Abstract
The Orchid flora of Madagascar is one of the most diverse with nearly 1000 orchid taxa, of which about 90 % are endemic to this biodiversity hotspot. The Itremo Massif in the Central Highlands of Madagascar with a Highland Subtropical climate range encompasses montane grassland, igneous and metamorphic rock outcrops, and gallery and tapia forests. Our study focused on identifying culturable mycorrhizae from epiphytic, lithophytic, and terrestrial orchid taxa to understand their diversity and density in a spatial matrix that is within the protected areas. We have collected both juvenile and mature roots from 41 orchid taxa for isolating their orchid mycorrhizal fungi (OMF), and to culture, identify, and store in liquid nitrogen for future studies. Twelve operational taxonomic units (OTUs), of three known orchid mycorrhizal genera, were recognized by analysis of internal transcribed spacer (ITS) sequences of 85 isolates, and, by comparing with GenBank database entries, each OTU was shown to have closely related fungi that were also found as orchid associates. Orchid and fungal diversity were greater in gallery forests and open grasslands, which is very significant for future studies and orchid conservation. As far as we know, this is the first ever report of detailed identification of mycorrhizal fungi from Madagascar. This study will help start to develop a programme for identifying fungal symbionts from this unique biodiversity hotspot, which is undergoing rapid ecosystem damage and species loss. The diversity of culturable fungal associates, their density, and distribution within the Itremo orchid hotspot areas will be discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About 44 % of all vascular plant species are confined to 34 global biodiversity hotspots (Mittermeier et al. 2005). Investing resources to the entire global hotspot could lead to spreading of the resources too thinly on the ground, making conservation of an entire hotspot area untenable, and therefore, conservation approaches must focus on selected areas of maximum diversity and/or endemism (Murray-Smith et al. 2009). Fenu et al. (2010) proposed the terms “micro-hotspots” (i.e., endemism-rich areas analogous to biogeographic units) and “nano-hotspots” (i.e., areas <3 km2 with an exceptional concentration of endemic taxa). By most estimates, Madagascar is home to 4 % of the world’s plant and animal species all confined to just 0.4 % of the Earth’s land surface, earning its reputation as one of the top five biodiversity “hotspots” (Tyson 2000). About 90 % of Madagascar’s natural vegetation has been cleared or permanently altered, and the remaining 10 % is by no means secure. Roos et al. (2004) studied the vascular plants in the Malesian Islands and observed that species–area relationships of families are dependent on species number; they found that island surface area is a predictor for island percent endemism in the study area. Madagascar, the world’s second largest island, has 10,000–12,000 vascular plant species, of which roughly 1 in 10 (about 1000) are orchids and about 90 % of which are endemic (Tyson 2000; Moat and Smith 2007). The Itremo Massif within the Central Highlands is a “micro-hotspot,” home to more than 50 orchid taxa of which the majority are endemic and some species are locally endemic (e.g., Angraecum protensum, Angraecum magdalenae).
Itremo Massif consists of a plateau of mixed igneous and metamorphic rock at an elevation of 1400–1923 m above sea level. The average temperature is in the range of 18–21 °C, annual rainfall of 1416 mm with a 4–6-month dry season. Savanna is the dominant habitat, with humid gallery forest, remnant tapia (Uapaca bojeri) forest and rocky and montane moorland habitats also present. The raising of cattle is commonplace in the region, and burning grassland is practiced annually. While some advocate carefully controlled burning as a management strategy for tapia forest in Itremo and elsewhere in the Central Highlands (Alvarado et al. 2014), other habitats can be badly affected (Whitman et al. 2011). We observed evidence of fire damage on two separate rocky ridges and speculate that adjacent man-made grass fires were the cause. In addition to this, illegal mining for precious stones is causing habitat degradation in some areas (Vorontsova et al. 2013). Following a proposal in 2008, a 273-km2 area was given Protected Area status in 2012, thanks to Itremo’s unique flora and fauna. The area covered by the study represented around 13 km2 both within and adjacent to the protected area (Fig. 1).
Map depicting the study sites near Itremo in the Central Highlands of Madagascar. Circles Gallery forest dominated by epiphytic orchids, also terrestrial orchids on river banks; squares rocky montane grassland with lithophytic and terrestrial orchids; triangles montane moorland with terrestrial orchids; T terrestrial, L lithophyte, E epiphyte, L/T lithophyte/terrestrial, Cer Ceratobasidium, Seb Sebacina, Tul Tulasnella. Numbers 2, 3, 4, 5, and 7 denote sites; solid line depicts core protected area. Site 1 is off the area of this map to the east. No Rhizoctonia-like fungi were isolated from collections at site 6. Image © 2014 Google Earth
In light of the ongoing demise of tropical ecosystems in Madagascar and worldwide, there is an urgent need to document and safeguard the full gamut of life forms in the landscape, and to understand how these biotic agents interact with one another in this age of extinction. Among angiosperms, orchids are particularly vulnerable given their dependency on other organisms—namely mycorrhizal fungi and insect pollinators—to complete their life cycles in nature (Swarts and Dixon 2009). Thus, studying these connections becomes crucial for orchid conservation. While orchids have received considerable study with respect to classification and phylogenetic relationships, other important aspects (e.g., pollination, propagation) have received less attention, and this is especially true of Orchidaceae in Madagascar (Cribb and Hermans 2009), with a few exceptions (e.g., Nilsson et al. 1992a, b; Whitman et al. 2011). To date, virtually nothing is known about the identity, distribution, and ecology of orchid mycorrhizal fungi (OMF) in Madagascar, or the physiological roles played by these fungi with native orchids. Madagascar’s remote location, coupled with its rugged terrain, continue to pose challenges to specialists seeking to gain access to orchid-rich habitats in search of such knowledge. This is especially true for mycologists faced with the burden of expediting fresh tissue samples harboring viable fungal material (e.g., pelotons) to the lab for further study.
The main objective of this study was to understand symbiotic relationships of orchids of the Itremo Massif in Madagascar. This study was conducted to achieve the ultimate goal of producing symbiotic propagules for reintroduction by understanding the role of mycorrhizal fungi in seed germination, seedling development, and establishment of plants in the wild. While orchids throughout Madagascar require study, we chose to focus on species inhabiting the Central Highlands—a region encompassing nearly 40 % of the island (Cribb and Hermans 2009)—and spontaneous seedlings in particular. The Itremo Massif has the largest area of exposed quartzitic substrate in Madagascar (du Puy and Moat 1996) with a mixture of ecosystems found nestled within a complex of hills and valleys separated by species-poor grasslands (Cribb and Hermans 2009). Several well-known, showy taxa are found in the region, many of which persist as lithophytes on sun-exposed rocks (e.g., Angraecum longicalcar). Others exist as terrestrials of grasslands (e.g., Benthamia cinnabarina), moist forests (e.g., Cynorkis purpurea), or well-drained soils (e.g., Habenaria ambositrana), and a modest number cling to gnarled branches of host trees in open areas as epiphytes (e.g., Bulbophyllum sp.).
The orchid seed baiting technique initially developed by Rasmussen and Whigham (1993) remains the most widely used method to capture fungal symbionts that support germination in situ. Although this technique has been applied to a large number of species with varied success, namely terrestrials (McKendrick et al. 2000; Batty et al. 2006; Phillips et al. 2011), the length of period required for successful baiting is one of its drawbacks, in addition to loss of baits (Gale et al. 2010). Given Madagascar’s large number of lithophytic taxa, affixing seed baits to exposed rocks also poses a serious practical challenge as we previously discovered. With these limitations, we opted to improve our odds for acquiring germination-phase fungi by targeting spontaneous seedlings on natural substrates. Additionally, we have collected roots from mature phase plants to identify the Rhizoctonia-like fungi utilized by these orchids. In this paper, we report the mycorrhizal fungi cultured from spontaneous seedlings and mature orchids in the Central Highlands of Madagascar using morphological and molecular characterization (ITS sequencing). We also provide discussion on the distribution of these isolates in the landscape linked to the specific microhabitats of the orchids. Furthermore, we describe the molecular confirmation of the field identification of spontaneous seedlings collected and propose it as a standard methodology for other collections of a similar nature. Collection and long-distance transportation of fresh orchid material from Madagascar to labs in Europe (Kew) and North America (Illinois) will also be discussed. To our knowledge, this is the first report that documents fungal associates from orchids of different life forms and diverse ecosystems from Madagascar.
Materials and methods
This joint study was conducted between Royal Botanic Gardens Kew (Kew) and Illionis College with logistic and taxonomic support from Kew Madagascar Conservation Centre (KMCC) and Parc Botanique et Zoologique de Tsimbazaza (PBZT). More than 40 taxa within 24 genera were selected for study. Root samples were shared between two partners, and to facilitate the legal collection and international transport of orchid material from Madagascar to the UK and USA, a Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) permit was obtained, which allowed three tubes each containing seedling and mature roots per species to be collected. Due to the limited availability of plants in the wild, further restrictions were put in place locally to collect from three each of juvenile and mature plants. This was followed by a phytosanitary certificate, which was secured prior to departure from the country. For the import of root samples (with soil) and seeds to the USA, two permits were needed from the US Department of Agriculture (USDA). The US Government considers all OMF to be plant pathogens due to the ties of some to the Rhizoctonia complex.
Study sites
Seven different sites within the Central Highlands were visited during 28 April to 3 May 2013 (Table 1). These sites were all within 5 km of one another with the exception of Analabeby (Fig. 1).
Collection and transportation
The collecting trip was conducted in April/May 2013 to collect spontaneous seedling roots on orchid-rich substrates and mature roots shortly after the rainy season (December to March). Roots of orchids, of both mature plants and spontaneous seedlings where possible, were collected from 41 taxa consisting of nine lithophytic, 17 epiphytic, and 15 terrestrial species, respectively. The collection of root samples was limited to three juvenile and three mature plants per species, which was the maximum allowance as stipulated by our CITES permit. Depending on the species and availability, between one and five roots per specimen were collected. Spontaneous seedlings were putatively identified on site based on subtle morphological features (e.g., presence of pseudobulbs in Bulbophyllum sp.; Fig. 2) as well as proximity to mature plants on or near the same substrate; the identities were later confirmed by DNA analysis as described later. To maximize our chances for isolating viable pelotons, younger-appearing roots were collected whenever possible. For epiphytic and lithophytic orchids, these roots were those that were translucent to white in color, often with slight greenish pigmentation near the apex (Fig. 2). Upon detachment in the field, each root was placed over a small, premoistened cotton ball within presterilized glass vial with screw cap. To permit gas exchange leading up to departure from Madagascar (5–10 days after collection), the caps on each vial were tightened only slightly, then placed within a 50-ml capacity centrifuge tube with screw cap. These tubes were then stored vertically within an insulated handbag for transport from field to shelter. Care was taken to keep the handbag out of direct sunlight at all times so that the root samples would remain as cool as possible (15–25 °C).
a Spontaneous seedlings of Angraecum species affixed to outer bark of a host tree adjacent to a foliose lichen from Itremo in the Central Highlands of Madagascar. b A small seedling, just past the protocorm stage (denoted by the arrow), was discovered beneath loose bark shown in the palm of a hand (b, top right). c An advanced stage seedling of Angraecum protensum displays a young healthy root system with green tipped apex (arrow). Younger roots of spontaneous seedlings were targeted for collection
The root collection procedure for terrestrial orchids differed slightly in that soil containing intact root systems (root ball) was also collected. This permitted the roots to remain in a seminatural state leading up to departure from Madagascar. A trowel or small shovel was used to gently excavate the soil around individual plants and to lift the root ball with minimal disturbance to the brittle root systems. Each root ball was then placed into its own separate plastic bag, and the bags were then carefully packed into an insulated handbag for transport. Upon arrival at the KMCC base in Antananarivo, Madagascar 2–7 days after field collection, all root samples and root balls were placed into a refrigerator (about 6 °C). Approximately 24 h before departure from the country, roots of terrestrial orchids were lifted from the soil and rinsed off with UV-irradiated and/or bottled water to remove soil particles and organic debris. Lateral branch roots, especially those that exhibited orange-yellow patches of coloration, were detached and placed over a premoistened cotton ball in a presterilized glass vial. Starch-filled tuberous roots were not retained because previous studies (L. Zettler, unpublished data) have shown that such roots are generally void of pelotons. The screw cap was then tightened firmly and wrapped with a strip of Parafilm “M.” Likewise, caps on glass vials containing roots of lithophytes and epiphytes were also tightened and wrapped with Parafilm “M” at that same time (about 24 h prior to departure by air). All sealed glass vials were then housed in 50-ml plastic (shatter-proof) centrifuge vials, which were also firmly tightened and sealed with Parafilm “M.” All vials were repacked into insulated handbags and transported back to labs in the USA and UK as cabin baggage.
Measurement of substrate acidity
Substrate samples were collected at several locations and consisted of soil and humus in the case of terrestrial habitats, and organic/inorganic debris on the surface and in the cracks of rocks where lithophytic orchids were found. On return to KMCC, the collected samples were analyzed using LaMotte STH Series Combination Soil Testing Outfit (LaMotte, Maryland, USA).
Fungal isolation, initial identification, and deposition
Immediately upon arrival at Kew and Illinois 24–48 h after departure from Madagascar, all root samples were placed in refrigeration (4–6 °C) for a period lasting up to 1 week, during which time fungal isolations took place. Fungi were isolated following the method of Zettler et al. (2003). Colonization of fungi in the cortical region of root sections was scored as percentage of cortical cells containing pelotons for all three life forms. Clumps of macerated cortical cells containing pelotons were immersed in fungal isolation medium (FIM; Mitchell 1989) containing streptomycin sulfate (Clements and Ellyard 1979) and incubated at 18 °C. After 1–4 days, hyphal tips that were observed emerging from cortical cells and/or pelotons under a dissection microscope were subcultured to FIM (Kew) or potato dextrose agar (PDA; Difco™, Becton, Dickinson and Co., Sparks, MD, USA) (Illinois) using a sterile scalpel. Cultures likely to be OMF were initially distinguished from common molds using previously published descriptions (Zettler et al. 2003). Those that yielded cultural characteristics (e.g., monilioid cells) resembling basidiomycetes in the Rhizoctonia complex (e.g., Tulasnellaceae, Ceratobasidiaceae) were retained for further identification by ribosomal DNA internal transcribed spacer (rDNA ITS) amplification and sequencing. Colony growth rates were recorded during early subculture. To safeguard these strains for the purposes of future work (e.g., symbiotic seed germination) and long-term conservation, fungi isolated in Illinois were deposited into the University of Alberta Microfungus Collection and Herbarium (UAMH), Edmonton, Canada, and Kew for permanent safekeeping.
Molecular identification of fungi by ITS sequencing
To identify each fungal sample, DNA was extracted and sequenced. Genomic DNA was isolated from mycelia using Sigma Extract-N-Amp™ Plant PCR Kit (Sigma Aldrich, St. Louis, MO, USA). ITS sequences were amplified using primer combinations ITS1F with ITS4, and ITS1 with ITS4-tul (White et al. 1990; Gardes and Bruns 1993; Taylor and McCormick 2008) and using Sigma Extract-N-AmpTM Plant PCR Kit (Sigma Aldrich, St. Louis, MO, USA). The reactions were performed using a programmable thermocycler for 35 cycles of 30 s at 94 °C, 35 s at 53 °C, and 1 min at 72 °C, with the extension step increased by 5 s per cycle. Amplification was verified on 2 % agarose gels containing 0.5 μg/ml ethidium bromide in 1× TBE (89 mM Tris, 89 mM boric acid, and 2 mM EDTA) or 1× SB (50 mM boric acid, pH 8.5 with sodium hydroxide).
The amplified DNA samples were sequenced by BigDye® (Life Technologies, Carlsbad, CA, USA) Sanger sequencing with both forward and reverse primers. The PCR products were cleaned using exonuclease I and shrimp alkaline phosphatase (Affymetrix, Santa Clara, CA, USA) to remove residual single-stranded primers and remaining dNTPs before performing the cycle sequencing reactions on a thermocycler for 25 cycles of 10 s at 96 °C, 5 s at 50 °C, and 4 min at 60 °C. Reaction products were purified by ethanol precipitation and analyzed using Applied Biosystems 3730xl DNA Analyzer (Life Technologies, Carlsbad, CA, USA).
All sequence analyses were performed using Geneious® software package (Biomatters, Auckland, New Zealand). The forward and reverse sequences were checked for accuracy and consensus, and compared to database sequences using BLAST (National Center for Biotechnology Information, Bethesda, MD, USA). Sequences that matched Rhizoctonia-like fungi were aligned and grouped in to operational taxonomic units (OTUs) based on a conservative similarity threshold of 95 %. Representative sequences of each OTU were used to requery the GenBank database using BLAST. Phylogenetic trees were separately constructed for each of the three genera, Tulasnella, Ceratobasidium, and Sebacina, together with closely matched sequences from GenBank database. The sequences were aligned with CLUSTALW algorithm, and neighbor-joining trees were made using Tamura–Nei genetic distance model.
Molecular confirmation of species identification of orchid seedlings
The DNA of orchid seedlings that yielded Rhizoctonia-like fungi was extracted and sequenced in order to confirm their species identification by matching the sequence of the chloroplast DNA region trnL-F to that of an identified plant. DNA was extracted from root tissue using Sigma Extract-N-Amp™ Plant PCR Kit (Sigma Aldrich, St. Louis, MO, USA) or from desiccated leaf tissue with a modified cetyltrimethylammonium bromide (CTAB) protocol (Doyle and Doyle 1987) followed by chloroform/isoamyl alcohol (24:1) extraction and precipitation in isopropanol. The trnL-F sequences were amplified using primer combinations c with d for the trnL intron, and e with f for the trnL-F intergenic spacer (Taberlet et al. 1991) and using Reddymix™ PCR master mix (Thermo Scientific ABgene, Pittsburgh, PA, USA) with additional 150 mM trehalose, 200 μg/ml bovine serum albumin, and 0.2 % Tween-20 (Samarakoon et al. 2013). The reaction was performed on a programmable thermocycler for 28 cycles of 1 min at 94 °C, 1 min at 48 °C, and 1 min at 72 °C. The PCR products were cleaned using QIAquick® columns (Qiagen Inc., East Crawley, UK) and sequenced as described above for fungus ITS sequencing.
Endophytic efficacy testing
To assess the mycorrhizal ability of some of the fungi that were acquired, preliminary in vitro symbiotic seed germination experiments were carried out using standard protocols (Dixon 1987; Zettler et al. 2013). Mature seed collected during the same trip were surface-sterilized and sown onto oat meal agar (2.5 g oats, 7 g agar, 1 l deionized water, pH 5.4 prior to autoclaving; Dixon 1987) in Petri plates (about 100–300 seeds per plate). Each plate was then inoculated with one of the 12 fungal isolates (Table 2), sealed with Parafilm “M” and incubated in the dark at 18 °C for 5 months. Seeds were sown on same medium as above, without fungal isolates, as the control. Plates were periodically inspected for germination. Germination is defined as the stage when the seeds have reached the protocorm stage. Protocorms were exposed to illumination provided by full spectrum bulbs (Sylvania Hg/32W Octron 410 0K, F032/841/ECO, 80 μmol m2 s−1, 12 h light/12 h dark). To verify the establishment of a mycorrhizal association in the seedling stage, roots from three Tylostigma nigrescens seedlings inoculated with fungus OTU tul6 (from T. nigrescens) were detached, macerated using a sterile scalpel, and immersed in FIM as described previously. Plates were then incubated at 18 °C for 48 h and inspected for actively growing pelotons. Resulting fungal colonies and hyphae were then compared to the original isolate for verification using light microscopy and visual comparisons.
Results
Analysis of substrate
The pH of substrates collected from three collection sites was measured. At Analabeby (site 1), lithophytic orchids (Angraecum longicalcar and Oeceoclades calcarata) had roots that were aerial or adherent on rock surfaces, of which many had reached and grown into the accumulated debris in the cracks of the rocks. These debris were noticeably basic, with three separate samples having pH values of 7.8, 8.0, and 8.2, although the soil taken from the surrounding grassland had a pH of 5.8. Debris collected from among the rocky outcrops at Ambatoantrano (site 2) had a pH of 5.4, while the marshy, wet soil at Tsinahabeomby (site 4) had a pH of 5.0.
Recovery and sequencing of fungal isolates
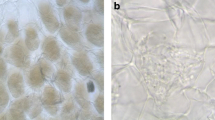
Percent of colonization with fungi of the root cortical regions of 23 orchids is represented in Fig. 3. Most colonization was found in terrestrial taxa followed by lithophytes and epiphytes. Although colonized in smaller percentages, epiphytes yielded more mycorrhizae than lithophytes. Of the 41 orchid taxa collected within seven collection sites in the Itremo region of central Madagascar, Rhizoctonia-like fungi were recovered from 11 orchid taxa (Table 2). This determination was based on morphological features matching Rhizoctonia-like fungi and confirmed by sequence data (Fig. 4). Many isolates fit the typical profile of OMF in pure culture on FIM and/or PDA. In general, these isolates had cream-colored to yellowish orange colonies and usually modest to fast growth rates at 18 °C. Colony margins were often entire and submerged slightly with raised aerial mycelia towards the (older) colony center (Fig. 5). Some isolates of Sebacina and Ceratobasidium yielded noticeable concentric zonation along the surface of the plate. Upon examination by light microscopy, many of the isolates produced ovoid to barrel-shaped monilioid cells in single or sparsely branched chains (Fig. 5). In some cases, numerous monilioid cells were evident within 1 week of subculturing. A few isolates, however, failed to yield monilioid cells, even aged (>2 months) cultures. Isolation of Rhzoctonia-like fungi was unsuccessful in 30 taxa due to very low colonization (Angraecum coutrixii, Graphorkis concolor, and few other species), high rate of digested pelotons (Satyrium trinerve) or in other cases, such as Bulbophyllum spp., the roots were extremely thin and difficult to isolate pelotons. In some other cases, dark septate endophytes (Angraecum rutenbergianum, Angraecum sp.) and other fast growing fungi made the isolation and culture of slow growing fungi difficult. Overall, epiphytes yielded less isolates compared to lithophytes and terrestrial.
The maximum observed percent colonization of cortical cells of root sections by pelotons in lithophytic (first eight taxa), epiphytic (second group of seven taxa) and terrestrial (last group of eight taxa) orchid roots collected from study sites near Itremo in the Central Highlands of Madagascar. Data were not recorded for all collections. Letters C (Ceratobasidium), T (Tulasnella), and S (Sebacina) represent Rhizoctonia-like fungi that were isolated from those taxa. Plain bars denote no fungi of the Rhizoctonia complex among the isolates cultured
Morphological and cultural characteristics of selected Rhizoctonia-like fungi collected from Itremo in the Central Highlands of Madagascar on Potato Dextrose Agar (PDA) >3 months incubation at 22 °C in 9 cm diameter Petri dishes. a Isolate OTUtul6 (UAMH 11781) from roots of Tylostigma nigrescens that grew on moist seepage mat in full sun at site 4. b Isolate OTUtul3 (UAMH 11789) acquired from roots of Cynorkis purpurea in site 7. c Isolate OTUcer1 from roots of an Aerangis sp., an epiphyte in forest at site 7
Most of the Rhizoctonia-like fungi that were isolated were assignable to Ceratobasidium, Sebacina, or Tulasnella (Table 2) based on BLAST searches of their ITS sequences against the GenBank database. Analysis of the ITS sequences of 85 Rhizoctonia-like fungal isolates revealed the presence of 12 OTUs: four Ceratobasidium, one Sebacina, and seven Tulasnella (Table 2). A phylogeny tree was constructed, separately for each genus, which included closely matched examples from the GenBank database (Figs. 6, 7, and 8).
Neighbor-joining phylogeny tree of aligned sequences of the seven Tulasnella OTUs (tul1–tul7) from orchid roots collected from Itremo in the Central Highlands of Madagascar. Also included in the tree are five closest BLAST matches in GenBank for each OTU, other close matches, and one representative of each of 10 OTUs from clades A and B described by Girlanda et al. (2011) (tulA1–4, tulB1–6). Sequences of clade B sensu Girlanda et al. (2011) were used to root the tree. Bootstrap percentages >50 %, after 1000 replicates, are shown
Neighbor-joining phylogeny tree of aligned sequences of the four Ceratobasidium OTUs (C1–C4) from orchid roots collected from Itremo in the Central Highlands of Madagascar. Also included in the tree are five closest BLAST matches in GenBank for each OTU and other close matches. Sequence of Sebacina isolate OTU seb1 was used to root the tree. Bootstrap percentages >50 %, after 1000 replicates, are shown
Neighbor-joining phylogeny tree of aligned sequences of the Sebacina OTU seb1 found from orchid roots collected from Itremo in the Central Highlands of Madagascar. Also included in the tree are close BLAST matches in GenBank. Two representative sequences of Sebacinales subgroup A sensu Weiss et al. (2004) were used to root the tree. Bootstrap percentages greater than 50 %, after 1000 replicates, are shown
All 12 OTUs had orchid-derived fungi among their closest matches from GenBank. In the Tulasnella phylogeny tree (Fig. 8), representative sequences from Girlanda et al. (2011) were also included, as the authors described two distinct clades of Tulasnella from terrestrial orchids. Similarly to Jacquemyn et al. (2012), all seven Tulasnella OTUs in the current study matched with clade A sensu Girlanda et al. (2011). The Ceratobasidium phylogeny tree (Fig. 7) was also well represented by orchid-derived fungi, although somewhat less than with Tulasnella; particularly noticeable was that some branches of the tree also had a number of fungi from Rosaceae and/or Poaceae. In the Sebacina phylogeny tree (Fig. 8), fungi found in orchids were clustered on some of the branches, while other branches were represented by fungi found in plants mainly of the Ericaceae and Aneuraceae. At the time of root collecting, seedling material was not available for collecting in majority of terrestrial taxa. However, the root materials collected from mature plants were colonized by Tulasnella OTUs (Table 3). Out of 12 OTUs, three were isolated from mature orchid roots, two from Tylostigma roots, and one from B. cinnabarina.
Roots of C. purpurea, collected from moist soil adjacent to a clear stream in a shaded forest in Antsirakambiaty, yielded the most diverse assemblage of isolates spanning all three Rhizoctonia groups (Ceratobasidium, Tulasnella, and Sebacina). The moist dense gallery forest had four orchids that yielded culturable Rhizoctonia-like fungi, as had the moist open grassland (Fig. 4a, b). The open grassland had more Tulasnella OTUs than other sites, all of which were isolated from terrestrial orchids. Site 5 (Small Gallery Forest) gave three culturable OTUs, while the exposed rocky areas of sites 2 and 3 had two species with culturable OTUs. The dense moist gallery forest had the most diverse collection of culturable fungi from the collection of root samples compared to any other habitat. Sites 4 and 5 yielded only Tulasnella and Ceratobasidium. The epiphytic orchid Aerangis punctata had an individual plant that harbored more than one OTU (cer1 and tul7). No other individual plant yielded more than one fungal OTU of the Rhizoctonia complex. In addition, pelotons in our samples also yielded non-Rhizoctonia fungi; in particular, slow-growing dark-pigmented colonies that were assignable to Toxicocladosporium, Cladophialophora, and Lophiostoma were isolated from more than one collection.
When the maturity of the plants that gave rise to the isolated OTUs was considered, it appears that roots of seedling-stage orchids harbored more Rhizoctonia-like fungi, both in lithophytic and epiphytic orchids (Table 3). On the other hand, terrestrial orchids, as a whole, seem able to yield Rhizoctonia-like isolates from either mature or juvenile plants. With restricted sample sizes due to stipulations on our collection permit, statistical tests of significance were not carried out on the data.
Endophytic efficacy testing
Seeds of nine orchid species collected in the trip were available for symbiotic germination tests. Apart from C. purpurea, none of these orchid species were able to develop to the protocorm stage on oat meal agar medium alone, while C. purpurea developed protocorms but stopped short of attaining leaf development in the absence of fungi. On the other hand, nine out of the 12 OTUs helped seeds to progress towards the protocorm stage and/or allowed C. purpurea protocorms to develop into seedlings within a period of 12 weeks, even in combination with orchid species other than its original host species. The OTU tul2 isolated from mature roots of the terrestrial B. cinnabarina induced germination of another terrestrial taxon, S. trinerve, which was also collected from the same site (site 4). Late stage leaf-bearing seedlings were produced from three terrestrial orchid species (T. nigrescens, C. purpurea, and H. ambositrana) from our preliminary symbiotic seed germination experiments. Roots of three T. nigrescens seedlings cultured in the presence of fungus OTU tul6 (isolated from mature T. nigrescens) yielded actively growing pelotons evident in FIM, and the resulting cultures matched morphology of the original fungus culture. Three OTUs (cer4, tul4 and tul7) did not help protocorm or seedling development of any of the orchids tested.
Discussion
Twelve OTUs, of three known orchid mycorrhizal genera, were recognized by analysis of ITS sequences of 85 isolates, and, by comparing with GenBank database entries, each OTU was shown to have closely related fungi that were also found as orchid associates. Of the 12 OTUs, three (cer1, tul3, and tul4) were isolated more than once, from different collections of a species (Tables 2 and 3), suggesting a certain level of specificity in the association within a background of a diversity of fungi that are apparently available to an individual orchid plant. Although this observation could alternatively suggest that the fungi are restricted to specific habitats that coincide with the preferred habitats of their host orchid species, it is very noteworthy that OTU tul3 was isolated from different habitats: from lithophytic Angraecum protensum and terrestrial C. purpurea. This indicates that at least some of the fungi are able to survive in, and colonize, a range of orchid species in a variety of habitats, but that there is an element of selection by the orchid. Some orchids harbored a surprisingly rich assemblage of different Rhizoctonia-like fungi; C. purpurea had a Ceratobasidium, Sebacina, and two OTUs of Tulasnella. With the exception of C. purpurea and Aerangis punctata (OTUs cer1 and tul7), all other orchids yielded only one type of Rhizoctonia-like fungus.
The phylogeny tree is based on isolates from a limited number of root samples due to extremely low number of plants available for collecting; therefore, this does not represent the full genetic diversity of the orchids within the remit of this study. However, this preliminary study suggested that a fungus seems able to associate with different types of orchids and some are specific to only certain orchids. Very notable was that the closest GenBank match of OTU tul7 (from Aerangis punctata) was a fungus that was identified in Aerangis punctata in Réunion (JF691324 and JF691326, Martos et al. 2012), a neighboring island to Madagascar but some 800 km to the east. OTU cer4, from the terrestrial C. purpurea, was found to be closely matched with sequences of fungi from Pterygodium catholicum (FJ788724, FJ788725, and FJ788812, Waterman et al. 2011), another African terrestrial orchid. It is planned to further investigate such indications of specificity; future collections will focus on roots of a greater number of individual plants and from several different sites for species of high conservation and scientific interests. Fungus–orchid relationships of selected orchids will also be studied by genomic analysis of mycorrhizal fungi in root sections with commonly used genetic markers in addition to ITS.
To our knowledge, this is the first report documenting culturable, potentially mycorrhizal endophytes from orchids of different life forms from diverse ecosystems from Madagascar, and one of the few from the African region (e.g., Jonsson and Nylund 1979; Mugambi 2001; Bonnardeaux et al. 2007; Waterman et al. 2011; Martos et al. 2012), most of which have used culture-independent molecular methods to detect and identify the fungi. Our success at isolating these fungi from remote and inaccessible locations suggests that our method used to collect and transport fresh root samples may have practical merit for other researchers faced with the daunting task of long-distance field work. For the terrestrial orchids in particular, the removal of a root ball (roots with surrounding soil), as opposed to immediate root detachment, followed by transport from the field to Antananarivo days later, may have contributed to the high number of isolates we recovered. For all samples, especially roots of small epiphytic seedlings prone to desiccation, placing fresh tissues over a moist cotton ball, coupled with (cool) incubation in darkness during transport, also may have contributed to the favorable outcome.
These, and numerous other, studies support the hypothesis that at least some tropical orchids, like their temperate counterparts, associate with basidiomycetes in the Cantharellales and Sebacinales (Bidartondo et al. 2004; Selosse et al. 2004). Within this group, the overwhelming majority of isolates fall under the category of “Rhizoctonia-like fungi” (Arditti 1992; Otero et al. 2002; Rasmussen 2002; Dearnaley 2007), encompassing teleomorphic genera Ceratobasidium (anamorphs = Ceratorhiza), Tulasnella (anamorphs = Epulorhiza), and Sebacina (Warcup and Talbot 1980). Tulasnelloid fungi, in particular, have been recovered from orchids with regularity throughout the world (e.g., Nontachaiyapoom et al. 2010; Zettler et al. 2013; Zi et al. 2014), and the orchids of Madagascar appear to fit the same general profile. The Ceratobasidiaceae was also well represented in Madagascar root samples, paralleling reports mostly from the New World (e.g., Richardson et al. 1993; Zettler and Piskin 2011), and Sebacinaceae fungi were also present among our isolates. Representatives of both Tulasnellaceae and Ceratobasidiaceae were isolated from several orchid species encompassing terrestrial, lithophytic, and epiphytic habits, but more epiphytes yielded Ceratobasidiaceae fungi while terrestrials had mostly Tulasnellaceae fungi (Table 2). It is interesting to note that gallery forest and the moist open grassland had the most hits for culturable Rhizoctonia-like fungi, predominantly from terrestrial taxa. The open grassland and adjacent lithophytic habitats are subjected to man-made fire (Whitman et al. 2011). Seedling recruitment and survival are likely to be adversely affected; therefore, conservation of orchids from these areas warrants immediate attention. Gallery forests are also affected, therefore, mapping the orchid diversity and their fungal symbionts need urgent study.
Results of soil/substrate analysis showed that most habitats where orchids were collected in this study showed an acidic pH of 5.0–5.8. One exception to this was the lithophytic habitat at Analabeby (site 1) where the pH of the accumulated substrate between the rocky outcrops, within which fungal symbionts might be expected to exist until they encounter their hosts, was consistently basic at 7.8–8.2. Analabeby (Fig. 1) collection sites feature rocky outcrops consisting mainly of marble, whereas the outcrops at the other study areas were of sandstone, granite, and quartzite, which explains the difference in pH of the immediate environment of lithophytic plants. It was particularly noticeable that the surfaces of the rocks at Analabeby were devoid of lichen, in contrast to its abundance on the rocks at Ambatoantrano, where the debris among the rocks had a pH of 5.4. Lithophytic habitats at other collection sites also had profuse growths of lichen, suggesting that these locations also had similarly acidic substrates. Rhizoctonia-like fungi were successfully isolated from lithophytic roots collected at Ambatoantrano (site 2), Ambatoantremo (site 3), and Tsinahabeomby (site 4), whereas in contrast, none were isolated from lithophytic roots collected from Analabeby. Epiphytic orchids were also often found in association with lichen; four collections from epiphytic habitats yielded OTUs of Rhizoctonia-like fungi. These observations could be indicating that fungal associates of the lithophytes at Analabeby may have a different physiology as a consequence of having adapted to a different environment, and they may therefore have different culture requirements. It could even be possible that orchids in these environments have symbiotic associations with types of fungi other than those of the Rhizoctonia complex. It may be necessary to adjust the isolation medium composition if mycorrhizae are to be successfully isolated from orchid roots from such environments.
As an alternative to in situ seed baiting, we made a conscious effort to collect roots from young orchid plants, including protocorms and very small seedlings. These juvenile plants were identified in the first instance by local botanical specialists based on morphology, habitat, and the proximity of mature specimens that could be the likely parents. We set out to bolster our confidence in the identities of the seedlings by seeking a definitive identification using DNA sequence analysis to compensate for the lack of mature morphological features. By matching DNA sequences of the seedlings to those of collections of mature plants and/or GenBank entries, we have been able to confirm the identities of the putatively identified seedlings. As one of our aims is to investigate the specificities in the orchid–fungus relationship, we consider the molecular confirmation of the seedling identity to be of critical importance and propose the methodology as standard procedure for our future collections of fungal associates from juvenile plants in Madagascar, with its diversity of orchid species.
Targeting spontaneous seedlings offers greater flexibility, compared to seed burial (baiting) technique, with positive identification being done within weeks instead of months or years of collecting the material. With the help of fingerprinting techniques as we used here, identifying the seedlings has been made more reliable. As orchid populations continue to decline worldwide, it is conceivable that cross-pollination between unrelated individuals will diminish resulting in lower seed viability. As a result, it may be necessary for future workers to rely more on spontaneous seedlings and less on the standard seed baiting techniques as a means to acquire early germination phase fungi. For critically endangered taxa, use of the seed baiting technique may also be viewed as too wasteful considering that few seed packets typically yield seedlings (protocorms) once retrieved. Nevertheless, we recommend that seed baiting techniques aimed at recovering protocorms not be abandoned, as the specific fungi supporting initial seed germination may actually be replaced by other types of fungi around the time that leaves are initiated in the seedling stage (fungal succession). Thus, it is conceivable that some of the fungi isolated from spontaneous seedlings may not be the same as those that initiated germination in situ. However, based on preliminary results involving in vitro symbiotic seed germination using the fungi isolated in this study (research in progress), majority of these fungi are indeed capable of initiating germination beyond the protocorm stage. This suggests that spontaneous seedlings—at least in part—harbor fungi of some physiological significance, and are not merely carriers of benign fungal assemblages. A thorough investigation into the possible mycorrhizal ability, or lack thereof, of the different fungi during orchid germination and subsequent growth is essential to better understand the diversity of fungi that have so far been isolated.
Our tally of Rhizoctonia-like fungi suggested that seedling roots of some lithophytic and epiphytic taxa were able to provide good quality pelotons for isolating Rhizoctonia-like isolates, whereas mature root material of terrestrial taxa was also a good source of isolates (Table 3). Regardless of whether these fungal OTUs are the same fungi that first allowed the successful germination of the orchid seed and establishment of the protocorm, the relative ease with which juvenile roots of some orchid species yielded Rhizoctonia-like fungi may be indicative of a greater dependence upon mycorrhizal symbiosis at particular stages of the life cycle. Older plants may be less dependent on Rhizoctonia-like fungi and/or may be switching to different fungi upon maturity. With the focus on Rhizoctonia-like fungi in this study, there is scope for further investigations into the possibility of fungal taxa other than Rhizoctonia-like fungi that may be OMF. Roots that were heavily colonized by fungi, as evidenced by a large number of visible pelotons, yet failed to yield Rhizoctonia-like isolates may have harbored different and/or difficult to culture fungi that were missed in this study and demands further attention. Furthermore, as the collection trip for this study was made at the start of the dry season and possibly at the start of plant dormancy, a collection made during wet season, when the orchids and fungi are anticipated to be participating in active symbiosis, will be investigated for future study. Collecting more material to obtain larger sample sizes would be needed for statistically meaningful survey data, which the authors are reluctant to recommend as a routine protocol to study exceptionally rare orchid species as reported here. Still, it would also be of interest to study the levels of fungal associations found on a single established plant over time, collecting samples at different times of year, and over a period of multiple years. This will help understand the nature and significance of fungi to orchid growth, recovery, and development, and its flux through the seasons and the orchid’s life cycle.
Conclusions and future studies
As the first detailed investigation into the identification of culturable orchid endophytes in Madagascar that are potential OMF, our findings will form the foundation of our future approach in understanding how the orchid–fungus relationship relates to their native environments. In addition to planned further collections from different locations, different orchid species, and at different times of the year, in vitro germination tests will be carried out to reveal the specificity/generality of the symbiosis. It is expected that the sum of knowledge will lead to a better conservation strategy that is designed to protect vulnerable orchid taxa by taking into account of their need for symbiotic partners. Furthermore, this will help to develop a phylogentic analysis of mycorrhizae, their evolution across the land mass of Madagascar, and how this affects natural regeneration of orchids in pristine and fragmented ecosystems.
Erratic pollination and low seed set were identified in Aerangis ellisii (Nilsson and Rabakonandrianina 1988; Nilsson et al. 1992a, b), but there may be similarly affected taxa in Madagascar. In the case of Aerangis ellisii, we have noticed very low or no germination of mature seeds by asymbiotic methods (unpublished data). To develop genetically diverse stocks for species restoration projects is a huge challenge as in vitro asymbiotic germination alone is not an effective tool. If mycorrhizal seed germination can be applied to improve not just seed germination but seedling survival in cases otherwise impossible may be a good step in the right direction. Although data are not available at the moment regarding fungal diversity and density for a specific habitat as part of this study, symbiotic seedlings can be used for reintroduction. Detailed studies to identify the mycobiont populations benefit the resilience of reintroduced orchids in Madagascar and other biodiversity hotspots areas. This will be developed as a major research area to contribute to ecosystem services, conservation, and phylogenetic studies.
References
Alvarado ST, Buisson E, Rabarison H, Rajeriarison C, Birkinshaw C, Lowry PP (2014) Comparison of plant communities on the Ibity and Itremo massifs, Madagascar, with contrasting conservation histories and current status. Plant Ecol Divers 7:497–508
Arditti J (1992) Fundamentals of orchid biology. Wiley, New York
Batty AL, Brundrett MC, Dixon KW, Sivasithamparam K (2006) In situ symbiotic seed germination and propagation of terrestrial orchid seedlings for establishment at field sites. Aust J Bot 54:375–381
Bidartondo MI, Burghardt B, Gebauer G, Bruns TD, Read DJ (2004) Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc R Soc Lond B 271:1799–1806
Bonnardeaux Y, Brundrett M, Batty A, Dixon K, Koch J, Sivasithamparam K (2007) Diversity of mycorrhizal fungi of terrestrial orchids: compatibility webs, brief encounters, lasting relationships and alien invasions. Mycol Res 111:51–61
Clements MA, Ellyard RK (1979) The symbiotic germination of Australian terrestrial orchids. Am Orchid Soc Bull 48:810–816
Cribb P, Hermans J (2009) Field guide to the orchids of Madagascar. Kew Publishing. Royal Botanic Gardens, Kew
Dearnaley JDW (2007) Further advances in orchid mycorrhizal research. Mycorrhiza 17:475–486
Dixon KW (1987) Raising terrestrial orchids from seed. In: Harris WK (ed) Modern orchid growing for pleasure and profit. Orchid Club of S Australia, Inc, Adelaide, pp 47–100
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
du Puy D, Moat J (1996) A refined classification of the primary vegetation of Madagascar based on the underlying geology: using GIS to map its distribution and to assess its conservation status. In: Lourenço WR (ed) Proceedings of the International Symposium on the ‘Biogeography de Madagascar’, Paris, September 1995, pp 205–218
Fenu G, Mattana E, Congiu A, Bacchetta G (2010) The endemic vascular flora of Supramontes (Sardinia), a priority plant conservation area. Candollea 65:347–358
Gale SW, Yamazaki J, Hutchings MJ, Yukawa T, Miyoshi K (2010) Constraints on establishment in an endangered terrestrial orchid: a comparative study of in vitro and in situ seed germinability and seedling development in Nervilia nipponica. Bot J Lin Soc 163:166–180
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Girlanda M, Segreto R, Cafasso D, Liebel HT, Rodda M, Ercole E, Cozzolino S, Gebauer G, Perotto S (2011) Photosynthetic Mediterranean meadow orchids feature partial mycoheterotrophy and specific mycorrhizal associations. Am J Bot 98:1148–1163
Jacquemyn H, Deja A, De hert K, Bailarote BC, Lievens B (2012) Variation in mycorrhizal associations with tulasnelloid fungi among populations of five Dactylorhiza species. PLoS One 7(8):e42212
Jonsson L, Nylund JE (1979) Favolaschia dybowskyana (Singer) Singer (Aphyllophorales), a new orchid mycorrhizal fungus from tropical Africa. New Phytol 83:121–128
Martos F, Munoz F, Pailler T, Kottke I, Gonneau C, Selosse MA (2012) The role of epiphytism in architecture and evolutionary constraint within mycorrhizal networks of tropical orchids. Mol Ecol 21:5098–5109
McKendrick SL, Leake JR, Taylor DL, Read DJ (2000) Symbiotic germination and development of myco-heterotrophic plants in nature: ontogeny of Corallorhiza trifida and characterization of its mycorrhizal fungi. New Phytol 145:523–537
Mitchell RB (1989) Growing hardy orchids from seeds at Kew. Plantsman 2:152–169
Mittermeier RA, Robles PG, Hoffman M, Pilgrim J, Brooks T, Mittermeier CG, Lamoreux J, da Fonseca GAB (2005) Hotspots revisited: earth’s biologically richest and most endangered terrestrial ecoregions. University of Chicago Press, Chicago
Moat J, Smith P (2007) Atlas of the vegetation of Madagascar. Royal Botanic Gardens, Kew
Mugambi GK (2001) Ensuring survival of Kenyan orchids: the ex situ conservation interventions. International Orchid Conservation Congress I (Conference Proceedings), p 102
Murray-Smith C, Brummitt NA, Oliviera-Filho AT, Bachman S, Moat J, Lughadha EM, Lucas EJ (2009) Plant diversity hotspots in the Atlantic coastal forests of Brazil. Conserv Biol 23:151–163
Nilsson LA, Rabakonandrianina E (1988) Hawk-moth scale analysis and pollination specialization in the epilithic malagasy endemic Aerangis ellisii (Reichenb fil) Schltr (Orchidaceae). Bot J Linn Soc 97:49–61
Nilsson LA, Rabakonandrianina E, Pettersson B (1992a) Exact tracking of pollen transfer and mating in plants. Nature 360:666–668
Nilsson LA, Rabakonandrianina E, Rotaharivelo R, Randriamanindry JJ (1992b) Long pollinia on eyes: hawk-moth pollination of Cynorkis uniflora Lindley (Orchidaceae) in Madagascar. Bot J Linn Soc 109:145–160
Nontachaiyapoom S, Sasirat S, Manoch L (2010) Isolation and identification of Rhizoctonia-like fungi from roots of three orchid genera, Paphiopedilum, Dendrobium, and Cymbidium, collected in Chiang Rai and Chiang Mai provinces of Thailand. Mycorrhiza 20:459–471
Otero JT, Ackerman JD, Bayman P (2002) Diversity and host specificity of endophytic Rhizoctonia-like fungi from tropical orchids. Am J Bot 89:1852–1858
Phillips RD, Barrett MD, Dixon KW, Hopper SD (2011) Do mycorrhizal symbioses cause rarity in orchids? J Ecol 99:858–869
Rasmussen HN (2002) Recent developments in the study of orchid mycorrhiza. Plant Soil 244:149–163
Rasmussen HN, Whigham DF (1993) Seed ecology of dust seeds in situ: a new study technique and its application to terrestrial orchids. Am J Bot 80:1374–1378
Richardson KA, Currah RS, Hambleton S (1993) Basidiomycetous endophytes from the roots of neotropical epiphytic Orchidaceae. Lindleyana 8:127–137
Roos MC, Kessler PJA, Gradstein SR, Baas P (2004) Species diversity and endemism of five major Malesian islands: diversity–area relationships. J Biogeogr 31:1893–1908
Samarakoon T, Wang SY, Alford MH (2013) Enhancing PCR amplification of DNA from recalcitrant plant specimens using a trehalose-based additive. Appl Plant Sci 1:1200236
Selosse MA, Faccio A, Scappaticci G, Bonfante P (2004) Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microb Ecol 47:416–426
Swarts ND, Dixon KW (2009) Terrestrial orchid conservation in the age of extinction. Ann Bot 104:543–556
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109
Taylor DL, McCormick MK (2008) Internal transcribed spacer primers and sequences for improved characterization of basidiomycetous orchid mycorrhizas. New Phytol 177:1020–1033
Tyson P (2000) The eighth continent: life, death and discovery in the lost world of Madagascar. William Morrow (Harper Collins) Publishers, New York
Vorontsova MS, Ratovonirina G, Randriamboavonjy T (2013) Revision of Andropogon and Diectomis (Poaceae: Sacchareae) in Madagascar and the new Andropogon itremoensis from the Itremo Massif. Kew Bull 68:1–15
Warcup JH, Talbot PHB (1980) Perfect states of Rhizoctonias associated with orchids III. New Phytol 86:267–272
Waterman RJ, Bidartondo MI, Stofberg J, Combs JK, Gebauer G, Savolainen V, Barraclough TG, Pauw A (2011) The effects of above- and belowground mutualisms on orchid speciation and coexistence. Am Nat 177:54–68
Weiss M, Selosse MA, Rexer KH, Urban A, Oberwinkler F (2004) Sebacinales: a hitherto overlooked cosm of heterobasidiomycetes with a broad mycorrhizal potential. Mycol Res 108:1003–1010
White TJ, Bruns TD, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Whitman M, Medler M, Randriamanindry JJ, Rabakonandrianina E (2011) Conservation of Madagascar’s granite outcrop orchids: the influence of fire and moisture. Lankesteriana 11:55–67
Zettler LW, Piskin KA (2011) Mycorrhizal fungi from protocorms, seedlings and mature plants of the Eastern Prairie Fringed Orchid, Platanthera leucophaea (Nutt.) Lindl.: a comprehensive list to augment conservation. Am Midl Nat 166:29–39
Zettler LW, Sharma J, Rasmussen F (2003) Mycorrhizal diversity. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ (eds) Orchid conservation. Natural History Publications (Borneo), Kota, pp 185–203
Zettler LW, Corey LL, Jacks AL, Gruender LT, Lopez AM (2013) Tulasnella irregularis (Basidiomycota: Tulasnellaceae) from roots of Encyclia tampensis in south Florida, and confirmation of its mycorrhizal significance through symbiotic seed germination. Lankesteriana 13:119–128
Zi XM, Sheng CL, Goodale UM, Shao SC, Gao JY (2014) In situ seed baiting to isolate germination-enhancing fungi for an epiphytic orchid, Dendrobium aphyllum (Orchidaceae). Mycorrhiza 24:487–499
Acknowledgments
We kindly thank the financial support received from Sainsbury Orchid Project, Bentham and Moxon Trust, and Margaret A Cargill Foundation. We acknowledge the invaluable assistance received from Gaëtan Ratovonirina and Landy Rajaovelona (KMCC), Solo Rapanarivo (PBZT) for field support during the collection; Korrie Edwards (Illinois College), and Helen Sandford, Margaret Ramsay, and Edward Jones (Kew) for technical assistance; Connie Gibas and Lynne Sigler (UAMH) for deposition of isolates; Stuart Cable (Kew) and Hanne Rasmussen for helpful suggestions; and Mike Fay and Robyn Cowan (Kew) for their advice on genetic fingerprinting of plant samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yokoya, K., Zettler, L.W., Kendon, J.P. et al. Preliminary findings on identification of mycorrhizal fungi from diverse orchids in the Central Highlands of Madagascar. Mycorrhiza 25, 611–625 (2015). https://doi.org/10.1007/s00572-015-0635-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-015-0635-6