Abstract
The development of arbuscular mycorrhiza (AM) is strongly suppressed under high-phosphate (Pi) conditions. To investigate AM fungal responses during the suppression of AM by high Pi, we performed an RNA-seq analysis of Rhizophagus irregularis colonizing Lotus japonicus roots at different levels of Pi (20, 100, 300, and 500 μM). AM fungal colonization decreased markedly under high-Pi conditions. In total, 163 fungal genes were differentially expressed among the four Pi treatments. Among these genes, a cell cycle-regulatory gene, cyclin-dependent kinase CDK1, and several DNA replication- and mitosis-related genes were repressed under high-Pi conditions. More than 20 genes encoding secreted proteins were also downregulated by high-Pi conditions, including the strigolactone-induced putative secreted protein 1 gene that enhances AM fungal colonization. In contrast, the expression of genes related to aerobic respiration and transport in R. irregularis were largely unaffected. Our data suggest that high Pi suppresses the expression of genes associated with fungal cell cycle progression or that encode secreted proteins that may be required for intercellular hyphal growth and arbuscule formation. However, high Pi has little effect on the transcriptional regulation of the primary metabolism or transport in preformed fungal structures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant–arbuscular mycorrhizal (AM) symbioses are established through molecular and genetic collaborations between plants and AM fungi. The development of AM symbioses is strongly affected by the external environment, and inorganic phosphate (Pi) is one of the most significant environmental factors involved (Gu et al. 2011; Carbonnel and Gutjahr 2014). Under low-Pi conditions, AM fungi can colonize plant roots and form arbuscules in the cortical cells, thus, enhancing Pi uptake by AM fungal hyphae for the host plant. In contrast, it has long been known that AM development is repressed by a high-Pi supply (Baylis 1967; Mosse 1973; Breuillin et al. 2010; Balzergue et al. 2011; Gu et al. 2011; Carbonnel and Gutjahr 2014; Kobae et al. 2016).
Under low-Pi conditions, the exudation of strigolactones from plant roots into the rhizosphere is enhanced (Yoneyama et al. 2007) and promotes hyphal branching and the metabolic activity of AM fungi in the soil (Akiyama and Hayashi 2006; Besserer et al. 2006; Besserer et al. 2008). AM fungi form hyphopodia on the surfaces of root epidermal cells and enter these cells through a pre-penetration apparatus, a tunnel-like structure derived from the host plant (Genre et al. 2005). After the hyphae penetrate the epidermal cells, AM fungi extend their hyphae into the intercellular space of the cortex and form arbuscules in the inner cortical root cells. In legume plants, the activation of the common symbiosis signaling pathway is essential for symbiosis with AM fungi as well as rhizobia (Kistner and Parniske 2002; Parniske 2008; Oldroyd 2013). The host plants perceive the lipochitooligosaccharides and chitooligosaccharides produced by AM fungi (Maillet et al. 2011; Genre et al. 2013). This activates the common symbiosis signaling pathway, which then induces calcium spiking in the root epidermal cells (Sun et al. 2015). The expression of many genes required for AM development is induced downstream from the common symbiosis signaling pathway (Pimprikar et al. 2016).
High Pi suppresses AM development in its early and late stages. In pea roots, the formation of hyphopodia by AM fungi is arrested by treatment with high Pi, suggesting that suppression occurs at a very early stage of AM development (Balzergue et al. 2011). In some cases, AM fungi can colonize plant roots even under high-Pi conditions, depending on the Pi level and the combination of the plant and AM fungal species involved (Breuillin et al. 2010; Gu et al. 2011). However, hyphal extension and the formation of arbuscules in the roots are generally reduced by high Pi (Branscheid et al. 2010; Breuillin et al. 2010). Furthermore, even in well-developed mycorrhizal roots that are grown under low-Pi conditions, AM fungal colonization is reduced once the roots are exposed to high Pi (Kobae et al. 2016), although the reduced colonization caused by high Pi is partly suppressed under low-nitrogen conditions (Nouri et al. 2014). AM suppression occurs within hours of exposure to high Pi, and the formation of new arbuscules is severely inhibited (Kobae et al. 2016). High Pi suppresses AM development in a systemic manner, as demonstrated with split-root experiments in which the addition of a high-Pi solution to one half of the root system also inhibited AM fungal colonization in the other half (Breuillin et al. 2010; Balzergue et al. 2011). Members of the miR399 family, which are involved in Pi-deficiency signaling pathways, are considered to be candidate signaling molecules in this systemic regulation because their expression is induced by AM fungal infection (Branscheid et al. 2010; Gu et al. 2014). However, the overexpression of miR399 did not restore AM fungal colonization at high-Pi levels (Branscheid et al. 2010), indicating that other mechanisms are also involved in this systemic regulation. The factors downstream from the systemic signaling pathway are largely unknown. It is unlikely that the common symbiosis signaling pathway is inactivated by high Pi because calcium oscillations are generated normally under high-Pi conditions (Balzergue et al. 2013). A possible downstream mechanism involves the phytohormone-mediated signaling pathways responsible for AM formation (Carbonnel and Gutjahr 2014). Treatment with high Pi led to the downregulation of gene sets involved in strigolactone biosynthesis (Breuillin et al. 2010; Balzergue et al. 2011). However, an exogenous supply of a synthetic strigolactone failed to improve AM fungal colonization under high-Pi conditions (Breuillin et al. 2010; Balzergue et al. 2011).
In contrast to the host plant responses, little is known about the AM fungal responses at the molecular level during AM suppression by high Pi. Recently, the genomic sequence of Rhizophagus irregularis was determined (Tisserant et al. 2013; Lin et al. 2014), and cDNA information for several AM fungal species is currently available (Tisserant et al. 2012; Tisserant et al. 2013; Kikuchi et al. 2014; Lin et al. 2014; Handa et al. 2015; Sędzielewska Toro and Brachmann 2016; Tang et al. 2016), allowing us to comprehensively explore the gene expression profiles of AM fungi. Using RNA-seq, Kikuchi et al. (2014) demonstrated that the transient application of a high-Pi solution to the hyphal compartment of a two-compartment culture system induced the expression of genes involved in the uptake of Pi and inorganic cations in the extraradical hyphae. This experiment was controlled to avoid inducing AM suppression to allow the characteristics of nutrient uptake by AM fungi to be accurately evaluated. We performed an RNA-seq analysis targeting R. irregularis genes to clarify the AM fungal responses during the suppression of AM that occurs in roots exposed to high Pi.
Materials and methods
Biological materials and growth conditions
Seedlings of Lotus japonicus B-129 were inoculated with 500 spores/plant of R. irregularis DAOM 197198 (Mycorise, Premier Tech, Rivière-du-Loup, Canada), produced under axenic conditions, in pots filled with river sand that had been autoclaved at 105 °C for 60 min. To generate highly colonized roots, the plants were supplied with a low concentration of KH2PO4 (100 μM) in a half-strength Hoagland’s solution (2.5 mM KNO3, 2.5 mM Ca(NO3)2, 1 mM MgSO4, 2.5 mg Fe L−1 Fe(III)-EDTA, 0.25 mg B L−1 H3BO3, 0.25 mg Mn L−1 MnCl2, 0.025 mg Zn L−1 ZnSO4, 0.01 mg Cu L−1 CuSO4, and 0.005 mg Mo L−1 NaMoO2) every other day for 3 weeks. The plants were then supplied with nutrient solution containing four different concentrations of KH2PO4 (20, 100, 300, or 500 μM) every other day for 1 week. The levels of potassium in the nutrient solutions were 2.52–3.00 mM. We considered that different potassium levels had little effect on AM symbiosis. All the plants were grown in a growth chamber at 25 °C with 16 h of light, with a photosynthetic photon flux density of 170 μmol m−2 s−1, and 8 h of darkness. The mycorrhizal roots were frozen in liquid nitrogen and stored at −80 °C until RNA extraction.
RNA-seq analysis
Total RNA was extracted from the frozen roots using RNAiso Plus (Takara, Shiga, Japan) in combination with Fruit-mate™ for RNA Purification (Takara), according to the manufacturer’s instructions. Genomic DNA was removed by digestion with RNase-free DNase (Qiagen, Hilden, Germany) on an RNeasy column (Qiagen), and the RNA was then purified on the column. The integrity of the total RNA was checked with the Agilent 2100 Bioanalyzer system (Agilent Technologies, Santa Clara, CA). The cDNA libraries for RNA-seq were constructed with the TruSeq RNA Sample Prep Kit (Illumina, San Diego, CA), according to the manufacturer’s instructions. Paired-end sequencing (2 × 100 bp) was performed with the Illumina HiSeq 2000 Sequencing System. Two biological replicates were prepared for the RNA-seq experiments. Data sets of the short reads were deposited in the DDBJ Sequence Read Archive (accession number: DRA004607). The genomic sequence and gene ontology (GO) annotation of R. irregularis was obtained from the JGI MycoCosm database (http://genome.jgi.doe.gov/Gloin1/Gloin1.home.html; Tisserant et al. 2013). Raw sequence reads were mapped to the R. irregularis genomic sequence with the TopHat program (Trapnell et al. 2009), and the annotated genes were counted, excluding putative rRNA genes (protein IDs: 67218, 67222, 73108, 102514, 235478, 247295, and 336739) with BEDTools (Quinlan and Hall 2010). The read counts were normalized with the iDEGES/edgeR method (Sun et al. 2013). Genes differentially expressed among the four different Pi conditions were detected with edgeR (Robinson et al. 2010), with a false discovery rate (FDR) cutoff of 0.01. A hierarchical clustering analysis was performed based on the normalized read counts of the differentially expressed genes (DEGs) using the average linkage method. A GO functional enrichment analysis of the DEGs was conducted using Fisher’s exact test with a weight algorithm in TopGO (Alexa and Rahnenführer 2016).
Assessment of AM colonization
Roots were cleared with 10 % KOH and stained with 0.05 % trypan blue in lactic acid. Hyphal, arbuscular, and vesicular colonization were determined as the percentage of root length colonized using the magnified intersection method (McGonigle et al. 1990). The data were arcsine transformed and analyzed with Tukey’s multiple comparisons test (P ≤ 0.05) using the R package.
Results
AM colonization
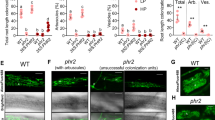
To assess the effects of the Pi concentration on AM colonization, we measured the proportions of the root lengths colonized by R. irregularis. Hyphal and arbuscular colonization decreased as the Pi levels increased (Fig. 1). In particular, the levels of arbuscular colonization under high-Pi conditions (300 and 500 μM) were less than one third of those under low-Pi conditions (20 and 100 μM). In contrast, vesicular colonization did not differ significantly among the different Pi treatments.
RNA-seq analysis
We investigated the gene expression profiles of R. irregularis in roots exposed to high Pi. Short reads were mapped against the R. irregularis genome sequence and 0.9–3.2 % of the reads were uniquely aligned (Table 1). This proportion of R. irregularis-derived reads is very similar to those reported in previous studies (Tisserant et al. 2013; Handa et al. 2015). In total, 163 genes were differentially expressed under the four different levels of Pi (Supplemental Table S1). Hierarchical clustering based on the expression levels of the DEGs showed that the samples were largely divided into two groups, according to their exposure to low Pi (20 and 100 μM) or high Pi (300 and 500 μM; Fig. 2). A GO analysis of the DEGs revealed an overrepresentation of genes involved in nucleosome and nucleosome assembly (Table 2). Nine DEGs related to these GO terms encoded histones, which play a crucial role in the packaging of DNA and allow the efficient replication and segregation of chromosomes. The expression of the histone genes was downregulated under high-Pi conditions relative to that under low-Pi conditions (Fig. 3a). The expression of several genes related to the regulation of the cell cycle was also repressed under high-Pi conditions: the cyclin-dependent kinase 1 gene (CDK1), DNA replication-related genes encoding DNA polymerase delta (Pol δ), proliferating cell nuclear antigen (PCNA), ribonucleotide reductase (RNR), and motor protein genes involved in mitosis (Fig. 3a). Twenty-nine genes encoding putative secreted proteins (18 % of the total DEGs) were differentially expressed during the high-Pi treatments (Fig. 3b). The expression of the majority of these secreted protein genes was suppressed under high-Pi conditions, including strigolactone-induced putative secreted protein 1 (SIS1) (Tsuzuki et al. 2016; Fig. 3b).
Gene expression profiles of differentially expressed genes (false discovery rate < 0.01) related to the cell cycle (a) and secreted proteins (b) under different Pi conditions. Putative secreted proteins of Rhizophagus irregularis are based on Tisserant et al. (2013) and Sędzielewska Toro and Brachmann (2016). Heat maps indicate the log2-fold changes in expression levels relative to the mean transcript abundance across the four Pi treatments
In contrast to the cell cycle-related genes and secreted protein genes, high concentrations of Pi had a negligible effect on the regulation of gene expression related to aerobic respiration in R. irregularis (Supplemental Table S2). Furthermore, most transporter genes were not differentially expressed during the Pi treatments, although the expression of four genes, encoding aquaporin AQP1, Pi transporter GintPT, iron permease FTR1, and mitochondrial oxaloacetate transport protein, were downregulated by the high-Pi treatments, whereas a gene of the major facilitator superfamily was upregulated (Table 3).
Discussion
High concentrations of Pi have long been known to inhibit AM formation (Baylis 1967; Mosse 1973; Breuillin et al. 2010; Balzergue et al. 2011; Gu et al. 2011; Carbonnel and Gutjahr 2014; Kobae et al. 2016). However, the AM fungal responses during the suppression of AM have been largely unknown. Here, we have shown that the expression of the R. irregularis histone genes is repressed in mycorrhizal roots under high-Pi conditions. Because transcripts of yeast histones transiently accumulate in early S-phase of the cell cycle (Bauer and Burgers 1990; Kurat et al. 2013), the downregulation of histone gene expression in R. irregularis may indicate changes in the regulation of the fungal cell cycle during exposure to high Pi. This is further supported by the repression of the cell cycle regulatory gene CDK1 and DNA-replication- and mitosis-related genes of R. irregularis in mycorrhizal roots exposed to high Pi. Information about the cell cycle regulation in AM fungi is limited. Flow cytometric studies and cell cycle-blocking experiments have shown that the AM fungal cell cycle is activated during its symbiotic phase in roots, during the production of germinating mycelia, and during the development of spores, whereas almost all the nuclei of resting spores are arrested in G0/G1 phase (Bianciotto and Bonfante 1993; Bianciotto et al. 1995; Marleau et al. 2011). Although almost nothing is known about the molecular mechanisms underlying the AM fungal cell cycle, it can be assumed that AM fungi possess the cell cycle control system that is conserved in most fungal species. In the present study, the expression of the genes encoding CDK1, Pol δ, PCNA, RNR, kinesin, and myosin genes was downregulated in mycorrhizal roots exposed to high Pi. Fungal CDK1 plays a central role in cell cycle progression and is associated with a cyclin protein. During the cell cycles of fission and budding yeasts, a conserved core set of genes related to DNA replication, mitosis, and cell division is expressed periodically (Rustici et al. 2004). DNA replication occurs in three steps: initiation, elongation, and termination. In the elongation step, Pol δ catalyzes the DNA chain elongation reaction in the presence of PCNA, which is a cofactor of Pol δ and increases the processivity of the polymerase. The expression of the genes encoding Pol δ and PCNA is transiently induced in the beginning of S phase of the cell cycle in budding yeast (Bauer and Burgers 1990). Furthermore, transcripts encoding RNR, which catalyzes the conversion of ribonucleotides to deoxyribonucleotides (the substrates for DNA synthesis by DNA polymerases), accumulate in the G1 phase in fission yeast (Rustici et al. 2004). Histones are synthesized during genome replication, which is followed by nuclear division, and these transcripts are tightly restricted to S phase (Hereford et al. 1981; Kurat et al. 2013). In addition to these DNA replication-related genes, the mitotic kinesin and myosin genes are also up-regulated in G1 phase in fission yeast (Rustici et al. 2004). The suppression of genes involved in DNA replication and mitosis observed in this study suggests that cell cycle progression is repressed in the intercellular hyphae of AM fungi by exposure to high Pi. We anticipated that the expression of genes involved in basic metabolism would be downregulated during the suppression of AM development by exposure to high Pi. Contrary to our expectation, the accumulation of aerobic respiration-related gene transcripts did not change across the Pi treatments. Kobae et al. (2016) demonstrated with vital staining of AM fungi that the mature arbuscules in Oryza sativa roots were metabolically active, even after the application of high Pi. Our data also show that the expression of most transporter genes in the intraradical hyphae was not repressed by high-Pi conditions. Taken together, these results suggest that fungal cell cycle progression, which may be required for intercellular hyphal growth and arbuscule development, is arrested by high Pi, whereas the transcriptional regulation related to metabolism and transport in preformed fungal structures is unaffected.
When we surveyed the transcriptome data of Rhizophagus sp. HR1 reported by Kikuchi et al. (2014), in which the extraradical mycelia in the hyphal compartment were exposed to high Pi, no cell cycle-related gene was repressed in the extraradical mycelia by the application of high Pi, indicating that high Pi does not directly inhibit fungal cell cycle progression. This evidence suggests that the cell cycle arrest in the intraradical hyphae caused by high Pi is probably attributable to indirect effects controlled by the host plant in response to high Pi. A possible explanation of these plant-mediated effects is the suppressed expression of the plant genes involved in AM development and maintenance. Pi replenishment repressed the plant genes related to strigolactone biosynthesis (Breuillin et al. 2010) and the production of strigolactones (Balzergue et al. 2011). The treatment of germinating hyphae with a synthetic strigolactone caused an increase in nuclear numbers in the hyphal tips, indicating that strigolactones stimulate AM fungal mitotic activity (Besserer et al. 2008). Taken together, these data suggest that the reduction of strigolactone levels by high Pi inhibit cell cycle progression in AM fungi. It has also been demonstrated that the R. irregularis SIS1 gene, which is upregulated by strigolactone treatment, plays a significant role in AM development (Tsuzuki et al. 2016). Therefore, AM suppression under high-Pi conditions may also be attributable to the reduced SIS1 expression that occurs with the reduced production of strigolactones.
Secreted fungal proteins known as “effectors” are important factors in plant–microbe interactions (Kamoun 2006). Recently, many secreted proteins have been identified in AM fungi with genomic surveys and transcriptome analyses (Tisserant et al. 2013; Lin et al. 2014; Fiorilli et al. 2016; Sędzielewska Toro and Brachmann 2016; Tang et al. 2016; Tsuzuki et al. 2016). Kloppholz et al. (2011) reported that the secreted AM fungal effector protein SP7, which interacts with the pathogenesis-related transcription factor ERF19 in the plant cell nucleus, supports the accommodation of the fungus within the plant roots. In the present study, the expression of a number of secreted fungal protein genes, including SIS1, was down regulated by high Pi. This group of secreted proteins may play a crucial role in successful root colonization by AM fungi.
Although the transcript levels of most transporter genes were constant across the Pi treatments, the four genes, AQP1, GintPT, FTR1, and a mitochondrial oxaloacetate transport protein gene, were downregulated in roots exposed to high Pi. The roles of these repressed transporter genes in mycorrhizal roots are largely unknown. The aquaporin AQP1 is known to be more highly expressed in intraradical hyphae than in extraradical hyphae (Aroca et al. 2009). However, AQP1 has no water transport activity when expressed in oocytes (Aroca et al. 2009). In this study, AQP1 expression was completely suppressed by high Pi, indicating that AQP1 can be used as a molecular marker of AM suppression. High-affinity Pi transporter genes, including GintPT, are expressed in both extraradical and intraradical hyphae (Harrison and van Buuren 1995; Benedetto et al. 2005; Tisserant et al. 2012; Fiorilli et al. 2013). GintPT was slightly downregulated in intraradical hyphae when exposed to high Pi (Fiorilli et al. 2013), which is consistent with our results. The reduced expression of GintPT may be attributable to the alleviation of P-starvation stress by the elevation of external or intracellular Pi. Among transporter genes, only a member of the major facilitator superfamily (protein ID 11960) was upregulated under high-Pi conditions. Transcripts of this major facilitator superfamily transporter accumulate in intraradical hyphae (Tisserant et al. 2012), but the molecules transported by the protein are unknown. If the transporter is related to the uptake of nutrients released from the host plant, the transporter gene may be expressed in response to the reduced supply of the nutrients associated with AM suppression.
In summary, we have shown here that high Pi represses the expression of genes associated with cell cycle progression and genes encoding secreted proteins, including SIS1, in AM fungi colonizing plant roots. These changes during AM suppression seem to be partly associated with a reduction in plant-derived strigolactones under high-Pi conditions. More research is required to determine whether strigolactones are involved in AM suppression, using plant mutants defective in the biosynthesis of strigolactones and by the application of exogenous strigolactones.
References
Akiyama K, Hayashi H (2006) Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann Bot 97:925–931
Alexa A, Rahnenführer J (2016) Gene set enrichment analysis with topGO. R package version 2.24.0. http://bioconductor.org/packages/release/bioc/html/topGO.html. Accessed 3 May 2016.
Aroca R, Bago A, Sutka M, Paz JA, Cano C, Amodeo G, Ruiz-Lozano JM (2009) Expression analysis of the first arbuscular mycorrhizal fungi aquaporin described reveals concerted gene expression between salt-stressed and nonstressed mycelium. Mol Plant-Microbe Interact 22:1169–1178
Balzergue C, Puech-Pagès V, Bécard G, Rochange SF (2011) The regulation of arbuscular mycorrhizal symbiosis by phosphate in pea involves early and systemic signalling events. J Exp Bot 62:1049–1060
Balzergue C, Chabaud M, Barker DG, Becard G, Rochange SF (2013) High phosphate reduces host ability to develop arbuscular mycorrhizal symbiosis without affecting root calcium spiking responses to the fungus. Front Plant Sci 4:426
Bauer GA, Burgers PM (1990) Molecular cloning, structure and expression of the yeast proliferating cell nuclear antigen gene. Nucleic Acids Res 18:261–265
Baylis GTS (1967) Experiments on the ecological significance of phycomycetous mycorrhizas. New Phytol 66:231–243
Benedetto A, Magurno F, Bonfante P, Lanfranco L (2005) Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza 15:620–627
Besserer A, Puech-Pagès V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais J-C, Roux C, Bécard G, Séjalon-Delmas N (2006) Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol 4:1239–1247
Besserer A, Bécard G, Jauneau A, Roux C, Séjalon-Delmas N (2008) GR24, a synthetic analog of strigolactones, stimulates the mitosis and growth of the arbuscular mycorrhizal fungus Gigaspora rosea by boosting its energy metabolism. Plant Physiol 148:402–413
Bianciotto V, Bonfante P (1993) Evidence of DNA replication in an arbuscular mycorrhizal fungus in the absence of the host plant. Protoplasma 176:100–105
Bianciotto V, Barbiero G, Bonfante P (1995) Analysis of the cell cycle in an arbuscualr mycorrhizal fungus by flow cytometry and bromodeoxyuridine labelling. Protoplasma 188:161–169
Branscheid A, Sieh D, Pant BD, May P, Devers EA, Elkrog A, Schauser L, Scheible W-R, Krajinski F (2010) Expression pattern suggests a role of miR399 in the regulation of the cellular response to local Pi increase during arbuscular mycorrhizal symbiosis. Mol Plant Microbe Interact 23:915–926
Breuillin F, Schramm J, Hajirezaei M, Ahkami A, Favre P, Druege U, Hause B, Bucher M, Kretzschmar T, Bossolini E, Kuhlemeier C, Martinoia E, Franken P, Scholz U, Reinhardt D (2010) Phosphate systemically inhibits development of arbuscular mycorrhiza in Petunia hybrida and represses genes involved in mycorrhizal functioning. Plant J 64:1002–1017
Carbonnel S, Gutjahr C (2014) Control of arbuscular mycorrhiza development by nutrient signals. Front Plant Sci 5:462
Fiorilli V, Lanfranco L, Bonfante P (2013) The expression of GintPT, the phosphate transporter of Rhizophagus irregularis, depends on the symbiotic status and phosphate availability. Planta 237:1267–1277
Fiorilli V, Belmondo S, Khouja HR, Abbà S, Faccio A, Daghino S, Lanfranco L (2016) RiPEIP1, a gene from the arbuscular mycorrhizal fungus Rhizophagus irregularis, is preferentially expressed in planta and may be involved in root colonization. Mycorrhiza 26:609–621
Genre A, Chabaud M, Timmers T, Bonfante P, Barker DG (2005) Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. Plant Cell 17:3489–3499
Genre A, Chabaud M, Balzergue C, Puech-Pagès V, Novero M, Rey T, Fournier J, Rochange S, Bécard G, Bonfante P, Barker DG (2013) Short-chain chitin oligomers from arbuscular mycorrhizal fungi trigger nuclear Ca2+ spiking in Medicago truncatula roots and their production is enhanced by strigolactone. New Phytol 198:190–202
Gu M, Chen A, Dai X, Liu W, Xu G (2011) How does phosphate status influence the development of the arbuscular mycorrhizal symbiosis? Plant Signal Behav 6:1300–1304
Gu M, Liu W, Meng Q, Zhang WQ, Chen AQ, Sun SB, Xu GH (2014) Identification of microRNAs in six solanaceous plants and their potential link with phosphate and mycorrhizal signalings. J Integr Plant Biol 56:1164–1178
Handa Y, Nishide H, Takeda N, Suzuki Y, Kawaguchi M, Saito K (2015) RNA-seq transcriptional profiling of an arbuscular mycorrhiza provides insights into regulated and coordinated gene expression in Lotus japonicus and Rhizophagus irregularis. Plant Cell Physiol 56:1490–1511
Harrison MJ, van Buuren ML (1995) A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 378:626–629
Hereford LM, Osley MA, Ludwig JR, McLaughlin CS (1981) Cell-cycle regulation of yeast histone mRNA. Cell 24:367–375
Kamoun S (2006) A catalogue of the effector secretome of plant pathogenic oomycetes. Annu Rev Phytopathol 44:41–60
Kikuchi Y, Hijikata N, Yokoyama K, Ohtomo R, Handa Y, Kawaguchi M, Saito K, Ezawa T (2014) Polyphosphate accumulation is driven by transcriptome alterations that lead to near-synchronous and near-equivalent uptake of inorganic cations in an arbuscular mycorrhizal fungus. New Phytol 204:638–649
Kistner C, Parniske M (2002) Evolution of signal transduction in intracellular symbiosis. Trends Plant Sci 7:511–518
Kloppholz S, Kuhn H, Requena N (2011) A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Curr Biol 21:1204–1209
Kobae Y, Ohmori Y, Saito C, Yano K, Ohtomo R, Fujiwara T (2016) Phosphate treatment strongly inhibits new arbuscule development but not the maintenance of arbuscule in mycorrhizal rice roots. Plant Physiol 171:566–579
Kurat CF, Recht J, Radovani E, Durbic T, Andrews B, Fillingham J (2013) Regulation of histone gene transcription in yeast. Cell Mol Life Sci 71:599–613
Lin K, Limpens E, Zhang Z, Ivanov S, Saunders DG, Mu D, Pang E, Cao H, Cha H, Lin T, Zhou Q, Shang Y, Li Y, Sharma T, van Velzen R, de Ruijter N, Aanen DK, Win J, Kamoun S, Bisseling T, Geurts R, Huang S (2014) Single nucleus genome sequencing reveals high similarity among nuclei of an endomycorrhizal fungus. PLoS Genet 10:e1004078
Maillet F, Poinsot V, André O, Puech-Pagès V, Haouy A, Gueunier M, Cromer L, Giraudet D, Formey D, Niebel A, Martinez EA, Driguez H, Bécard G, Dénarié J (2011) Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 469:58–63
Marleau J, Dalpé Y, St-Arnaud M, Hijri M (2011) Spore development and nuclear inheritance in arbuscular mycorrhizal fungi. BMC Evol Biol 11:51
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501
Mosse B (1973) Plant growth responses to vesicular-arbuscular mycorrhiza. IV. in soil given additional phosphate. New Phytol 72:127–136
Nouri E, Breuillin-Sessoms F, Feller U, Reinhardt D (2014) Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrida. PLoS One 9:e90841
Oldroyd GED (2013) Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol 11:252–263
Parniske M (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol 6:763–775
Pimprikar P, Carbonnel S, Paries M, Katzer K, Klingl V, Bohmer Monica J, Karl L, Floss Daniela S, Harrison Maria J, Parniske M, Gutjahr C (2016) A CCaMK-CYCLOPS-DELLA complex activates transcription of RAM1 to regulate arbuscule branching. Curr Biol 26:987–998
Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140
Rustici G, Mata J, Kivinen K, Lio P, Penkett CJ, Burns G, Hayles J, Brazma A, Nurse P, Bahler J (2004) Periodic gene expression program of the fission yeast cell cycle. Nat Genet 36:809–817
Sędzielewska Toro K, Brachmann A (2016) The effector candidate repertoire of the arbuscular mycorrhizal fungus Rhizophagus clarus. BMC Genomics 17:101
Sun J, Nishiyama T, Shimizu K, Kadota K (2013) TCC: an R package for comparing tag count data with robust normalization strategies. BMC Bioinformatics 14:219
Sun J, Miller JB, Granqvist E, Wiley-Kalil A, Gobbato E, Maillet F, Cottaz S, Samain E, Venkateshwaran M, Fort S, Morris RJ, Ane JM, Denarie J, Oldroyd GE (2015) Activation of symbiosis signaling by arbuscular mycorrhizal fungi in legumes and rice. Plant Cell 27:823–838
Tang N, San Clemente H, Roy S, Becard G, Zhao B, Roux C (2016) A survey of the gene repertoire of Gigaspora rosea unravels conserved features among Glomeromycota for obligate biotrophy. Front Microbiol 7:233
Tisserant E, Kohler A, Dozolme-Seddas P, Balestrini R, Benabdellah K, Colard A, Croll D, Da Silva C, Gomez SK, Koul R, Ferrol N, Fiorilli V, Formey D, Franken P, Helber N, Hijri M, Lanfranco L, Lindquist E, Liu Y, Malbreil M, Morin E, Poulain J, Shapiro H, van Tuinen D, Waschke A, Azcón-Aguilar C, Bécard G, Bonfante P, Harrison MJ, Küster H, Lammers P, Paszkowski U, Requena N, Rensing SA, Roux C, Sanders IR, Shachar-Hill Y, Tuskan G, Young JPW, Gianinazzi-Pearson V, Martin F (2012) The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol 193:755–769
Tisserant E, Malbreil M, Kuo A, Kohler A, Symeonidi A, Balestrini R, Charron P, Duensing N, Frey NFD, Gianinazzi-Pearson V, Gilbert LB, Handa Y, Herr JR, Hijri M, Koul R, Kawaguchi M, Krajinski F, Lammers PJ, Masclauxm FG, Murat C, Morin E, Ndikumana S, Pagni M, Petitpierre D, Requena N, Rosikiewicz P, Riley R, Saito K, Clemente HS, Shapiro H, Van Tuinen D, Becard G, Bonfante P, Paszkowski U, Shachar-Hill YY, Tuskan GA, Young PW, Sanders IR, Henrissat B, Rensing SA, Grigoriev IV, Corradi N, Roux C, Martin F (2013) Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc Natl Acad Sci USA 110:20117–20122
Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25:1105–1111
Tsuzuki S, Handa Y, Takeda N, Kawaguchi M (2016) Strigolactone-induced putative secreted protein 1 is required for the establishment of symbiosis by the arbuscular mycorrhizal fungus Rhizophagus irregularis. Mol Plant-Microbe Interact 29:277–286
Yoneyama K, Yoneyama K, Takeuchi Y, Sekimoto H (2007) Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta 225:1031–1038
Acknowledgments
We thank the National BioResource Project for supplying the L. japonicus B-129 seeds through the Frontier Science Research Center of the University of Miyazaki, Japan. We appreciate the great help by research assistants Ms. Reika Oguchi and Ms. Satomi Shimizu. This work was supported by the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and the Food industry from the Ministry of Agriculture, Forestry and Fisheries of Japan (grant no. 26036A), and ACCEL from the Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 120 kb)
Rights and permissions
About this article
Cite this article
Sugimura, Y., Saito, K. Transcriptional profiling of arbuscular mycorrhizal roots exposed to high levels of phosphate reveals the repression of cell cycle-related genes and secreted protein genes in Rhizophagus irregularis . Mycorrhiza 27, 139–146 (2017). https://doi.org/10.1007/s00572-016-0735-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-016-0735-y