “AMF,” individually. All statistical analyses were performed with the Statgraphic Centurion XV 2.06 (Statpoint®) software.
Results
Only significant results for individual factors or interactions are presented but means and standard errors of all treatments from all variables measured are presented in Supplementary Table 1.
Shoot and root dry weight
Shoot and root dry weight showed significant effects from the “Maize genotype × AMF” interaction (Table 1). Plants fertilized with P had the highest growth both in terms of shoot and root dry weight independent of maize genotype (Fig. 1a, b). Inoculation with the AMF field population caused shoot growth promotion compared to the control, although strongest with the genotype PUMA (Fig. 1a, b).
Shoot P and N concentration
For shoot P concentration, significant effects were observed for all three factors examined “Maize genotype,” “Insect herbivory,” and “AMF” although no interactions were significant (Table 1). The shoot P concentration was significantly higher in plants inoculated with AMF compared to the non-inoculated controls and plants fertilized with mineral P (Fig. 2a). The shoot P concentration was higher in PUMA (1.48 mg P g−1) than in H-318 (1.33 mg P g−1). Herbivory increased the shoot P concentration by 11.3% compared to that of plants without S. frugiperda.
Shoot P concentration (a) means of the factor “AMF” (n = 20) and shoot N concentration (b) means of the “Maize genotype × AMF” interaction (n = 10) of 8-week-old maize plants. Bars topped by the same letter do not differ significantly by post hoc LSD test (α = 0.05). Error bars represent standard error of the mean
Shoot N concentration showed a significant interaction effect of “Maize genotype × AMF,” but no effect of herbivory (Table 1). The shoot N concentration in H-318 was unaffected by AMF inoculation and P fertilization but was reduced in PUMA by P fertilization compared to the treatment without AMF, and to the treatment with AMF, which also presented higher shoot N concentrations than in the corresponding treatments with H-318 (Fig. 2b).
AMF root colonization
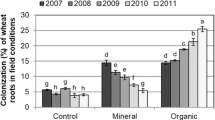
A significant “Maize genotype × Insect herbivory” interaction was obtained for AMF root colonization (Table 1), which was slightly reduced and increased by insect herbivory in the maize genotypes H-318 and PUMA, respectively (Fig. 3). Plants without AMF inoculation remained without mycorrhizas at harvest.
Herbivory, larval weight, and recovery of larvae
Herbivory, larval weight, and recovery of larvae were significantly affected by AMF (Table 1). Herbivory was highest with AMF, intermediate without AMF, and lowest without AMF with P fertilization (Table 2). The same pattern was observed for mean larval weight (Table 2) and recovery of larvae (Table 2) although with much stronger contrasts between the treatments without AMF and without AMF with P fertilization.
Significant positive correlations were observed between larval weight and shoot P concentration (r = 0.70, P < 0.001) (Fig. 4) and between larval weight and herbivory as damage categories (r = 0.74, P < 0.001) (Fig. 4).
Discussion
Arbuscular mycorrhizal fungi provide key ecosystem services in agroecosystems in terms of improved crop nutrition and health (Gianinazzi et al. 2010). However, here we show that such plant beneficial features of AMF in terms of improved foliar P concentration coincided with increased foliar damage from herbivores and higher biomass of S. frugiperda larvae. Nevertheless, increased herbivory in mycorrhizal plants did not result in reduced plant growth performance, confirming our hypothesis that AMF help to compensate damage from foliar herbivory, most likely by improving nutrient uptake.
The observed plant growth and shoot P promotion by native field communities of AMF is a common maize mycorrhiza response (Zitlalpopoca-Hernandez et al. 2017; Alvarado-Herrejón et al. 2019) although depending on maize genotype (Sawers et al. 2017) and agricultural practices such as fertilization (Sarabia et al. 2017; López-Carmona et al. 2019). The soil P level available for plant growth in the present study (7.3 mg P kg−1) was below the critical soil P level recommended for maize growth, which has been reported to be <15 mg P kg soil (Tang et al. 2009). When P is limiting plant growth as was the case in the present study, maize may benefit from associating with AMF principally in terms of plant growth promotion (Deng et al. 2017).
As was the case in the present study, effects of AMF on maize N acquisition seem to be less important than those on P. In scenarios with N deficiency, mycorrhiza plant growth suppression may even occur (López-Carmona et al. 2019). In a field experiment, competition for N between AMF and maize was suggested to be responsible for an observed increase in kernel N concentration after elimination of the native AMF community by fungicide application (Wang et al. 2018). In the present study, when improving maize P acquisition, either induced by AMF or by application of mineral P to the sterile soil substrate, shoot N dilution occurred, suggesting that the mineral N application rate during maize growth was not enough to avoid N deficiency, though only statistically significant in the PUMA maize genotype.
S. frugiperda herbivory had no significant effect on the plant growth parameters examined, although herbivory tended to reduce shoot and root dry weight, and as expected, did so more markedly in the susceptible genotype H-318. However, mycorrhizal plants were subjected to higher levels of herbivory than non-mycorrhizal plants both with and without P fertilization. In sterile soil, herbivory was lowest in P fertilized plants. In addition, recovery of the larvae added to each plant was very low in non-mycorrhizal plants, but whether the larvae had died or escaped is unfortunately unaccounted.
The improved larval performance in mycorrhizal plants in terms of herbivory and biomass is opposite to the findings of Koricheva et al. (2009) reporting that arbuscular mycorrhizal associations reduce the performance of foliar chewing insects like S. frugiperda. This effect was suggested to be linked to AMF induced host plant defense responses increasing the amounts of bioactive metabolites toxic to such herbivores, although depending on AMF species (Koricheva et al. 2009). The observed improved herbivore performance in the present study with a maize mycorrhiza association suggests that interactions between foliar chewing insects and AMF are complex, depending on plant and AMF species and most likely also growth substrate nutritional status, which needs to be further examined. In general, information on maize–mycorrhiza–herbivores is limited (Zitlalpopoca-Hernandez et al. 2017; Murrell et al. 2019)
Our results suggest that AMF-mediated increased maize shoot P status is most likely responsible for the increased food quality for S. frugiperda. Similarly, Goverde et al. (2000) found that AMF increased shoot P concentration of Lotus coniculatus coincided with improved life history traits of the lepidopteran herbivore, Polyommatus icarus. On the other hand, Bernaola et al. (2018) reported that improved performance of S. frugiperda and other pests in rice was not related to alterations in shoot nutrient composition, but rather alterations in defense-related pathways. This may also be the case in our study, but as in the case of Bernaola et al. (2018), plant defense was not measured in the present study. Future studies on plant–AMF–herbivore interactions should include more detailed analyses of both plant nutrition and defense, to unravel underlying mechanisms of the observed interactions.
It is well known that N fertilization improving shoot N concentration can increase performance of insect herbivores (Mattson 1980), whereas information on the effects of P fertilization on insect herbivores is less studied (Huberty and Denno 2006). However, in vitro studies where P was added to the diet have been shown to increase the growth of lepidopteran larvae (Clancy and King 1993). Also, improved growth of Manduca sexta larvae was observed when feeding on leaves of P-fertilized Datura wrightii (Perkins et al. 2004). Similarly, Janssen (1994) reported increased larval growth of Spodoptera exempta with increased P concentration in maize leaves.
Interestingly, recovery of larvae feeding on shoots from plants fertilized with P without mycorrhiza was very low and the few recovered ones had lower average biomass compared to that of larvae feeding on shoots of mycorrhizal plants or non-mycorrhizal plants without P fertilization. Overall, the shoot N concentration was low, and from visual observations, symptoms of maize N deficiency were apparent with chlorotic leaves. The observed reduction in larval performance may be due to induced resistance from N deficiency, which is common for a wide range of herbivores (Tingey and Singh 1980). Plant deficiency from nutrients such as N, P, and K has been shown to induce alterations in the production of secondary metabolites such as the increase in phenolic compounds used in plant defense (Gershenzon 1984; Chishaki and Horiguchi 1997; Fortier et al. 2006), which may explain the low recovery of larvae observed in the present study. Similarly, Slansky (1993) reported that lepidopteran larvae avoid feeding on nutrient deficient foliage, which may even have repellant effects against herbivores. Moreover, Estiarte et al. (1994) found that larvae of Helicoverpa armigera feeding on N-deficient plants had lower biomass than those feeding on plants with normal N concentration. Future studies on S. frugiperda herbivory as affected by AMF and/or P fertilization should employ confined herbivory compartments where larvae can be fully accounted.
Overall, the observed herbivory did not result in plant growth reduction, which indicates some degree of tolerance allowing the plant to recover from herbivore infestation (Núñez-Farfán et al. 2007; Peterson et al. 2017; Wang et al. 2018). Although mycorrhizal plants suffered from higher levels of herbivory than non-mycorrhizal plants regardless of P fertilization, no effect on plant growth was observed, suggesting a higher tolerance level in mycorrhizal plants. This concurs with other studies on mycorrhiza mediated tolerance to pest infestation, which seems to be related to improved nutrient acquisition and/or increased photosynthetic activity (Vannette and Hunter 2009).
Herbivory slightly reduced and increased the percentage of AMF root colonization in H-318 and PUMA, respectively, while herbivory had no effect on root dry weight. The observed changes in AMF root colonization seems to be associated with altered resource allocation to the mycorrhizal association depending on maize genotype. This coincides with Gehring and Bennett (2009), who reported varying effects of foliar herbivory on AMF, although the consensus is that foliar herbivory diminishes AMF performance due to reduction of photosynthetic area, resulting in lower allocation of carbohydrates to the roots and thereby reduced resources for biotrophic fungal symbiont growth (Gange et al. 2002; Gehring and Bennett 2009). However, in general, effects of foliar herbivory on the performance of mycorrhizal associations have been shown to be limited as was the case in the present study (Barto and Rillig 2010).
Ryan and Graham (2018) recently questioned the relevance of managing AMF in agroecosystems while calling for additional field-based evidence of the claimed crop benefits of AMF. Likewise, field-based studies on the effects of AMF on plant–arthropod interactions are limited (Heinen et al. 2018). The observed improved performance of S. frugiperda larvae feeding on leaves of mycorrhizal maize may result in reduced crop health and hence yield reduction, but studies on effects of AMF on natural enemies of S. frugiperda in the field would provide a more balanced idea of effects of AMF on crop health. Plants with AMF may also promote the performance of natural enemies of S. frugiperda, as has been shown with predator spiders regulating spider mite infestation in mycorrhizal bean (Hoffmann et al. 2011).
In conclusion, our results show that field communities of AMF promoting maize growth and nutrition, may also increase the insect damage by improving the food quality of maize leaves for larval growth, which seems to be linked to increased P uptake by the maize mycorrhizal association. Such plant biotic interactions seem to be important to consider when developing pest management strategies in maize agroecosystems. However, this needs to be investigated under field conditions in future experiments.
References
Altieri M, Nicholls C (2004) Biodiversity and pest management in agroecosystems. CRC Press, Boca Raton
Alvarado-Herrejón M, Larsen J, Gavito ME, Jaramillo-López PF, Vestberg M, Martínez-Trujillo M, Carreón-Abud Y (2019) Relation between arbuscular mycorrhizal fungi, root-lesion nematodes and soil characteristics in maize agroecosystems. Appl Soil Ecol 135:1–8
Barto EK, Rillig MC (2010) Does herbivory really suppress mycorrhiza? A meta-analysis. J Ecol 98:745–753
Bennetzen JL, Hake SC (eds) (2009) Handbook of maize: genetics and genomics. Springer Science & Business Media, Berlin
Bernaola L, Cosme M, Schneider RW, Stout M (2018) Belowground inoculation with arbuscular mycorrhizal fungi increases local and systemic susceptibility of rice plants to different pest organisms. Front Plant Sci 9:747
Bremner JM (1996) Nitrogen-total. In: Sparks DL (ed) Methods of soil analysis, part 3. Soil Science Society of America, Madison, pp 1085–1121
Chishaki N, Horiguchi T (1997) Responses of secondary metabolism in plants to nutrient deficiency. Soil Sci Plant Nutr 43:987–991
Clancy KM, King R (1993) Defining the western spruce budworm’s nutritional niche with response surface methodology. Ecology 74:442–454
Deng Y, Feng G, Chen X, Zou C (2017) Arbuscular mycorrhizal fungal colonization is considerable at optimal Olsen-P levels for maximized yields in an intensive wheat–maize cropping system. Field Crop Res 209:1–9
Domínguez CA, Dirzo R (1995) Rainfall and flowering synchrony in a tropical shrub: variable selection on the flowering time of Erythroxylum havanense. Evol Ecol 9:204–216
Estiarte M, Filella I, Serra J, Penuelas J (1994) Effects of nutrient and water stress on leaf phenolic content of peppers and susceptibility to generalist herbivore Helicoverpa armigera (Hubner). Oecol 99:387–391
Fortier E, Desjardins Y, Tremblay N, Bélec C, Côté M (2006) Influence of irrigation and nitrogen fertilization on broccoli polyphenolics concentration. In International Symposium on Vegetable Safety and Human Health 856, pp 55–62
Gange AC (2007) Insect mycorrhizal interactions: patterns, processes and consequences. In: Ohgushi T et al (eds) Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press, Cambridge, pp 124–143
Gange AC, Bower E, Brown VK (2002) Differential effects of insect herbivory on arbuscular mycorrhizal colonization. Oecol 131:103–112
García-Rodríguez Y, Bravo-Monzón A, Martínez-Díaz Y, Torres-Gurrola G, Espinosa-García FJ (2012) Variación Fitoquímica Defensiva en Ecosistemas Terrestres. In: Rojas JC, Malo EA (eds) Temas Selectos en Ecología Química de Insectos. El Colegio de la Frontera sur, Lerma Campeche, pp 217–252 446 p
Garrido E, Bennett AE, Fornoni J, Strauss SY (2010) Variation in arbuscular mycorrhizal fungi colonization modifies the expression of tolerance to above-ground defoliation. J Ecol 98:43–49
Gavito ME, Miller MH (1998) Changes in mycorrhiza development in maize induced by crop management practices. Plant Soil 198:185–192
Gehring C, Bennett A (2009) Mycorrhizal fungal–plant–insect interactions: the importance of a community approach. Environ Entomol 38:93–102
Gershenzon J (1984) Changes in the levels of plant secondary metabolites under water and nutrient stress. In: Phytochemical adaptations to stress. Springer, Boston, pp 273–320
Gianinazzi S, Gollotte A, Binet MN, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20:519–530
Giovannetti M, Mosse (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500
Goverde M, van der Heijden M, Wiemken A, Sanders I, Erhardt A (2000) Arbuscular mycorrhizal fungi influence life history traits of a lepidopteran herbivore. Oecol 125:362–369
Heinen R, Biere A, Harvey JA, Bezemer M (2018) Effects of soil organisms on aboveground plant–insect interactions in the field: patterns, mechanisms and the role of methodology. Front Ecol Evol 6:106
Hoffmann D, Vierheilig H, Peneder S, Schausberger P (2011) Mycorrhiza modulates aboveground tri-trophic interactions to the fitness benefit of its host plant. Ecol Entomol 36:574–581
Huberty AF, Denno RF (2006) Consequences of nitrogen and phosphorus limitation for the performance of two planthoppers with divergent life-history strategies. Oecol 149:444–455
Janssen JAM (1994) Impact of the mineral composition and water content of excised maize leaf sections on fitness of the African armyworm, Spodoptera exempta (Lepidoptera: Noctuidae). Bull Entomol Res 84:233–245
Koricheva J, Gange AC, Jones T (2009) Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecol 90:2088–2097
Kormanik PP, McGraw AC (1982) Quantification of vesicular arbuscular mycorrhiza in plant roots. In: Schenck NC (ed) Methods and principles of mycorrhizal research. American Phytopathological Society, St Paul, pp 37–45
Kumar A, Verma JK (2018) Does plant–microbe interaction confer stress tolerance in plants?: a review. Microbiol Res 207:41–52
López-Carmona DA (2013) Respuesta de micorrizas de maíz (Zea mays) a un gradiente de estrés hídrico y fertilización mineral. MSc thesis. Universidad Nacional Autónoma de México. 77 p
López-Carmona DA, Alarcón A, Martínez-Romero E, Peña-Cabriales JJ, Larsen J (2019) Maize plant growth response to whole rhizosphere microbial communities in different mineral N and P fertilization scenarios. Rhizosphere 9:38–46
Machado RA, Ferrieri AP, Robert CA, Glauser G, Kallenbach M, Baldwin IT, Erb M (2013) Leaf-herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. New Phytol 200:1234–1246
Mattson WJ Jr (1980) Herbivory in relation to plant nitrogen content. Ann Rev Ecol Syst 11:119–161
Mauch-Mani B, Baccelli I, Luna E, Flors V (2017) Defense priming: an adaptive part of induced resistance. Annu Rev Plant Biol 68:485–512
Médiène S, Valantin-Morison M, Sarthou JP, De Tourdonnet S, Gosme M, Bertrand M, Pelosi C (2011) Agroecosystem management and biotic interactions: a review. Agron Sustain Dev 31:491–514
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Murrell EG, Ray S, Lemmon ME, Luthe DS, Kaye JP (2019) Cover crop species affect mycorrhizae-mediated nutrient uptake and pest resistance in maize. Renew Agr food Syst:1–8
Núñez-Farfán J, Fornoni J, Valverde PL (2007) The evolution of resistance and tolerance to herbivores. Annu Rev Ecol Evol Syst 38:541–566
Pedigo LP, Rice M (2009) Entomology and pest management. Pearson, London
Perkins MC, Woods HA, Harrison JF, Elser JJ (2004) Dietary phosphorus affects the growth of larval Manduca sexta. Arch Insect Biochem Physiol 55:153–168
Peterson RKD, Varella AC, Higley LG (2017) Tolerance: the forgotten child of plant resistance. PeerJ. https://doi.org/10.7717/peerj.3934
Poitout S, Bues R (1974) Elevage de chenilles de vingt-huit espèces de Lépidoptères Noctuidae et de deux espèces d'Arctiidae sur milieu artificiel simple. Particularités de l'élevage selon les espèces In Annales de zoologie: Ecologie animale
Pozo MJ, Azcón AC (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:393–398
Ramírez Gómez M, Rodríguez A (2012) Mecanismos de defensa y respuestas de las plantas en la interacción micorrícica: una revisión. Rev Colomb Biotecnol 14:271–284
Ryan MH, Graham JH (2018) Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol 220:1092–1107
Sarabia M, Cornejo P, Azcón R, Carreón-Abud Y, Larsen J (2017) Mineral phosphorus fertilization modulates interactions between maize, rhizosphere yeasts and arbuscular mycorrhizal fungi. Rhizosphere 4:89–93
Sarabia M, Jakobsen I, Grønlund M, Carreon-Abud Y, Larsen J (2018) Rhizosphere yeasts improve P uptake of a maize arbuscular mycorrhizal association. Appl Soil Ecol 125:18–25
Sawers RJ, Svane SF, Quan C, Grønlund M, Wozniak B, Gebreselassie MN, Jakobsen I (2017) Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root-external hyphae and the accumulation of transcripts encoding PHT1 phosphate transporters. New Phytol 214:632–643
Slansky F Jr (1993) Nutritional ecology: the fundamental quest for nutrients. In: Stamp NE, Casey TM (eds) Caterpillars, ecological and evolutionary constraints on foraging. Chapman & Hall, New York, pp 29–91
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic, Cambridge
St-Arnaud M, Vujanovic V (2007) Effect of the arbuscular mycorrhizal symbiosis on plant diseases and pests. Mycorrhizae in crop production. Haworth, New York, pp 67–122
Tang X, Ma Y, Hao X, Li X, Li J, Huang S, Yang X (2009) Determining critical values of soil Olsen-P for maize and winter wheat from long-term experiments in China. Plant Soil 323:143–151
Tingey WM, Singh SR (1980) Environmental factors influencing the magnitude and expression of resistance. In: Maxwell FG, Jennings PR (eds) Breeding plants resistant to insects. Wiley, New York, pp 89–113
Vannette RL, Hunter MD (2009) Mycorrhizal fungi as mediators of defense against insect pests in agricultural systems. Agric For Entomol 11:351–358
Veresoglou SD, Rillig MC (2012) Suppression of fungal nematode plant pathogens through arbuscular mycorrhizal fungi. Biol Lett 8:214–217
Wang S, Ding T, Xu M, Zhang B (2018) Bidirectional interactions between beet armyworm and its host in response to different fertilization conditions. PLoS One 13:e0190502
Whipps JM (2004) Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can J Bot 82:1198–1227
Zitlalpopoca-Hernandez G, Najera-Rincon MB, del-Val E, Alarcon A, Jackson T, Larsen J (2017) Multitrophic interactions between maize mycorrhizas, the root feeding insect Phyllophaga vetula and the entomopathogenic fungus Beauveria bassiana. Appl Soil Ecol 115:38–43
Acknowledgments
We thank the Biological Sciences Postgraduate program of the National Autonomous University of Mexico for facilitating PhD training for Raúl Omar Real Santillan. We also thank the National Council for Science and Technology (CONACYT) for funding the basic science project 179319. Finally, we extend our gratitude to Maribel Nava Mendoza for excellent technical support for P and N measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Real-Santillán, R.O., del-Val, E., Cruz-Ortega, R. et al. Increased maize growth and P uptake promoted by arbuscular mycorrhizal fungi coincide with higher foliar herbivory and larval biomass of the Fall Armyworm Spodoptera frugiperda. Mycorrhiza 29, 615–622 (2019). https://doi.org/10.1007/s00572-019-00920-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-019-00920-3