Abstract
The presence and quality of the belowground mycorrhizal fungal community could greatly influence plant community structure and host species response. This study tests whether mycorrhizal fungal communities in areas highly impacted by anthropogenic disturbance and urbanization are less species rich or exhibit lower host root colonization rates when compared to those of less disturbed systems. Using a soil bioassay, we sampled the ectomycorrhizal fungal (EMF) communities associating with Quercus rubra (northern red oak) seedlings in soil collected from seven sites: two mature forest reference sites and five urban sites of varying levels of disturbance. Morphological and polymerase chain reaction–restriction fragment length polymorphism analyses of fungi colonizing root tips revealed that colonization rates and fungal species richness were significantly lower on root systems of seedlings grown in disturbed site soils. Analysis of similarity showed that EMF community composition was not significantly different among several urban site soils but did differ significantly between mature forest sites and all but one urban site. We identified a suite of fungal species that occurred across several urban sites. Lack of a diverse community of belowground mutualists could be a constraint on urban plant community development, especially of late-successional woodlands. Analysis of urban EMF communities can add to our understanding of urban plant community structure and should be addressed during ecological assessment before pragmatic decisions to restore habitats are framed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Expansion of urban and suburban land is at the expense of natural forests, wetlands, and agricultural land (Robinson et al. 2005). Continued urbanization is anticipated (McDonnell et al. 1997) along with a growing proportion of the global human population living in cities and suburbs (Sadik 1999; Pavao-Zuckerman 2008). Though urban ecology studies have focused on urbanization effects on animals (Ditchkoff et al. 2006) and plants (Lundholm and Marlin 2006; Neil and Wu 2006), there has been little emphasis on analysis of urban fungi (Newbound et al. 2010). Weiher (2007) calls attention to the bias of ecological restoration studies toward plants and animals, with a considerably lower proportion targeting fungi and soil microbes (less than 3% of papers reviewed).
The diversity of EMF communities in natural systems is impressive, and most studies have shown little overlap in species assemblages between replicate plots or sites (Horton and Bruns 2001; Taylor 2002). Mycorrhizal inoculum is pervasive in undisturbed soils (Allen et al. 2003). However, severely disturbed soils could be characterized by reduced mycorrhizal inoculum or altered fungal diversity (Newbound et al. 2010) and a characteristic urban EMF suite, much like many plant species ubiquitously encountered across disturbed sites (Del Tredici 2010). For example, high levels of N deposition have been found to decrease fungal abundance and change community composition (Baxter et al. 1999; Lilleskov and Bruns 2001; Lilleskov et al. 2002). Cousins et al. (2003) found a lack of arbuscular mycorrhizal fungal spores in soils of unvegetated urban lots, and Bainard et al. (2011) found reduced mycorrhizal colonization of urban trees compared to rural trees. Parsons et al. (1998) cite the lack of mycorrhizal associations on tree seedlings planted on a closed landfill where only three ectomycorrhizal morphotypes were found.
This study used a soil bioassay and morphological and molecular methods to examine the ectomycorrhizal fungal (EMF) communities associating with Quercus rubra (northern red oak) seedlings in soils of seven sites: five urban sites of varying disturbance severity and two less disturbed forest reference sites. We test the hypotheses that the less disturbed reference site soils exhibit higher EMF colonization of seedling root systems, higher EMF species richness, and different EMF species composition than the disturbed site soils.
Methods
Study sites
Seven sites differing in degree of anthropogenic disturbance and surrounding urbanization were used. The two least disturbed sites were mature (>170 years old) forests: Kilmer Woods (REF1) in Piscataway, Middlesex County, New Jersey (latitude 40°31′4″ N, longitude 74°26′23″ W) and Helyar Woods (REF2) in New Brunswick, Middlesex County, New Jersey (latitude 40°28′33″ N, longitude 74°25′17″ W). In this metropolitan area, these sites are among the closest extant systems to undisturbed forest sites. We call these two sites “reference sites” because they are representative of local mature woodlands and their biotic and abiotic structure could serve as a target for forest restoration. Sites characterized by intermediate disturbance were Duke Farms (URB1), a post-agricultural woodland in Hillsborough, Somerset County, New Jersey (latitude 40°33′9″ N, longitude 74°37′25″ W), and Greenbelt (URB2), a remnant woodland patch adjacent to a plant nursery operation in Staten Island, New York (latitude 40°35′46″ N, longitude 74°10′50″ W). A third disturbed site was a former arsenal/superfund site in Edison, Middlesex County, New Jersey (latitude 40°30′41″ N, longitude 74°21′22″ W; URB3). The most urban sites were a recreational park with a fabricated substrate of rubble and excavation material in Brooklyn, New York (latitude 40°35′2″ N, longitude 73°59′34″ W; URB4) and a sparsely vegetated lot adjacent to an oil refinery in Bayonne, Hudson County, New Jersey (latitude 40°39′34″ N, longitude 74°6′5″ W; URB5), in which the soil was obviously contaminated with oil. Quercus individuals were found at five study sites, but not at URB4 and URB5. Collectively, the disturbed sites are referred to as “urban.” A summary of site characteristics and soil properties is provided in Table 1.
Though the majority of woodlands in this metropolitan region have been logged several times over, our urban study sites, if restrictive anthropogenic influences were absent, would likely sustain oak-dominated woodlands. Uplands in northern and central New Jersey are dominated by mixed oak forest (Collins and Anderson 1994), and before European settlement, even the uplands of the island of Manhattan were dominated by oak woodlands (Sanderson 2009). Post-agricultural succession in this area typically leads to oak-dominated forests, with the Duke Farms site (URB1) being such an example.
Experimental design
Soil collection
To bioassay the EMF communities, soil was collected from each of the seven sites in April 2008. Using a drain spade shovel rinsed with a 10% bleach solution after collection at each site, 15 soil cores approximately 15 cm in depth and diameter were removed from the ground. Cores were randomly collected from five points along three parallel transects, each transect being 20–25 m long and 1 m apart (total sampling area of approximately 50 m2). Soil cores were placed into sterilized standard plastic nursery pots 15 cm in height and diameter while keeping the vertical structure of the cores intact.
Although Taylor and Bruns (1999) showed that EMF bioassay experiments in which soil is removed from field sites and the experiment is performed in pots selects for the resistant fungal propagule community, Avis and Charvat (2005) showed that keeping the soil vertical profile intact during collection more accurately reflects field conditions and helps to retain field similarities in the resulting soil EMF community. Bioassays conducted in such a way provide mycorrhizal inoculum that could come from spores, sclerotia, soil hyphae, and colonized root fragments (Avis and Charvat 2005).
Bait plants
Q. rubra was chosen as a host species as its distribution includes virtually all of eastern North America (USDA NRCS 2007), and it is common at our reference sites. Since most EMF species form mycorrhizal associations with a broad range of host tree species, usually within a family (Horton and Bruns 1998), EMF found in association with Q. rubra are likely to associate with other members of Fagaceae.
Q. rubra acorns (Sheffield’s Seed Co., Inc., Locke, NY, USA; sourced in Michigan) were surface-sterilized with a 10% bleach solution, cold-stratified for 60 days in moistened peat moss, and germinated on steam-sterilized potting soil. Upon the first flush of leaves, one seedling was transplanted into each pot of collected soil, 15 pots of soil from each study site (105 pots), within 3 days of soil collection. Seedlings were also transplanted into three pots of steam-sterilized potting soil to check for EMF contamination in the greenhouse. Seedlings were maintained in the greenhouse and watered with tap water three times weekly for 26 weeks. Newton and Pigott (1991) showed EMF colonization rates of approximately 40–50% on oak seedlings planted in pots of collected field soil after 20 weeks. We allowed a few more weeks to approximate the length of the local growing season. Pots were arranged randomly and periodically rotated to minimize effects of bench position. Whole intact root systems were harvested from each soil pot and stored in individually sealed plastic bags at 4°C. Ten root systems from each site were randomly selected for EMF sampling.
EMF sampling and analysis
EMF sampling
Each root system was subsampled for EMF colonization within 3 weeks of harvest. Root systems were gently rinsed free of adhering soil by submersion and slight agitation in water. Fine roots were clipped from the root system into fragments 1 to 7 cm in length. Effort was made to clip roots from all around the root system at varying distances from the root collar. Root fragments were placed into three gridded petri dishes filled with water until fragments became crowded and overlapped.
Each petri dish contained 36 1.3 × 1.3 cm squares, arranged in six rows by six columns. In each dish, one square per row was randomly selected and root tips within that square were sampled for EMF colonization. Root tips (×6 to ×50 magnification) that were visibly colonized by EMF were classified as “colonized” and bare tips as “uncolonized.” Each EMF root tip was counted and classified into a morphotype as per the criteria of Agerer (1987–1996) based on external characteristics. Effort was made to overestimate morphotype diversity based on minor differences. For each root system sample, at least two (if available) representative EMF root tip samples of each morphotype were collected and stored in 2× CTAB in separate sterile 1.5 mL microcentrifuge tubes for subsequent molecular analysis.
EMF colonization was measured as a percentage by dividing the number of EMF-colonized root tips by the number of colonized plus uncolonized root tips. Measures of EMF species richness and community composition were based on subsequent molecular analysis.
DNA extraction and PCR amplification
DNA was extracted from EMF root tips representing each morphotype from each seedling root system using the CTAB extraction protocol (Gardes and Bruns 1993). Polymerase chain reaction (PCR) was performed in a GeneAmp 9700 thermal cycler (Applied Biosystems, Foster City, CA, USA) according to the methods of Gardes and Bruns (1993), with the following modifications: A PCR mastermix (GoTaq Colorless Mastermix, Promega Corporation, Madison, WI, USA) was used along with the primers ITS1F (Gardes and Bruns 1993) and ITS4 (White et al. 1990) to amplify the internal transcribed spacer (ITS) ribosomal DNA region. Samples that did not successfully amplify were noted, and replicates (EMF root tips of the same morphotype from the same root system) were extracted and run through PCR in the same way until amplification was successful or until no more replicate samples were available.
RFLP analysis and DNA sequencing
Successfully amplified samples were digested for restriction fragment length polymorphism (RFLP) using the restriction enzymes HinfI and DpnII (New England Biolabs, Beverly, MA, USA). Fragments were separated using agarose gel (3%) electrophoresis, visualized under UV light, and photographed on a ChemiDoc XRS molecular imager (Bio-Rad Laboratories, Hercules, CA, USA). Band patterns were visually analyzed, and samples with identical band patterns with both enzymes were grouped together into RFLP types. The ITS regions of representative samples of each RFLP type were sequenced at Cornell University’s DNA Sequencing Facility using an Automated 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA). Each ITS sequence was entered into a BLAST (NCBI) query to provide records of similar sequences in GenBank (Altschul et al. 1997). RFLP types whose sequences were at least 97% similar along at least 90% of the sequence length in a pairwise alignment comparison were combined under one taxon type (O’Brien et al. 2005). Taxonomic identities were assigned to each RFLP type based on affinity to BLAST records as follows: greater than or equal to 97% similarity = species level match, 95–96% similarity = genus level match, 90–94% similarity = family level match, and less than 90% similarity = order level match. Root tip morphology, in addition to sequence data, was used to identify Cenococcum sp. Some RFLP types failed to yield sequence data and were not given a taxonomic identity. DNA sequences from this study have been submitted to GenBank (accession numbers GU907781–GU907810).
Taxonomic (or RFLP type) identities were retroactively assigned to EMF morphotypes from each root system. From here, these identities will be referred to as separate EMF “species,” as they are all less than 97% similar to each other in ITS sequence comparisons. Morphotypes that failed to amplify were included in percent colonization counts but were excluded from community-level measures (species richness and community composition). RFLP typing was conducted on up to three tips for any particular morphotype within a root system. When RFLP analysis revealed more than one genetic type, the taxonomic identity was assigned equally among the tips in that morphotype.
Statistical analysis
Differences in EMF colonization and EMF species richness between site soils were determined by ANOVA (JMP®, version 8, SAS Institute Inc., Cary, NC, 1989–2009) followed by the post hoc Tukey–Kramer HSD test. Analysis of similarity (ANOSIM), devised by Clarke (1993), was performed using the program PRIMER v6 (Clarke and Gorley 2006) to compare EMF community composition among site soils. ANOSIM deals with non-parametric data and is especially geared toward species counts with multiple zero values. It tests the null hypothesis that there are no differences in community composition among sites. In this study, this null hypothesis is rejected at p < 0.002 (alpha value of 0.05 with Bonferroni correction for multiple comparisons). Community similarity was analyzed among site soils by aggregating EMF species into genera and creating a resemblance matrix using the Bray–Curtis similarity index on presence/absence data. Using generic identities is appropriate because sequences of many fungal species are not available in GenBank. When a list of species was retrieved with a BLAST query and all were of the same genus, we were confident of the generic identification. PRIMER v6 was also used to create a cluster diagram (CLUSTER) based on common EMF species presence/absence in each site soil.
Results
EMF sampling
In 11 cases, RFLP patterns could not be determined for a morphotype within a sample due to failed or weak amplification. This resulted in 35 EMF tips classified as “unknown.” Fifty-four different RFLP pattern types were identified. EMF tips from the same root system classified into different morphotypes often revealed identical RFLP patterns. No colonization was observed on seedlings maintained in sterilized potting soil.
After combining RFLP types with at least 97% similarity over at least 90% of their ITS sequence, 34 distinct EMF “species” remained. Comparisons of RFLP types found in this study to records in GenBank are shown in Table 2. Four RFLP types failed to yield sequence data, and so their original RFLP type numbers were retained as their designations (RFLP types 16, 23, 39, 43).
EMF colonization
An ANOVA comparing the percentage of EMF-colonized root tips showed significant differences among site soils (F [6,63] = 10.445, p < 0.0001). Seedlings planted in URB4 and URB5 soils had significantly lower root colonization rates than those in the other five site soils (Tukey–Kramer HSD, α = 0.05; Fig. 1). EMF colonization in URB3 soil was distinctly bimodal, with root systems having either 30–60% colonization or 0–10% colonization.
EMF species richness
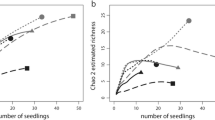
Significant differences were found with an ANOVA comparing the number of EMF species present per seedling root system among site soils (F [6,63] = 13.02, p < 0.0001). An almost 17-fold gradient in richness is shown among sites (Fig. 2). On average, reference site soils had 3.0 EMF species per root system and urban site soils had 1.2 species per system, a significant difference (Student’s t test, p < 0.001).
Site soils differed in total number of observed EMF species and jackknife estimates of species richness, with the greatest number of species found in REF1 soil (Table 3). Of the 34 total species found, 47% occurred exclusively in the reference site soils, 32% occurred exclusively in the urban site soils, and 21% occurred in both reference and urban site soils.
EMF community composition
The ANOSIM test indicated significant differences in EMF community composition on host roots between 11 site soil pairwise comparisons. Ten site pairs did not show significantly different EMF communities. In comparison to each other, the two reference sites showed non-significant differences in EMF community composition but were significantly different from all but one urban site (URB3). Several urban sites did not significantly differ from each other. Figure 3 graphically depicts EMF community similarity relationships between site soils.
A cluster analysis of EMF species based on their presence/absence in each of the seven site soils indicates species assemblages (Fig. 4). Since sites were defined a priori, this analysis serves only to show affinities between EMF species in terms of common site soils in which they occurred. The analysis shows separation first of a group of EMF found primarily in URB2 soil and then splits the remainder of EMF species between those found only in REF1 and REF2 and those found among several study site soils. The second cluster, containing EMF species Scleroderma citrinum, Pezizales sp., Tuber sp. 4, Thelephora terrestris, Tuberaceae sp., Scleroderma sp., and Cenococcum sp., represents species that were found broadly across the seven site soils. Relative abundance of each EMF species as a percentage of total colonized root tips across replicate seedlings in each site soil is also shown in Fig. 4. Of note is the lack of rare EMF species colonizing small proportions of the root systems in URB4 and URB5 soils.
Cluster analysis among EMF species using presence/absence data. Species are clustered based on common site soil presence. Relative abundance of each EMF species as percent of total root tips colonized across the ten replicate seedlings for each site soil is shown. Although the proportion of colonized root tips does not indicate the abundance of genetic fungal individuals, it can still suggest a level of dominance of fungal species within a root system. Four major species assemblages are indicated: reference site group, URB3 group, common group, and URB2 group
Discussion
EMF community differences
Our results indicate that the level of site disturbance affects the type of impact on EMF communities. In URB1 and URB2, sites of intermediate disturbance, EMF root colonization rates were not significantly affected, but EMF community composition was different in comparison to the reference sites. High disturbance soils (URB4 and URB5), however, were associated with both altered community composition and significantly reduced root colonization rates. These results suggest that inoculum abundance or spread of fungi on a root system is not significantly limited unless the soil is extremely disturbed (polluted, high pH, low organic matter, compacted) or specific host plants are absent, potentially leading to fewer inoculum sources. Dickie and Reich (2005) found a similar relationship between EMF diversity and colonization rates in which reductions in fungal community diversity were observed before declines in seedling colonization rates as distance from forest trees increased. Additionally, other studies have found that disturbances such as clear-cutting affect EMF community composition but not root colonization rates (Byrd et al. 2000; Jones et al. 2003).
Jackknife estimates of overall EMF species richness show that with increased sampling, REF1 would likely retain its standing as the most species-rich soil and URB4 and URB5 as the most species-poor. These estimates also indicate that the full pool of species at each site was not observed given the sampling effort, particularly at the more species-rich sites, as has been observed repeatedly in EMF community studies (Horton and Bruns 2001; Taylor 2002). If a greater diversity of EMF mutualists associated with a seedling’s root system confers greater benefit to the seedling, then seedlings at URB4 and URB5 would be at a disadvantage. In these site soils, seedlings that exhibited any EMF colonization were colonized by only one species. Seedlings in REF1 and REF2 soils had up to six and five species per root system, respectively. EMF species differ in their physiological functioning traits (Baxter and Dighton 2005), capacities to acquire soil nutrients (Baxter and Dighton 2001), and tolerance to environmental factors (Baxter and Dighton 2005). Increasing EMF diversity on a host’s root system could allow for increased overall EMF functioning under changing conditions.
The ANOSIM results suggest some degree of convergence among EMF communities of the urban site soils, whereas communities of the reference site soils converge with each other and share significant similarities with only the most species-rich urban site (URB3). We suggested earlier the possibility of an “urban EMF suite”—an assemblage of EMF species found commonly across disturbed sites much like a suite of plant species predictably found in urban areas (Del Tredici 2010). As indicated in Fig. 4, a distinct set of fungal species was found on host roots across multiple sites (“common group”), perhaps indicating that these particular species are more tolerant of a wide range of environmental conditions. Amanitaceae sp., Russula sp. (as Russula sp. 2), and Tomentella sublilacina, the three EMF found exclusively on host roots in both REF1 and REF2 soils, are characteristic of late-successional or mature forest stands (Dighton and Mason 1985; Keizer and Arnolds 1994; Taylor and Bruns 1999; Lilleskov and Bruns 2005). Multiple Russula species were found in REF1, REF2, and, interestingly, URB3 soils. Cenococcum was found in five of our seven site soils, including the soils of three disturbed sites. Its distribution is known to be broad (Trappe 1964; LoBuglio 1999; Cripps 2003), and propagules of this genus have been found to persist for years even after clear-cutting (Shaw and Sidle 1982). Scleroderma, present in all but one site’s soil (as either Scleroderma sp. or S. citrinum), has been found to be typical of disturbed sites (Danielson 1984). It is not surprising that fungal species such as S. citrinum, Tuber sp., Thelephora terrestris, and Cenococcum sp. were found in REF1 and REF2 soils in addition to those of the disturbed sites, just as ruderal plants such as Daucus carota (Queen Anne’s lace) and Solidago rugosa (rough goldenrod) can be found in both urban and rural meadows. Also, although REF1 and REF2 are representative of relatively undisturbed forest systems in our study area, being located in a metropolitan region means that they are still impacted by some degree of disturbance, particularly nitrogen deposition (this could be a reason for the absence of Cortinarius spp. in these soils, as this genus has been shown to be among the first to drop out of high nitrogen soils (Wallenda and Kottke 1998; Lilleskov et al. 2001; Peter et al. 2001)). We would expect to see some disturbance-associated species at these sites.
Of note is the bimodal pattern of EMF colonization in URB3 soil. The distribution of colonization rates suggests that at a small scale (e.g., within meters), sources of EMF colonization at this site are either present at moderately high levels or are completely absent. Implications for establishing EMF-dependent seedlings could be significant; depending on where a seed lands and germinates, it could encounter either abundant EMF inocula or none at all. Boerner et al. (1996) found similar patchiness in disturbed sites, in which small patches of moderate inoculum availability were surrounded by areas completely lacking in inoculum. Dickie and Reich (2005) found spatial heterogeneity in EMF inoculum distribution, with decreasing ectomycorrhizal colonization of oak seedlings as distance from a forest edge increased. At URB3, we believe two historical factors may be responsible for zones of low inoculum availability: use of the site for an arsenal and soil dumping. Such heterogeneity in inoculum distribution could regulate spatial patterns of seedling establishment or growth. For example, Dickie et al. (2005) observed greater oak seedling growth at intermediate distances from mature trees, where canopy shading was not prohibitive but mycorrhizal inoculum remained abundant.
Reasons for different EMF communities
Many possible explanations could account for differences in EMF colonization, species richness, and community composition on host roots among site soils. Jumpponen and Egerton-Warburton (2005) discuss a series of “filters” fungal species must pass through until site-specific mycorrhizal communities are established: the host filter, in which compatibility between host plants and fungal species determines mycorrhizal communities; the environmental filter, which imposes abiotic constraints; and the biotic filter, in which facilitative or competitive interactions between fungal species determine community composition. Although an array of fungal species may be present in a site’s propagule bank, only a selection will be represented in the active mycorrhizal community, determined by successful passage through the above site-specific filters (Jumpponen and Egerton-Warburton 2005). Peay et al. (2010), for example, observed effects of soil type on EMF richness and community structure in a tropical forest and attributed these effects to either physical and chemical differences among soil types (abiotic filtering) or differences in host plant communities associated with the soil types (host filtering).
Differences among individual site histories likely contribute to observed differences in EMF communities. Past disturbance at our urban sites could have disrupted the mycelial network and may have caused extensive lags in EMF community recovery. We would expect an urban park fabricated with excavation material to have a different EMF community structure from that of a post-agricultural woodland. However, even though the particular sources of disturbance vary among the urban sites, we still see convergence in EMF communities among several of these site soils.
Other possible explanations of EMF community differences include adverse soil conditions, distance from reference sites, and landscape fragmentation. Poor soil conditions at the more highly disturbed sites could be prohibitive toward EMF survival or spread on host roots. Additionally, long distances from less disturbed woodlands and fragmentation of sites isolated within an urban matrix could present barriers to EMF spore dispersal. Although wind-dispersed spores could potentially travel across broad areas (Molina et al. 1992), we do not know whether urban environments physically interfere with such dispersal. Interestingly, Tuber spp. were highly represented in the urban sites and hypogeous fungi such as Tuber can be dispersed over long distances by mammals (Ashkannejhad and Horton 2006). It is also notable that of all the urban sites, URB3 is within closest proximity to the reference sites and is the only urban site whose EMF community composition is not significantly different from that of the reference sites.
Differences in successional stages and aboveground plant community composition could also explain EMF community differences among site soils. Highly disturbed and early successional systems are often dominated by non-mycorrhizal and facultatively mycorrhizal plants (Jumpponen and Egerton-Warburton 2005). Lack of reference site EMF species in the urban soils could be partly due to a lack of inoculum otherwise available through mycelial transfer from pre-existing mature root systems (Fleming 1983; Kranabetter and Frieson 2002) and fragments of such in our bioassay soils. However, successional state does not explain the depauperate EMF community in URB1 soil. Given the passing of 70+ years since agricultural activities and the subsequent development of a closed-canopy woodland, we would expect the EMF community of this site to be more similar to that of REF1 or REF2. URB2, also, has mature Quercus individuals and was lacking in EMF species typical of mature woodlands.
It is important to keep in mind that in this study we have observed EMF colonization only of Q. rubra seedlings and cannot make assumptions about fungi that might colonize other host plant species. Additionally, Q. rubra is known to associate with both ectomycorrhizal fungi and arbuscular mycorrhizal fungi (AMF; Dickie et al. 2001). It is possible that in the urban soils, any reduction in benefits provided to host seedlings due to a lack of EMF associates could be ameliorated through root colonization by AMF. In fact, Bainard et al. (2011) suggested that trees in urban environments may rely more on AMF than EMF.
Implications for community ecology
Lack of mycorrhizal mutualists at disturbed urban sites could be a constraint to plant community development (Dickie and Reich 2005), particularly if spore dispersal into isolated urban patches is limited. Nuñez et al. (2009) found that invasion of surrounding forests by non-native trees from Argentinian Pinaceae plantations was prevented by the low abundance of EMF propagules in soils far from the plantations. In our study, low potential for EMF colonization could be one of several urban stressors that prevents establishment of EMF-dependent plant species in severely disturbed sites.
Given the demonstrated importance of mycorrhizal fungi to plant survival and community structure (Read and Birch 1987; Horton and van der Heijden 2008), determining the status of urban mycorrhizal fungal communities will contribute to our understanding of urban plant community assembly. The mycorrhizal community should also be considered during ecological restoration of urban sites, as successful restoration of a target plant community could benefit from, if not require, the presence of an appropriate mycorrhizal fungal community (Eviner and Hawkes 2008; van der Heijden and Horton 2009; Newbound et al. 2010) that, as our study shows, is likely to be depauperate at urban sites.
References
Agerer R (1987–1996) Colour atlas of Ectomycorrhizae. Eichhorn-Verlag Eduard Dietenberger, Schwäbisch Gmünd
Allen EB, Allen MF, Egerton-Warburton L, Corkidi L, Gomez-Pompa A (2003) Impacts of early- and late-seral mycorrhizae during restoration in seasonal tropical forest, Mexico. Ecol Appl 13:1701–1717
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Ashkannejhad S, Horton TR (2006) Ectomycorrhizal ecology under primary succession on coastal sand dunes: interactions involving Pinus contorta, suilloid fungi and deer. New Phytol 169:345–354
Avis PG, Charvat I (2005) The response of ectomycorrhizal fungal inoculum to long-term increases in nitrogen supply. Mycologia 97:329–337
Bainard LD, Klironomos JN, Gordon AM (2011) The mycorrhizal status and colonization of 26 tree species growing in urban and rural environments. Mycorrhiza 21:91–96
Baxter JW, Dighton J (2001) Ectomycorrhizal diversity alters growth and nutrient acquisition of grey birch (Betula populifolia) seedlings in host-symbiont culture conditions. New Phytol 152:139–159
Baxter JW, Dighton J (2005) Diversity–functioning relationships in ectomycorrhizal fungal communities. In: Dighton J, White JF, Oudemans P (eds) The fungal community. Its role and organization in the ecosystem. Taylor and Francis Group, Boca Raton, pp 383–398
Baxter J, Pickett STA, Carreiro MM, Dighton J (1999) Ectomycorrhizal diversity and community structure in oak forest stands exposed to contrasting anthropogenic impacts. Can J Bot 77:771–782
Boerner RE, DeMars JBG, Leicht PN (1996) Spatial patterns of mycorrhizal infectiveness of soils long a successional chronosequence. Mycorrhiza 6:79–90
Byrd KB, Parker VT, Vogler DR, Cullings KW (2000) The influence of clear-cutting on ectomycorrhizal fungus diversity in a lodgepole pine (Pinus contorta) stand, Yellowstone National Park, Wyoming, and Gallatin National Forest, Montana. Can J Bot 78:149–156
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Collins BR, Anderson KH (1994) Plant communities of New Jersey: a study in landscape diversity. Rutgers University Press, New Brunswick
Cousins JR, Hope D, Gries C, Stutz JC (2003) Preliminary assessment of arbuscular mycorrhizal fungal diversity and community structure in an urban ecosystem. Mycorrhiza 13:319–326
Cripps CL (2003) Native mycorrhizal fungi with aspen on smelter-impacted sites in the northern Rocky Mountains: occurrence and potential use in reclamation. National Meeting of the American Society of Mining and Reclamation and the 9th Billing Land Reclamation Symposium, Billings, Montana. ASMR, Lexington
Danielson RM (1984) Ectomycorrhizal association in jack pine stands in northeastern Alberta. Can J Bot 62:932–939
Del Tredici P (2010) Wild urban plants of the northeast: a field guide. Cornell University Press, Ithaca
Dickie IA, Reich PB (2005) Ectomycorrhizal fungal communities at forest edges. J Ecol 93:244–255
Dickie IA, Koide RT, Fayish AC (2001) Vesicular–arbuscular mycorrhizal infection of Quercus rubra seedlings. New Phytol 151:257–264
Dickie IA, Schnitzer SA, Reich PB, Hobbie CE (2005) Spatially disjunct effects of co-occurring competition and facilitation. Ecol Lett 8:1191–1200
Dighton J, Mason PA (1985) Mycorrhizal dynamics during forest tree Development. In: Moore D, Casselton LA, Wood DA, Frankland JC (eds) Developmental biology of higher fungi. Cambridge University Press, Cambridge, pp 117–139
Ditchkoff SS, Saalfeld ST, Gibson CJ (2006) Animal behavior in urban ecosystems: modifications due to human-induced stress. Urban Ecosyst 9:5–12
Eviner VT, Hawkes CV (2008) Embracing variability in the application of plant–soil interactions to the restoration of communities and ecosystems. Restor Ecol 16:713–729
Fleming LV (1983) Succession of mycorrhizal fungi on birch: infection of seedlings planted around mature trees. Plant Soil 71:263–267
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Horton TR, Bruns TD (1998) Multiple-host fungi are the most frequent and abundant ectomycorrhizal types in a mixed stand of Douglas fir (Pseudotsuga menziesii) and bishop pine (Pinus muricata). New Phytol 139:331–339
Horton TR, Bruns TD (2001) The molecular revolution in ectomycorrhizal ecology: peeking into the molecular black-box. Mol Ecol 10:1855–1871
Horton TR, van der Heijden M (2008) The role of symbioses in seedling establishment and survival. In: Leck MA, Parker VT, Simpson RL (eds) Seedling ecology and evolution. Cambridge University Press, Cambridge, pp 189–213
Jones MD, Durall DM, Cairney JWG (2003) Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytol 157:399–422
Jumpponen A, Egerton-Warburton LM (2005) Mycorrhizal fungi in successional environments: a community assembly model incorporating host plant, environmental, and biotic filters. In: Dighton J, White FJ, Oudemans P (eds) The fungal community. Its role and organization in the ecosystem. Taylor and Francis Group, Boca Raton, pp 139–168
Keizer PJ, Arnolds E (1994) Succession of ectomycorrhizal fungi in roadside verges planted with common oak (Quercus robur L.) in Drenthe, The Netherlands. Mycorrhiza 4:147–159
Kranabetter JM, Frieson J (2002) Ectomycorrhizal community structure on western hemlock (Tsuga heterophylla) seedlings transplanted from forests into openings. Can J Bot 80:861–868
Lilleskov EA, Bruns TD (2001) Nitrogen and ectomycorrhizal fungal communities: what we know, what we need to know. New Phytol 149:156–158
Lilleskov EA, Bruns TD (2005) Spore dispersal of a resupinate ectomycorrhizal fungus, Tomentella sublilacina, via soil food webs. Mycologia 97:762–769
Lilleskov EA, Fahey TJ, Lovett GM (2001) Ectomycorrhizal fungal aboveground community change over an atmospheric nitrogen deposition gradient. Ecol Appl 11:397–410
Lilleskov EA, Fahey TJ, Horton TR, Lovett GM (2002) Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska. Ecology 83:104–115
LoBuglio KF (1999) Cenococcum. In: Cairney JWG, Chambers SM (eds) Ectomycorrhizal fungi: key genera in profile. Springer, Berlin, pp 287–309
Lundholm JT, Marlin A (2006) Habitat origins and microhabitat preferences of urban plant species. Urban Ecosyst 9:139–159
McDonnell MJ, Pickett STA, Groffman P, Bohlen P, Parmelee RW, Carreiro MM, Medley K (1997) Ecosystem processes along an urban-to-rural gradient. Urban Ecosyst 1:21–36
Molina R, Massicotte H, Trappe JM (1992) Specificity phenomena in mycorrhizal symbiosis: community-ecological consequences and practical implications. In: Allen MF (ed) Mycorrhizal functioning. An integrative plant–fungal process. Chapman and Hall, New York, pp 357–423
Neil K, Wu J (2006) Effects of urbanization on plant flowering phenology: a review. Urban Ecosyst 9:243–257
Newbound M, Mccarthy MA, Lebel T (2010) Fungi and the urban environment: a review. Landsc Urban Plan 96:138–145
Newton AC, Pigott CD (1991) Mineral nutrition and mycorrhizal infection of seedling oak and birch. I. Nutrient uptake and the development of mycorrhizal infection during seedling establishment. New Phytol 117:37–44
Nuñez MA, Horton TR, Simberloff D (2009) Lack of belowground mutualisms hinders Pinaceae invasions. Ecology 90:2352–2359
O’Brien HE, Parrent JL, Jackson JA, Moncalvo J, Vilgalys R (2005) Fungal community analysis by large-scale sequencing of environmental samples. Appl Environ Microbiol 71:5544–5550
Parsons WJ, Ehrenfeld JG, Handel SN (1998) Vertical growth and mycorrhizal infection of woody plant roots as potential limits to the restoration of woodlands on landfills. Restor Ecol 6:280–289
Pavao-Zuckerman MA (2008) The nature of urban soils and their role in ecological restoration in cities. Restor Ecol 16:642–649
Peay KG, Kennedy PG, Davies SJ, Tan S, Bruns TD (2010) Potential link between plant and fungal distributions in a dipterocarp rainsforest: community and phylogenetic structure of tropical ectomycorrhizal fungi across a plant and soil ecotone. New Phytol 185:529–542
Peter M, Ayor F, Egli S (2001) Nitrogen addition in a Norway spruce stand altered macromycete sporocarp production and below-ground ectomycorrhizal species composition. New Phytol 149:211–325
Read DJ, Birch CPD (1987) The effects and implications of disturbance of mycorrhizal mycelial systems. Proc R Soc Edinb 94B:13–24
Robinson L, Newell JP, Marzluff JM (2005) Twenty-five years of sprawl in the Seattle region: growth management responses and implications for conservation. Landsc Urban Plan 71:51–72
Sadik N (1999) The state of world population 1999–6 billion: a time for choices. United Nations Population Fund, New York
Sanderson EW (2009) Mannahatta: a natural history of New York City. Abrams, New York
Shaw CG, Sidle RC (1982) Evaluation of planting sites common to a southeast Alaska clear-cut. II. Available inoculum of the ectomycorrhizal fungus Cenococcum geophilum. Can J For Res 13:9–11
Taylor AFS (2002) Fungal diversity in ectomycorrhizal communities: sampling effort and species detection. Plant Soil 244:19–28
Taylor DL, Bruns TD (1999) Community structure of ectomycorrhizal fungi in a Pinus muricata forest: minimal overlap between the mature forest and resistant propagule communities. Mol Ecol 8:1837–1850
Trappe JM (1964) Mycorrhizal hosts and distribution of Cenococcum graniforme. Lloydia 27:100–106
USDA NRCS (2007) The PLANTS Database. National Plant Data Center. http://plants.usda.gov. Accessed 16 Dec 2007
van der Heijden MGA, Horton TR (2009) Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J Ecol 97:1139–1150
Wallenda T, Kottke I (1998) Nitrogen deposition and ectomycorrhizas. New Phytol 139:169–187
Weiher E (2007) On the status of restoration science: obstacles and opportunities. Restor Ecol 15:340–343
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis N, Gelfand D, Sninsky J, White T (eds) PCR protocols and applications: a laboratory manual. Academic, New York, pp 315–322
Acknowledgments
This research was made possible by funding from the Doris Duke Charitable Foundation and the New Jersey Mycological Association. Thank you to Lena Struwe, Jean Molina, and Sasha Eisenman for access to lab space and procedural advice. We appreciate Peter Morin’s input on statistical analyses. Thank you to Duke Farms, Helyar Woods, Greenbelt Native Plant Center, and the US EPA Edison facility for site access. Discussions with Blake Mathys, Victor Medore, Carrie Norin, and Elena Tartaglia were very helpful. Comments and suggestions from two anonymous reviewers greatly improved this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karpati, A.S., Handel, S.N., Dighton, J. et al. Quercus rubra-associated ectomycorrhizal fungal communities of disturbed urban sites and mature forests. Mycorrhiza 21, 537–547 (2011). https://doi.org/10.1007/s00572-011-0362-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-011-0362-6