Abstract
To advance our understanding of ectomycorrhizal fungal communities in mining areas, the diversity and composition of ectomycorrhizal fungi associated with Masson pine (Pinus massoniana Lamb.) and soil chemistry were investigated in Taolin lead–zinc (Pb–Zn) mine tailings (TLT), two fragmented forest patches in a Huayuan Pb–Zn mineland (HY1 and HY2), and a non-polluted forest in Taolin in central south China. Ectomycorrhizal fungal species were identified by morphotyping and sequence analyses of the internally transcribed spacer regions of ribosomal DNA. The two study sites in the Huayuan mineland (HY1 and HY2) were significantly different in soil Pb, Zn, and cadmium (Cd) concentrations, but no significant difference was observed in ectomycorrhizal colonization, ectomycorrhizal fungal richness, diversity, or rank–abundance. In addition, the similarity of ectomycorrhizal fungal communities between HY1 and HY2 was quite high (Sørensen similarity index = 0.47). Thus, the concentration of heavy metals may not be determining factors in the structure of these communities. In the tailings, however, significantly lower ectomycorrhizal colonization and ectomycorrhizal fungal richness were observed. The amounts of Pb and Zn in the tailing sand were higher than the non-polluted forest but far lower than in HY1. Thus, these heavy metals did not account for the reduced colonization and ectomycorrhizal fungal richness in TLT. The ectomycorrhizal fungal community in TLT was dominated by four pioneer species (Rhizopogon buenoi, Tomentella ellisii, Inocybe curvipes, and Suillus granulatus), which collectively accounted for 93.2 % of root tip colonization. The immature soil conditions in tailing (low N and P, sand texture, and lack of organic matter) may only allow certain pioneer ectomycorrhizal fungal species to colonize the site. When soil samples from four sites were combined, we found that the occurrences of major ectomycorrhizal fungal taxa were not clearly related to the concentrations of Pb, Zn, and Cd. In conclusion, our results suggest that ectomycorrhizal fungal communities in mining areas are not necessarily affected by heavy metals themselves but could be largely determined by soil maturity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mining activities destroy vegetation and remove surface soil to reach buried ore deposits. Mineral extraction processes are often accompanied by pulverization of rocks and chemical use. The residue of the extracted substrates is stored in tailings, producing large areas of bare ground and piles of tailings. Ideally, these mine areas should be restored to the original vegetation (Bradshaw and Johnson 1992). However, the toxicity of heavy metals, lack of macronutrients, and abnormal soil structures in mining areas make restoration difficult without careful planning (e.g., storage of surface soil to cover the bare ground after mining activities). In China, many mining areas have been abandoned, causing severe ecological and environmental problems (Liu et al. 2005; Shu et al. 2005; Li 2006). Soils in these abandoned mine areas are highly contaminated by heavy metals, which are dispersed by wind or dissolved in water, causing human health disorders in the surrounding regions.
Many of the abandoned mine areas in China were originally covered by forests dominated by Pinaceae and Fagaceae. These tree species depend on ectomycorrhizal fungi (EMF) for nutrient absorption and cannot grow in their absence. Some reports have also demonstrated that EMF help trees survive in soils contaminated by heavy metals (Dickinson et al. 1992; Wilkinson and Dickinson 1995) by alleviating the toxicity of the metals (Jentschke and Godbold 2000; Meharg and Cairney 2000; Adriaensen et al. 2004; Adriaensen et al. 2005; Adriaensen et al. 2006; Colpaert et al. 2011). Thus, application of EMF in abandoned mine areas may help with forest restoration. Unfortunately, our knowledge about the function of EMF in heavy metal tolerance is largely from in vitro experiments using a few easily culturable strains.
In the field, EMF always exist and function as a community composed of a diverse range of species. Although ectomycorrhizal fungal communities have been studied in many different forests throughout the world, we know little about these communities in mining areas. A few reports document significantly reduced diversity of ectomycorrhizal fungal communities in heavy metal-contaminated areas (Staudenrausch et al. 2005; Ruotsalainen et al. 2009). In contrast, other studies have shown highly diverse ectomycorrhizal fungal communities in heavy metal-contaminated sites, with no strong indication of selection for heavy metal-tolerant species (Blaudez et al. 2000; Cripps 2003; Krpata et al. 2008; Colpaert et al. 2011; Hui et al. 2011). Because we do not know the reasons for the inconsistent results in previous studies, more research is needed to identify the determinant factors that structure ectomycorrhizal fungal communities in heavy metal-contaminated soils.
Masson pine (Pinus massoniana Lamb.) has been frequently planted in deforested areas in central southern China because of its tolerance to drought and arid conditions (Zhu et al. 2010). Many natural forests and plantations of Masson pine are distributed in this region. Even in mining areas, we can find some remaining forest patches or naturally established trees, as well as planted trees. Masson pine is a typical ectomycorrhizal conifer species and maintains symbioses with a diverse range of ectomycorrhizal fungal species in natural forests (Chen 1989; Ke and Liu 2005). To develop an effective reforestation strategy in the mining areas, we need better understanding of EMF on Masson pine in mining areas.
In this study, ectomycorrhizal fungal communities on Masson pine were studied in the largest abandoned lead–zinc (Pb–Zn) mine (Taolin Pb–Zn mine) and an operating Pb–Zn mine (Huayuan Pb–Zn mine) in Hunan Province in central southern China. The goals of this study were to characterize ectomycorrhizal fungal communities on Masson pines growing in two Pb–Zn mining areas and to evaluate the effects of heavy metals and other soil factors on these communities.
Materials and methods
Sampling sites
The study areas are located in Linxiang City and Huayuan County (about 700 km apart) in Hunan Province, China. Three distinct habitats [fragmented forest patches (two sites, HY1 and HY2) in excavated Huayuan Pb–Zn mineland, Taolin Pb–Zn mine tailing (TLT), and non-polluted forest (TLC) in Linxiang City] were selected for present study. The position of Taolin mine and Huayuan mine are shown in Fig. 1. The study sites are described in detail in the following text.
Taolin Pb–Zn tailing (TLT; 29°22′ N, 113°28′ E) of Taolin Pb–Zn mine is located in a low mountain and hill region with elevations ranging from 200 to 300 m and slopes between 25 ° and 35 °. The average annual temperature and precipitation are 16.4–16.8 °C and 1,325 mm, respectively. The mine was operated from 1901 to 2002. The tailing dam with a top surface area of approximately 79 ha, which was built in 1960, stores 5,000 million tons of processed residues and reaches an ultimate height of about 30 m from its foot. The residue is silver white tailing sand mainly composed of SiO2 (about 70 %). The tailing surface soils were mainly composed of sand and silt, with lack of macronutrients and organic matter. The tailing surface is often drought because of poor water holding capacity and high evaporation (1,424.2 mm/year) on tailing surface (Guo et al. 2007). Most parts of the top surface of this huge tailing were still completely bare except for about 200 individuals of planted 7-year-old Masson pine (P. massoniana) and a few naturally established herbaceous plant species. The pine trees showed no symptoms of disorder and were selected for the present research.
The non-polluted mature forest (TLC) on the slope of a hill is about 7 km far from the Taolin tailing. The forest was mainly composed of regenerated Masson pine (about 30 years old), Chinese fir (Cunninghamia lanceolata Lamb.), and Quercus spp. The forest land is in the typical red soil hilly regions in southern China.
Huayuan Pb–Zn mine (28°31′ N, 109°22′ E) is located in Huayuan County in western Hunan Province and is the largest Pb–Zn ore deposit processed in this province. The region has an average altitude of 800–1,200 m with subtropics mountainous moist climate; the average annual temperature and precipitation in this area are 16 °C and 1,418 mm, respectively. Vegetative cover in the mining area was severely destroyed by mining activities. Two fragmented secondary forest patches (HY1 and HY2) in the mining area, about 500 m apart, were selected as sampling sites. The HY1 forest patch is composed of regenerated Masson pine trees (about 15 years old) and Chinese fir (C. lanceolata Lamb.). The HY2 patch, a secondary Masson forest (about 30 years old), was located 200 m away from an extraction plant. Many unprocessed ore rocks were dispersed around the forest patch.
Sampling
Sampling at the Huayuan Pb–Zn mining sites (HY1 and HY2) and Taolin sites (TLT and TLC) was carried out in March 2009 and April 2010, respectively. Because of the limited number of pine trees in HY1 and HY2, only ten Masson pine trees were selected for sampling. From each of the selected trees, three root systems (each approximately 15 cm in length) and three corresponding rhizospheric soil samples (each 50 ml) were collected within approximately 3-m distance from the focal tree. In the Taolin area, we selected 40 trees in the tailing (TLT) and 24 trees in the control forest (TLC) sites and collected one root and one soil sample from each tree. All root systems were traced from the trunk to confirm the identity of the roots. Pine needles were also sampled from each selected tree for analysis of heavy metal contents.
Ectomycorrhizal root tip morphotyping and molecular analysis
Root samples were gently washed in tap water to remove soil particles and debris, cut into approximately 9-cm-long sections, and placed in a glass plate filled with tap water. Root tips that were colonized by EMF were counted to determine percent colonization and classified into ectomycorrhizal fungal morphotypes based on their mantle color, surface texture, branching patterns, rhizomorph characteristics, and emanating hyphae using a dissecting microscope (Agerer 1987–1993). About one tenth of the ectomycorrhizal root tips for each morphotype from each root system were randomly selected, individually placed in 2.0-ml tubes, and vacuum-dried for DNA extraction.
Crude genomic DNA was extracted from dried ectomycorrhizal root tips using a modified cetyl-trimethylammonium bromide (CTAB) method (Lian et al. 2003). A single root tip sample was homogenized in a 2.0-ml tube containing one zirconia ball and 50 μl of CTAB (2 % CTAB, 100 mM Tris (pH 8.0), 20 mM EDTA (pH 8.0), 1.4 NaCl, and 0.5 % β-mercaptoethanol) solution using a beadbeater (MicroSmash; Tomy Seiko Co. Ltd., Tokyo, Japan). After confirming that the sample was completely pulverized, 350 μl of CTAB solution was added to the tube and then incubated at 65 °C for 1 h. DNA was isolated using chloroform–isoamyl alcohol mixture (24:1) extraction, precipitation in isopropanol, and washing in 75 % ethanol. The extracted DNA was dissolved in 30 μl sterilized water and stored at –30 °C until use.
The fungal internal transcribed sequence (ITS) regions were amplified by polymerase chain reaction (PCR) using Ampli Taq Gold (Applied Biosystems, Foster City, CA, USA) and fungal-specific primers ITS1-F and ITS4 (White et al. 1990; Gardes and Bruns 1993; Lian et al. 2003). When the PCR products were faint, absent, or multibanded, three alternative methods were tested. For the multiband samples with a morphotype like Cenococcum sp., ITS1 and ITS4 primers were used in the PCR reaction (White et al. 1990). For the other multiband samples, the basidiomycete-specific primer pair, ITS1-F and ITS4B (Gardes and Bruns 1993), was used, and the ITS fragments were amplified by MightyAmp Polymerase (Takara, Dalian City, Japan) or Ampli Taq Gold. For samples of faint and absent PCR products, a nested-PCR system was used with two primer sets (first PCR step: NLA3 and NLC2 primers; second PCR step: NSI1 and NLB4 primers) using Ampli Taq Gold (Martin and Rygiewicz 2005). This nested-PCR system was also employed for the unsuccessful samples after applying ITS1-F and ITS4B primers.
To decrease the number of sequencing samples, restriction fragment length polymorphism (RFLP) was used to identify molecular types within each root sample. Five microliters of each PCR product was digested with AluI or HinfI (1.5 U; Takara Otsu, Shiga, Japan) in the reaction mixture for 8 h at 37 °C. Restriction fragments were separated by electrophoresis on 1.2 % agarose gels. One representative sample with a unique RFLP pattern within a root sample was used for direct sequencing using ITS1-F, ITS1, ITS4, and ITS3 primers, respectively. The PCR products were purified using a PCR Product Pre-Sequencing Kit (USB Co., Cleveland, OH, USA) according to the manufacturer’s instructions. After a sequencing reaction with BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems), sequences were determined in Applied Biosystems 3130xl Genetic Analyzers. When direct sequencing resulted in failure, PCR products were subcloned using pT7 Blue Perfectly Blunt Cloning kit (Novagen, Madison, WI, USA). Successful inserts were sequenced using T7 and U19 primers as described earlier.
The obtained sequences were edited and manually corrected in BioEdit 7.0.8 and then clustered into species-level operational taxonomic units (OTUs) by BLASTclust with 97 % sequence similarity for species delimitation (http://toolkit.tuebingen.mpg.de/blastclust) (Peay et al. 2009). Sequences from OTUs were identified by querying GenBank and UNITE (Koljalg et al. 2005) online databases using the Blastn search option. Sequences having ITS similarities ≥97 % to known fungal species were given the same species name (Tedersoo et al. 2003; Krpata et al. 2008; Tedersoo et al. 2008; Peay et al. 2009). Sequences with 90–97 % similarities to known species were identified at the genus level (Pestana Nieto and Santolamazza Carbone 2009). When Blast results showed poor matches (<90 % ITS similarities), we treated the OTUs as unknown species. All sequences, except a few short ITS sequences, were submitted to the DNA Data Bank of Japan (DDBJ).
Chemical analyses of soil and pine leaves
Soil samples were air-dried, ground, and passed through a 2-mm mesh screen. Pine needles were washed with tap water, rinsed with deionized water (DW), oven-dried at 105 °C for 5 min and at 60 °C for 24 h, and finally milled. Soil pH value was determined by HM-30 G pH meter (TOA Electronics Ltd., Kobe, Japan) after mixing the soil sample with DW at 1:2 ratio by volume. Electrical conductivity (EC) was measured with a B-173 compact conductivity meter (Horiba, Ltd., Tokyo, Japan) using soil suspensions (soil/DW = 1:5). After digestion by sulfuric acid and perchloric acid, total nitrogen was determined by the indophenol blue method (Dora 1976) and total phosphorus was determined by the molybdenum blue method (Olsen and Sommers 1982) using a U-2010 spectrophotometer equipped with an AS-1000 auto sampler (Hitachi Instruments, Inc., Tokyo, Japan). The total amounts of potassium (K), Pb, cadmium (Cd), copper (Cu), and Zn in soil and pine needle samples were determined by a Z-6100 Polarized Zeeman atomic absorption spectrometer (Hitachi, Co., Tokyo, Japan) after wet digestion with HNO3 and HClO4 mixture (4:1, v/v for soil; 7:1, v/v for leaves). We also analyzed plant-available heavy metal (Pb, Cd, Cu, and Zn) concentrations by extracting metal ions from the soil with DTPA solution (5 mM DTPA, 10 mM CaCl2, and 100 mM triethanolamine at pH 7.3) (Lindsay and Norvell 1978).
Data analysis
The relative abundance of an ectomycorrhizal fungal species in a tree or site was defined as the ratio of ectomycorrhizal tips colonized by that species within a tree or site, respectively. Frequency was shown by the number of trees colonized by each EMF. Observed species richness was the total number of detected ectomycorrhizal fungal species in a site. Because detecting all EMF at a site is impossible, the sufficiency of sampling effort was evaluated by species accumulation curves produced with EstimateS version 8.0 (Colwell 2006). Species richness estimators (i.e., Jackknife1, Jackknife2, and Chao2) and diversity indices [i.e., Simpson’s index (1/D) and Shannon–Wiener Index (H′)] were calculated for each study site using EstimateS, and then one-way ANOVAs were used to compare the difference in those indices among different sites. Sørensen’s similarity index was used to compare the ectomycorrhizal fungal composition between the study sites.
One-way ANOVA followed by Tukey’s test at 5 % significance level was used to compare soil and plant chemical data among the four sites. By pooling all of the soil and plant heavy metal data from the four sites, correlation between soil and leaf heavy metals was analyzed. To further analyze the effect of each soil factor on ectomycorrhizal fungal diversity after alleviating a large variance within a site and difference in sample sizes among the sites, we created another dataset by grouping 12 soil subsamples from TLC and ten samples from TLT according to the level of each soil factor. The resultant dataset included two subsample groups from TLC and four groups from TLT, each of which was represented by ten or 12 soil samples with similar levels of the soil factor. Then, the correlation between each soil factor and ectomycorrhizal fungal diversity was analyzed among HY1, HY2, and the subsample groups from TLT and TLC (eight data points in total). All of the above statistical analyses were conducted with SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). One-way ANOVA followed by Tukey’s test at 5 % significance level was used for comparing the distribution range of nitrogen (N), Cu, Cd, and Zn content in soils for each major EMF taxa. A nonparametric test (Steel–Dwass test) was performed online (http://www.gen-info.osaka-u.ac.jp/MEPHAS/s-d-e.html) to compare the Pb concentration of soil samples in which the major ectomycorrhizal fungal taxa were found.
Results
Rhizosphere soil characteristics and heavy metal concentrations in Masson pine leaves
The results of soil analyses are summarized in Table 1. Soils were slightly acidic to neutral at the three mining sites, HY1 (mean ± stand error, 6.4 ± 0.2) and HY2 (6.9 ± 0.3) in Huayuan and the tailing site TLT (6.4 ± 0.6) in the Taolin area, while the soils of the control forest (TLC) adjacent to the Taolin tailing were acidic (pH 4.7 ± 0.4). Soil EC values were within the nonsaline range in all study sites. The concentrations of macronutrients in HY1 and HY2 soils were within the normal range, but the concentrations of heavy metals in HY1, especially Pb, Zn, and Cd, were high. Tailing (TLT) soils contained slightly higher levels of Pb, Zn, and Cu, accompanied by very low concentrations of total N and phosphorus (P). Soils in the control forest (TLC) were characterized by the lowest concentrations of heavy metals and the highest amounts of N among the four study sites. The concentrations of Zn in HY1, HY2, and TLT, Pb in HY1, and Cd in HY1 and HY2 exceeded grade III levels of the China Environmental Quality Standard (Zn, 500 mg kg–1; Pb, 500 mg kg–1; Cd, 1 mg kg–1). In addition, Cu in TLT exceeded grade II levels (Cu, 50 mg kg–1; indicating the threshold of pollution) but did not reach the grade III level (Cu, 400 mg kg–1). The amount of DTPA-extractable heavy metals was significantly correlated with the total amount in soil (R = 0.986, P < 0.0001 for Cd; R = 0.776, P < 0.0001 for Cu; R = 0.905, P < 0.0001 for Zn; R = 0.659, P < 0.0001 for Pb) and tended to be high in TLT and HY1.
In Masson pine leaves, the highest concentrations of Zn, Pb, and Cu were observed at TLT, reaching up to 652.1, 55.9, and 6.5 mg kg–1, respectively (Table 2). With the exception of Cu, the lowest concentrations of heavy metals in leaves were recorded in the control forest. The correlation between soil heavy metal concentrations and their concentration in leaves was not significant for any heavy metal examined (Pb: R 2 = 0.06, P = 0.1; Zn: R 2 = 0.003, P = 0.8; Cu: R 2 = 0.04, P = 0.2; Cd: R 2 = 0.02, P = 0.4).
Mycorrhizal colonization
The ectomycorrhizal colonization rate of Masson pine root tips in TLT (25.9 ± 21.0 %) was significantly lower than that in TLC (61.4 ± 29.0 %), HY1 (53.4 ± 19.8 %), and HY2 (61.1 ± 15.6 %) with P < 0.05. The values of the latter three sites were not significantly different. At the TLT site, 11 of 40 root samples had no ectomycorrhizal root tips.
Ectomycorrhizal fungal communities
In total, 1,610, 1,065, 999, and 913 of ectomycorrhizal tips were morphotyped in HY1, HY2, TLT, and TLC samples, respectively. By selecting one-tenth of the root tips from each morphotype, 126, 188, 265, and 291 samples of ectomycorrhizal root tips from sites HY1, HY2, TLT, and TLC, respectively, were used in RFLP analysis. All of the unique RFLP patterns in each root sample were subjected to sequencing, totaling 212 (HY1: 38, HY2: 43, TLT: 66, TLC: 65). After pairwise alignment, we obtained 88 unique sequences, which were deposited in DDBJ with accession numbers AB634253–AB634284 and AB636414–AB636468. Sequences of non-ectomycorrhizal taxa were excluded from the following analyses. By applying 97 % ITS sequence similarity for species delimitation, 47 molecular OTUs were identified and a representative sequence from each OTU were listed in Table 3. Of the 47 OTUs, 41 belonged to Basidiomycetes and six to Ascomycetes. Thelephoraceae was the most species-rich family, represented by 14 OTUs, followed by Russulaceae with 10 OTUs. All other fungal families had less than three OTUs.
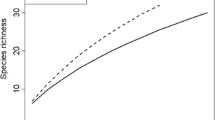
In total, 17, 13, 8, and 23 OTUs were found at HY1, HY2, TLT, and TLC, respectively. OTU richness accumulation was significantly lower in TLT than the other three sites (Fig. 2). The accumulation curves for HY1 and HY2 were not significantly different (P > 0.05); both were located in the middle between the curves for TLT and TLC. Sørensen similarity indices between three root samples from the same pine tree were 0.58 ± 0.39 in HY1 and 0.53 ± 0.33 in HY2, both of which were significantly higher than the values between root samples from different trees at each site (HY1: 0.14 ± 0.20, P = 0.0004; HY2: 0.26 ± 0.22, P = 0.0008). This result indicates that three root samples from the same tree could not be treated as independent samples. Thus, to avoid violating sample independence, we pooled the three root samples in the following analyses. OTU accumulations for HY1 and HY2 sites after the pooling were not significantly different from the curve for the control forest (P > 0.05). Species richness estimators (i.e., Chao2, Jackknife1, and Jackknife2) and diversity indices (i.e., Shannon’s H′ and Simpson’s 1/D) were lowest in TLT among the four sites. Estimators and diversity indices for HY1 and HY2 were not significantly different from the values for the control forest (Table 4).
Accumulation curves for ectomycorrhizal fungal OTUs in the Huayuan and Taolin mining areas. The observed number of OTUs is given as Mao Tau estimates (Sobs) with 95 % confidence intervals. The mean cumulative number of ectomycorrhizal fungal OTUs in each site is plotted after 50 randomizations. HY1 and HY2 Sobs were calculated based on 30 samples without combination at Huayuan sites 1 and 2, TLT Taolin tailing, TLC Taolin control site, HY1 (pooled) and HY2 (pooled) three samples were pooled for each tree
Of the 47 OTUs, 35 occurred at only one site. Only one OTU, Atheliaceae sp., occurred at all of the study sites. Three OTUs (Ascomycota sp., Phialocephala fortinii, and Suillus granulatus) were shared between the Huayuan and Taolin areas. Two sites (HY1 and HY2), located a close distance apart in the Huayuan area, shared seven OTUs, resulting in very similar communities (0.47 in the Sørensen index; Table 5), although soil heavy metal concentrations were significantly different between the two sites (Table 1).
Rank–abundance relationships revealed that the ectomycorrhizal fungal community in TLT was dominated by a few dominant species (Fig. 3); specifically, Rhizopogon buenoi (34.1 % in relative abundance), Tomentella ellisii (32.4 %), Inocybe curvipes (17.1 %), and S. granulatus (9.6 %) accounted for 93.2 % of the total relative abundance (Table 3). They were found in 28 of 29 ectomycorrhizal root samples. In contrast, the ectomycorrhizal fungal community in TLC was represented by many less dominant species, in which six OTUs of Thelephoraceae (30.3 % relative abundance in total) and six OTUs of Russulaceae (22.3 %) were relatively abundant at the family level and Atheliaceae sp. (16.0 %) was most abundant at the species level. The rank–abundance relationships for HY1 and HY2 were almost similar to each other and also to the curve for TLT, represented by the higher dominance of a few ectomycorrhizal fungal species.
After grouping similar soil samples (i.e., similar level of soil N, P, K, Pb, Zn, Cd, or Cu concentration) within each of TLT and TLC, we analyzed the correlation between individual soil factors and ectomycorrhizal fungal diversity among the subgroups, HY1 and HY2. This analysis revealed that the Chao2 richness estimator and Simpson diversity index were significantly correlated with soil N of the site/subgroups (R = 0.874, P = 0.005 for Chao2 vs. N; R = 0.729, P = 0.04 for Simpson vs. N, Fig. 4a, b). Although the correlation between the Chao2 richness estimator or Simpson value and soil Cu amount were also significant (R = –0.771, P = 0.025 for Chao2 vs. Cu; Fig. 4c), ectomycorrhizal fungal diversity or richness was not significantly correlated with other soil factors such as Pb, Zn, or Cd.
a Chao2 richness estimator and b Simpson diversity index (1/D) in correlation with N concentration (R = 0.874, P = 0.005 for Chao2 vs. N; R = 0.729, P = 0.04 for Simpson index vs. N), and c Chao2 richness estimator of ectomycorrhizal fungal species negatively correlated with Cu concentration in rhizosphere soil (R = –0.771, P = 0.025 for Chao2 vs. Cu). The dataset included HY1, HY2, two subsample groups from TLC, and four groups from TLT. Twelve and ten soil subsamples from TLC and TLT, respectively, were combined according to the level of each soil factor
The occurrence of each of the major fungal taxa revealed some biased occurrence to a certain soil condition. For example, the occurrence of R. buenoi and I. curvipes was restricted to lower soil N conditions and never appeared in soil containing >229 mg N kg–1 soil (Fig. 4a). ANOVA revealed that the N concentrations of soil samples that harbored R. buenoi, I. curvipes, T. ellisii, and S. granulatus were significantly lower than those with Atheliaceae sp., T. terreum, Thelephoraceae (excluding T. ellisii), and Russulaceae (Fig. 5a). Conversely, the Cu content of soil samples associated with those four fungi was significantly higher than those for Atheliaceae sp., T. terreum, Thelephoraceae (excluding T. ellisii), and Russulaceae (Fig. 5c). Cenococcum geophilum did not differ significantly with the other major taxa except for R. buenoi and I. curvipes. We found no clear patterns of biased occurrence in other soil factors for those major taxa (Fig. 5b, d, e).
The occurrence of major ectomycorrhizal taxa in different concentrations of N (a), Zn (b), Cu (c), Pb (d), and Cd (e). Taxa 1 = R. buenoi, 2 = I. curvipes, 3 = T. ellisii, 4 = S. granulatus, 5 = Atheliaceae, 6 = C. geophilum, 7 = Tricholoma terreum, 8 = Thelephoraceae (excluding T. ellisii), 9 = Russulaceae. Different letters refer to significant differences according to Tukey's test at P < 0.05. HY1 Huayuan site 1, HY2 Huayuan site 2, TLT Taolin tailing, TLC Taolin control site
Discussion
Most in vitro studies concerning the effects of heavy metals on EMF symbioses have provided strong evidence of reduced ectomycorrhizal fungal infection in higher heavy metal concentrations (Jones and Hutchinson 1986; Bell et al. 1988; Dixon 1988; Dixon and Buschena 1988; Chappelka et al. 1991; Hartley et al. 1999; Hartley-Whitaker et al. 2000). However, these in vitro experiments only used a limited number of ectomycorrhizal fungal species and young seedlings for short experimental periods. Thus, it is inappropriate to extrapolate the results of these in vitro studies to natural settings, which are characterized by high species/genetic diversity of EMF associated mainly with mature trees.
In the field, conflicting results have been found for the effect of heavy metals on EMF associations. Around a former lead/zinc smelter, Populus tremula was intensively colonized by EMF (up to 95 %) and was associated with highly diverse (H′ = 2) ectomycorrhizal fungal communities (Krpata et al. 2008). In a former uranium mining area, birch (Betula pendula) growing on the waste heap covered with an organic soil layer was also well colonized (61.5 %) by diverse ectomycorrhizal fungal communities comparable to non-polluted sites, while the colonization rate (27.7 %) and ectomycorrhizal fungal diversity at the bare heap site was significantly reduced (Staudenrausch et al. 2005). Naturally established Salix caprea on a former ore with high concentrations of Pb and Cu were poorly colonized (3 to 36 %) by EMF (Hrynkiewicz et al. 2008). No significant changes in diversity and richness of the ectomycorrhizal fungal communities were observed in a shotgun shooting range heavily contaminated with Pb up to 18,780 mg kg–1 (Hui et al. 2011). More field research in heavy metal-contaminated areas is required to determine the causes of these conflicting results.
In our study, two forest patches in Huayuan minelands were quite different in Pb, Zn, and Cd concentrations. Pb was about 70 times higher, Zn 12 times higher, and Cd eight times higher in HY1 soil compared to HY2 soil. However, ectomycorrhizal colonization rates were not significantly different between the forest patches (53.4 ± 19.8 % in HY1, 61.1 ± 15.6 % in HY2). These values were also similar to the colonization level in the non-polluted control forest TLC (61.4 ± 29.0 %). In addition, ectomycorrhizal fungal communities in HY1 and HY2 were similar in species accumulation curves, rank–abundance patterns, diversity indices, and richness estimators. Moreover, the similarity of ectomycorrhizal fungal communities between HY1 and HY2 was higher than any of the other combinations of the four sites (Sørensen index = 0.47). These results clearly indicate that Pb, Zn, and Cd amounts are not necessarily major determinants in structuring these fungal communities.
At the tailing site, however, ectomycorrhizal colonization was very poor (25.9 ± 21.0 %) and ectomycorrhizal fungal richness was apparently lower than that in HY1, HY2, and the control forest TLC, as shown in the species accumulation curve (Fig. 2). Although the tailing soil contained higher amounts of Pb, Zn, and Cd than the non-polluted forest, the heavy metal concentrations were significantly lower than at HY1 (Table 1), where the species accumulation curve was significantly higher than at TLT. Thus, heavy metals may not be responsible for the reduced ectomycorrhizal colonization and ectomycorrhizal fungal richness at TLT. On the other hand, the immature tailing soil was composed of heavily processed residues, lack of macronutrients, and contained significantly higher Cu (Table 1) and characterized by sandy texture, low organic matter, and drought (Guo et al. 2007), and possibly by insufficient EMF propagules. These soil properties would adversely affect ectomycorrhizal fungal colonization and may account for the poor ectomycorrhizal colonization and low EMF richness in the tailings.
Besides, the pine trees in young stage (7-year-old) might be also responsible for the low richness and diversity of EMF in the tailing site compared with the 15- or 30-year-old forest sites. In a rehabilitated bauxite mine site, the numbers of EMF species associated with 7-year-old Eucalyptus stands were less than half of those associated with native forest (Gardner and Malajczuk 1988). Low ectomycorrhizal fungal species richness was also observed in 4-year-old jarrah forest in the same mine sites in a recent study by Glen et al. (2008) and in a 5-year-old red oak (Quercus rubra) plot in disturbed sites (Gebhardt et al. 2007). Glen et al. (2008) also revealed a clear trend of increasing ectomycorrhizal fungal species richness with increasing time since rehabilitation and found no significant difference in species richness in the rehabilitated mine sites in 12- and 16-year-old classes compared with their unmined reference sites in similar age. Thus, the character of an ectomycorrhizal fungal community in TLT is probably indicative of the early stage of development of the rehabilitated sites. Meanwhile, some other confounding factors (e.g., geographical location, land history) may also account for the observed patterns to some extent.
The ectomycorrhizal fungal community of the non-polluted TLC forest was dominated by Thelephoraceae and Russulaceae, which were represented by six OTUs each and accounted for 30.3 and 22.3 % of the relative abundance, respectively. The dominance of these two families has been frequently reported from many forest ecosystems including boreal Pinaceae forests (Horton and Bruns 2001). Ectomycorrhizal fungal communities in HY1 and HY2 also followed this general pattern and harbored six OTUs of Thelephoraceae (8.8 % in relative abundance) and four OTUs of Russulaceae (4.9 %) collectively. Thus, as in ectomycorrhizal colonization rates and EMF richness, the occurrence of Thelephoraceae and Russulaceae was not strictly affected by higher amounts of Pb, Zn, and Cd (Fig. 5b, d, e).
In contrast, the ectomycorrhizal fungal community of the tailings was devoid of Russulaceae and was less dominated by Thelephoraceae, except T. ellisii. Instead, R. buenoi (34.1 %), T. ellisii (32.4 %), I. curvipes (17.1 %), and S. granulatus (9.6 %) collectively occupied over 93 % of the ectomycorrhizal root tips. Many Rhizopogon spp. and suilloid fungi have been frequently found in association with young trees in early successional sites (Nara 2006; Ishida et al. 2008), even under heavy metal-contaminated conditions (Turnau et al. 1996; Vrålstad et al. 2002; Adriaensen et al. 2005; Johansson et al. 2005; Colpaert et al. 2011). Some Inocybe species also frequently occur in early successional sites and mine sites (Kalin and Stokes 1981; Malloch 1982; Cripps 1997, 2003; Nara et al. 2003; Krpata et al. 2008). T. ellisii appears after fire disturbance because of its heat-tolerant propagules (Buscardo et al. 2010). Therefore, the ectomycorrhizal fungal community in the tailing can be regarded as an early successional community. Immature soil conditions of the tailing sand, which contained less organic matter, may only allow pioneer EMF to colonize the site.
The importance of soil maturity irrespective of heavy metals in structuring ectomycorrhizal fungal communities in mining areas was also supported by the significant correlation between soil N and ectomycorrhizal fungal diversity/richness (Fig. 4a, b), while soil Pb, Zn, and Cd amounts were not correlated with ectomycorrhizal fungal richness or diversity. Further evidence was found in the results of our analyses based on individual soil samples and occurrence data of each major EMF taxon. Each of the pioneer EMF, which were dominant in the tailings, only appeared in soil samples with lower N (Fig. 5a), and their occurrence was not related to other soil factors (Fig. 5b–e). In contrast, major taxa in non-polluted forests and fragmented forest patches in Huayuan mineland [i.e., Russulaceae and Thelephoraceae (except T. ellisii)] were only found in higher N soil samples, while their occurrence was not clearly related to Pb, Zn, and Cd. Therefore, we may be able to conclude that soil immaturity may be a more prevalent determinant than heavy metals in structuring ectomycorrhizal fungal communities.
References
Adriaensen K, van der Lelie D, Van Laere A, Vangronsveld J, Colpaert JV (2004) A zinc-adapted fungus protects pines from zinc stress. New Phytol 161(2):549–555. doi:10.1046/j.1469-8137.2003.00941.x
Adriaensen K, Vangronsveld J, Colpaert JV (2006) Zinc-tolerant Suillus bovinus improves growth of Zn-exposed Pinus sylvestris seedlings. Mycorrhiza 16(8):553–558. doi:10.1007/s00572-006-0072-7
Adriaensen K, Vralstad T, Noben JP, Vangronsveld J, Colpaert JV (2005) Copper-adapted Suillus luteus, a symbiotic solution for pines colonizing Cu mine spoils. Appl Environ Microbiol 71(11):7279–7284. doi:10.1128/AEM.71.11.7279-7284.2005
Agerer R (1987–1993) Colour atlas of Ectomycorrhizae, vol 1–7th del. Einhorn-Verlag Eduard Dietenberger, Schwäbisch Gmünd, Germany
Bell R, Evans C, Roberts E (1988) Decreased incidence of mycorrhizal root tips associated with soil heavy-metal enrichment. Plant Soil 106(1):143–145. doi:10.1007/bf02371206
Blaudez D, Jacob C, Turnau K, Colpaert JV, Ahonen-Jonnarth U, Finlay R, Botton B, Chalot M (2000) Differential responses of ectomycorrhizal fungi to heavy metals in vitro. Mycol Res 104(11):1366–1371. doi:10.1017/s0953756200003166
Bradshaw AD, Johnson M (1992) Revegetation of metalliferous mine wastes: the range of practical techniques used in Western Europe. Elsevier, Manchester
Buscardo E, Rodriguez-Echeverria S, Martin MP, De Angelis P, Pereira JS, Freitas H (2010) Impact of wildfire return interval on the ectomycorrhizal resistant propagules communities of a Mediterranean open forest. Fungal Biol 114(8):628–636. doi:10.1016/j.funbio.2010.05.004
Chappelka AH, Kush JS, Runion GB, Meier S, Kelley WD (1991) Effects of soil-applied lead on seedling growth and ectomycorrhizal colonization of loblolly pine. Environ Pollut 72(4):307–316. doi:10.1016/0269-7491(91)90004-g
Chen LQ (1989) Studies on symbiotic mycorrhiza fungi with Masson pine. Forest Res 2(4):357–362 (in Chinese)
Colpaert JV, Wevers JHL, Krznaric E, Adriaensen K (2011) How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution. Ann For Sci 68(1):17–24. doi:10.1007/s13595-010-0003-9
Colwell RK (2006) EstimateS: statistical estimation of species richness and shared species from samples. Version 8.0. doi: http://viceroy.eeb.uconn.edu/EstimateSPages/
Cripps CL (1997) The genus Inocybe in Montana aspen stands. Mycologia 89(4):670–688. doi:10.2307/3761005
Cripps CL (2003) Native mycorrhizal fungi with aspen on smelter-impacted sites in the northern Rocky Mountains: occurrence and potential use in reclamation. Paper presented at the National Meeting of the American Society of Mining and Reclamation and the 9th Billings Land Reclamation Symposium, Billings MT, June 3–6, 2003
Dickinson NM, Turner AP, Watmough SA, Lepp NW (1992) Acclimation of trees to pollution stress—cellular metal tolerance traits. Ann Bot 70(6):569–572
Dixon R (1988) Response of ectomycorrhizal Quercus rubra to soil cadmium, nickel and lead. Soil Biol Biochem 20(4):555–559. doi:10.1016/0038-0717(88)90072-7
Dixon R, Buschena C (1988) Response of ectomycorrhiza Pinus banksiana and Picea glauca to heavy metals in soil. Plant Soil 105(2):265–271. doi:10.1007/bf02376791
Dora S (1976) Determination of ammonia and Kjeldahl nitrogen by indophenol method. Water Res 10(1):31–36. doi:10.1016/0043-1354(76)90154-8
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118. doi:10.1111/j.1365-294X.1993.tb00005.x
Gebhardt S, Neubert K, Wollecke J, Munzenberger B, Huttl RF (2007) Ectomycorrhiza communities of red oak (Quercus rubra L.) of different age in the Lusatian lignite mining district, East Germany. Mycorrhiza 17(4):279–290. doi:10.1007/s00572-006-0103-4
Gardner JH, Malajczuk N (1988) Recolonisation of rehabilitated bauxite mine sites in western Australia by mycorrhizal fungi. Forest Ecol Manag 24(1):27–42. doi:10.1016/0378-1127(88)90022-9
Glen M, Bougher NL, Colquhoun IJ, Vlahos S, Loneragan WA, O’Brien PA, Hardy GESJ (2008) Ectomycorrhizal fungal communities of rehabilitated bauxite mines and adjacent, natural jarrah forest in Western Australia. Forest Ecol Manag 255(1):214–225. doi:10.1016/j.foreco.2007.09.007
Guo J, Wu F, Xie S, Yao L, Xie Y (2007) Environmental conditions and exploitation of lead–zinc tailings in Linxiang County, Hunan Province. Chin J Soil Sci 38:553–557 (in Chinese)
Hartley-Whitaker J, Cairney J, Meharg A (2000) Sensitivity to Cd or Zn of host and symbiont of ectomycorrhizal Pinus sylvestris L. (Scots pine) seedlings. Plant Soil 218(1):31–42. doi:10.1023/a:1014989422241
Hartley J, Cairney JWG, Freestone P, Woods C, Meharg AA (1999) The effects of multiple metal contamination on ectomycorrhizal Scots pine (Pinus sylvestris) seedlings. Environ Pollut 106(3):413–424. doi:10.1016/s0269-7491(99)00095-0
Horton TR, Bruns TD (2001) The molecular revolution in ectomycorrhizal ecology: peeking into the black-box. Mol Ecol 10(8):1855–1871. doi:10.1046/j.0962-1083.2001.01333.x
Hrynkiewicz K, Haug I, Baum C (2008) Ectomycorrhizal community structure under willows at former ore mining sites. Eur J Soil Biol 44(1):37–44. doi:10.1016/j.ejsobi.2007.10.004
Hui N, Jumpponen A, Niskanen T, Liimatainen K, Jones KL, Koivula T, Romantschuk M, Strommer R (2011) EcM fungal community structure, but not diversity, altered in a Pb-contaminated shooting range in a boreal coniferous forest site in Southern Finland. FEMS Microbiol Ecol 76(1):121–132. doi:10.1111/j.1574-6941.2010.01038.x
Ishida TA, Nara K, Tanaka M, Kinoshita A, Hogetsu T (2008) Germination and infectivity of ectomycorrhizal fungal spores in relation to their ecological traits during primary succession. New Phytol 180(2):491–500. doi:10.1111/j.1469-8137.2008.02572.x
Jentschke G, Godbold DL (2000) Metal toxicity and ectomycorrhizas. Physiol Plantarum 109(2):107–116. doi:10.1034/j.1399-3054.2000.100201.x
Johansson L, Xydas C, Messios N, Stoltz E, Greger M (2005) Growth and Cu accumulation by plants grown on Cu containing mine tailings in Cyprus. Appl Geochem 20(1):101–107. doi:10.1016/j.apgeochem.2004.07.003
Jones MD, Hutchinson TC (1986) The effect of mycorrhizal infection on the response of Betula papyrifera to nickel and copper. New Phytol 102(3):429–442. doi:10.1111/j.1469-8137.1986.tb00820.x
Kalin M, Stokes PM (1981) Macrofungi on uranium mill tailings—associations and metal content. Sci Total Environ 19(1):83–94. doi:10.1016/0048-9697(81)90120-0
Ke LX, Liu BR (2005) Resource and ecological distribution of ectomycorrhizal fungi under pine forests of Huangshan Mountain district. Chin J Appl Ecol 2005(16):445–458 (in Chinese)
Koljalg U, Larsson KH, Abarenkov K, Nilsson RH, Alexander IJ, Eberhardt U, Erland S, Hoiland K, Kjoller R, Larsson E, Pennanen T, Sen R, Taylor AF, Tedersoo L, Vralstad T, Ursing BM (2005) UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol 166(3):1063–1068. doi:10.1111/j.1469-8137.2005.01376.x
Krpata D, Peintner U, Langer I, Fitz WJ, Schweiger P (2008) Ectomycorrhizal communities associated with Populus tremula growing on a heavy metal contaminated site. Mycol Res 112(Pt 9):1069–1079. doi:10.1016/j.mycres.2008.02.004
Li MS (2006) Ecological restoration of mineland with particular reference to the metalliferous mine wasteland in China: a review of research and practice. Sci Total Environ 357(1–3):38–53. doi:10.1016/j.scitotenv.2005.05.003
Lian C, Hogetsu T, Matsushita N, Guerin-Laguette A, Suzuki K, Yamada A (2003) Development of microsatellite markers from an ectomycorrhizal fungus, Tricholoma matsutake, by an ISSR-suppression-PCR method. Mycorrhiza 13(1):27–31. doi:10.1007/s00572-002-0193-6
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42(3):421–428. doi:10.2136/sssaj1978.03615995004200030009x
Liu YG, Zhang HZ, Zeng GM, Huang BR, Li X, Xu WH (2005) Characteristics of tailings from metal mines in Hunan Province, China. J Cent South Univ Technol 12(2):225–228
Malloch D (1982) An undescribed species of Inocybe from mine wastes in Ontario. Can J Bot 60(1):40–45. doi:10.1139/b82-006
Martin KJ, Rygiewicz PT (2005) Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol (5). doi:10.1186/1471-2180-5-28
Meharg AA, Cairney JWG (2000) Ectomycorrhizas—extending the capabilities of rhizosphere remediation? Soil Biol Biochem 32(11–12):1475–1484. doi:10.1016/s0038-0717(00)00076-6
Nara K (2006) Ectomycorrhizal networks and seedling establishment during early primary succession. New Phytol 169(1):169–178. doi:10.1111/j.1469-8137.2005.01545.x
Nara K, Nakaya H, Wu B, Zhou Z, Hogetsu T (2003) Underground primary succession of ectomycorrhizal fungi in a volcanic desert on Mount Fuji. New Phytol 159(3):743–756. doi:10.1046/j.1469-8137.2003.00844.x
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties. Agronomy, No. 9. American Society of Agronomy and Soil Science Society of America, Madison, pp 403–430
Peay KG, Garbelotto M, Bruns TD (2009) Spore heat resistance plays an important role in disturbance-mediated assemblage shift of ectomycorrhizal fungi colonizing Pinus muricata seedlings. J Ecol 97(3):537–547. doi:10.1111/j.1365-2745.2009.01489.x
Ruotsalainen AL, Markkola AM, Kozlov MV (2009) Mycorrhizal colonisation of mountain birch (Betula pubescens ssp. czerepanovii) along three environmental gradients: does life in harsh environments alter plant–fungal relationships? Environ Monit Assess 148(1-4):215–232. doi:10.1007/s10661-007-0152-y
Pestana Nieto M, Santolamazza Carbone S (2009) Characterization of juvenile maritime pine (Pinus pinaster Ait.) ectomycorrhizal fungal community using morphotyping, direct sequencing and fruitbodies sampling. Mycorrhiza 19(2):91–98. doi:10.1007/s00572-008-0207-0
Shu WS, Ye ZH, Zhang ZQ, Lan CY, Wong MH (2005) Natural colonization of plants on five lead/zinc mine tailings in Southern China. Restor Ecol 13(1):49–60. doi:10.1111/j.1526-100X.2005.00007.x
Staudenrausch S, Kaldorf M, Renker C, Luis P, Buscot F (2005) Diversity of the ectomycorrhiza community at a uranium mining heap. Biol Fert Soils 41(6):439–446. doi:10.1007/s00374-005-0849-4
Tedersoo L, Kõljalg U, Hallenberg N, Larsson K-H (2003) Fine scale distribution of ectomycorrhizal fungi and roots. New Phytol 159:153–165. doi:10.1046/j.0028-646x.2003.00792.x
Tedersoo L, Jairus T, Horton BM, Abarenkov K, Suvi T, Saar I, Koljalg U (2008) Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol 180(2):479–490. doi:10.1111/j.1469-8137.2008.02561.x
Turnau K, Kottke I, Dexheimer J (1996) Toxic element filtering in Rhizopogon roseolus/Pinus sylvestris mycorrhizas collected from calamine dumps. Mycol Res 100(1):16–22. doi:10.1016/s0953-7562(96)80094-3
Vrålstad T, Myhre E, Schumacher T (2002) Molecular diversity and phylogenetic affinities of symbiotic root-associated ascomycetes of the Helotiales in burnt and metal polluted habitats. New Phytol 155(1):131–148. doi:10.1046/j.1469-8137.2002.00444.x
White TJ, Bruns TD, Lee S, Taylor J (1990) Analysis of phylogenetic relationships by amplification and direct sequencing of ribosomal RNA genes. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Wilkinson DM, Dickinson NM (1995) Metal resistance in trees—the role of mycorrhizae. Oikos 72(2):298–300. doi:10.2307/3546233
Zhu LH, Wu XQ, Qu HY, Ji J, Ye JR (2010) Micropropagation of Pinus massoniana and mycorrhiza formation in vitro. Plant Cell Tiss Org 102(1):121–128. doi:10.1007/s11240-010-9711-y
Acknowledgements
This research was supported by grants-in-aid from the Japan Society of the Promotion of Sciences (JSPS). We sincerely thank Mr. Jun Wang, a doctoral student of Central South University, China, for assistance in field sampling. We also thank Dr. Takahide A. Ishida and Prof. Taizo Hogetsu for critical reading of this manuscript and helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, J., Nara, K., Lian, C. et al. Ectomycorrhizal fungal communities associated with Masson pine (Pinus massoniana Lamb.) in Pb–Zn mine sites of central south China. Mycorrhiza 22, 589–602 (2012). https://doi.org/10.1007/s00572-012-0436-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-012-0436-0