Abstract
A novel microfluidic chip with integrated Teflon AF1600 surface liquid core optical waveguide (LCW) modified with nano gold was proposed and fabricated in this article. Physical deposite method was used to integrate Teflon AF1600 LCW into microchannel. After that, the inner surface of Teflon AF1600 LCW was chemically modified at temperature of 40 °C, subsequent nano gold were in situ immobilied on the silanized inner surface of Teflon AF LCW precoated with a thin layer of poly(diallyldimethylammonium chloride) within microchannel by a chemical self-assembly method. Under the optimized conditions, the prepared microfluidic SERS chip exhibited high sensitivity for R6G with detection limit of 10−11 mol/L and SERS enhancement factor (EF) of 2.7 × 108. Compared to single nano gold SERS enhancement substrate within a microfluidic chip, the SERS detection sensitivity for R6G was improved 4 orders of magnitude. Apart from high SERS enhancement effect, the as-prepared integrated microstructure had extremely good SERS detection reproducibility and duration stability. Furthermore, it was successfully used to detect the bovine serum albumin (BSA), and exhibited excellent SERS response. The research showed great prospects and technical support for design and fabrication of integrated SERS microfluidic chip and sensitive detection of trace biochemical samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Surface-enhanced Raman spectroscopy (SERS) have attracted substantial research interest, since it was first observed in 1974 by Fleischmann et al. (1974), with its advantages, such as narrow spectroscopic bands with excellent molecular specificity, reduced photo bleaching, simple pretreatment, ultra-high sensitivity and so on. Unfortunately, the application of SERS was frequently restricted by instability and poor SERS signal reproducibility due to the inhomogeneous distributions of sample caused by “coffee-ring” effect (Mao et al. 2014; Lin and Chang et al. 2014). In addition, before and during measurements, the analytes on the open SERS substrate surfaces can be easily contaminated, so that impacting on the results of spectral analysis. In recent years, however, it was discovered that embedding SERS substrates into microfluidic channel could serve as an integrated platform to make SERS detection more reproducible, efficient, safe, and environmentally friendly (Li et al. 2013; Lee et al. 2014; Anne Marz et al. 2011). At present, SERS detection for various biological and environmental analytes in a microfluidic chip has been a research focus (Ashok et al. 2013; Talian and Huebner 2013; Hoonejani et al. 2015; Fu et al. 2015; Novara et al. 2016). However, excitation light spot is only 1–3 µm in diameter under conventional detection mode, the application of microfluidic SERS chip is limited owing to its low detection sensitivity which was caused by a small amount of excited sample molecules and metal nanoparticles within the scope of exciting light. Thus, an integrated photonic structure in a SERS detection microchannel for extension of the detection volume and increasing the number of exicted analyte-nanostructure conjugates could be desirable. Recently, several methods using liquid-core fibers (Qi and Berger 2004), and photonic crystal fibers (Han et al. 2010; Yang et al. 2010; Eftekhari et al. 2012) have been demonstrated but are difficult to integrate. Liquid core waveguide (LCW) is particularly attractive because it can provide high excitation intensities over long distances and be integrated into microchannel. The liquid core is both as test sample and as light transmission medium. At present, the structure type of LCW that has been integrated into microfluidic chip is very limited. Measor et al. utilized an antiresonant reflecting optical waveguide (ARROW) structure to create an integrated LCW on microfluidic chip (Measor et al. 2007). Silver colloidal was used for enhancement media. Detection sensitivity of R6G to a minimum concentration of 30 nmol/L was found. The disadvantages were, however, that the ARROW had not only special requirements for operating conditions and accuracy of dielectric layer thickness in a fabrication process, but also was hard to form a microfluidic channel without leaking. Otherwise, suspended metal colloidals were used as SERS enhancement medium in microfluidic SERS chips with integrated LCW due to their easy preparation. However, the SERS detection reproducibility have more challenges, that is because uncontrolled aggregation of metal colloidals are formed after mixing with sample molecules. Meanwhile, precipitates could be formed after aggregation of metal colloidals, which may lead to a significant decrease of the SERS signal in a few minutes. Therefore, to improve the practicability of microfluidic SERS chips with integrated LCW, the more integrated LCW microstructures that are easy to be prepared need to be developed. Morever, to improve SERS detection reproducibility, metal nanoparticles need to be immobilized on the inner surface of integrated LCW to keep the structure stability of metal nanoparticles.
Teflon AF LCW is a widely-used LCW with simple structure (Tian et al. 2007; Eftekhari et al. 2011; Altkorn et al. 1997, 1999). Teflon AF cladding layer films have a refractive index (1.29–1.31) that are lower than the refractive index of water (1.33), therefore light could be delivered through the same physical path as the fluid flows by total internal reflection when the core are filled with water or aqueous solutions. Teflon AF LCW can be easily integrated into microchannel due to their simple structure. At present, some references have reported the methods that integrated Teflon AF films into microchannel to form Teflon AF LCW microstructures without leaking (Datta et al. 2003; Manor et al. 2003; Wu and Gong 2008; Cho et al. 2009). However, there was no relevant reports about both SERS detection by using the microfluidic SERS chip with integrated Teflon AF LCW and the immobilization method of metal nanoparticles on inner surface of integrated Teflon AF LCW because of high chemical stability of Teflon AF.
In this paper, a high-efficiency microfluidic SERS chip with integrated Teflon AF1600 LCW modified with nano gold was proposed and prepared. Teflon AF1600 LCW was integrated into microchannel by physical deposition method, and it was treated with a mixture of potassium permanganate solution and nitric acid under water bath to introduce hydroxyl group. Afterwards, gold nanoparticles were immobilized from solution onto the silanized inner surface of Teflon AF1600 LCW precoated with a thin layer of poly(diallyldimethylammonium chloride), PDDA. The thickness and smoothness of coated Teflon AF1600 films were affected by vacuum force and response time. The change of chemical structure of Teflon AF1600 film depended on oxidation treatment temperature. And the coverage densities of gold nanoparticles were decided by assembly time. Those influence factors would be investigated and optimized. According to characterizing results of fabricated microstructures by scanning electron microscope and Raman spectrometer, both the performance of as-prepared microfluidic SERS chip and the relationship among coverage densities of nano gold, length of LCW, and SERS enhancement effect of the microfluidic SERS chip were investigated with model molecule R6G, and the potential of the microfluidic SERS chip for measure the biological samples was demonstrated by using bovine serum albumin (BSA) as a sample.
2 Materials and methods
2.1 Materials
Teflon AF1600 was obtained from DuPont (Wilmington, DE). Perfluoro(n-butyltetrahydrofuran) (FC-75) was obtained from 3 M Corp (Minneapolis, MN). The Sylgard 184 (including PDMS monomer and curingagent) was purchased from Dow Corning (Midland, MI, USA). Hydrogen tetrachloroaurate hydrate (HAuCl4·4H2O), Shanghai Chemical reagent CO., Rhodamine 6G (R6G, Sigma), H2SO4 (98 %, Chongqing Chuandong chemical (group) CO. Ltd), H2O2 (30 %, Chongqing Chuandong chemical (group) CO. Ltd). Trisodium citrate (Chongqing ChuanDong chemical (group) CO. Ltd), poly dimethyl diallyl ammonium chloride (PDDA, wt35 %, AR), 1H,1H,2H,2H-perfluorodecyltriethoxysilane (Sigma Aldrich Inc.). Stainless steel micro wires (Hebei Angtai wire mesh manufacturing co. Ltd). And all the other regents were of analytical regent (AR) grade and purchased from Chongqing ChuanDong chemical group.
Preparation of gold colloid solution:Gold colloids were prepared by using the method developed by Frens (1973). Briefly, 100 mL of 0.01 % HAuCl4·4H2O was brought to a vigorous boil while stirring, then 8 mL of 1 % trisodium citrate was rapidly added to the solution, and boiled for another 15 min. After that, the prepared gold colloid was cooled to room temperature and kept at 4 °C until use.
2.2 Fabrication of microfluidic SERS chip integrated LCW modified with nano gold
2.2.1 Fabrication of PDMS microfluidic chip
PDMS microfluidic chip was prepared by microwire-molding fabrication technology (Jia et al. 2008). The whole experiment for forming microchannel was shown in Fig. S1 (supporting information). First, glass substrate and microwires should be ultrasonic cleaned by acetone, ethanol and deionized water for 10 min respectively. Sequently, the clean 100 µm-diameter microwire for forming channel was aligned on other microwires which were aligned on clean glass slide, so as to control the distance between microchannel and substrate. Following, a pane-shaped PMMA cover frame was covered to the surface of the glass substrate, then liquid PDMS (prepolymer: curing agent = 10:1 w/w) was poured into, and allowed the deployed microwire immerged in. Then PDMS with the immerged aligning microwire was degassed in vacuum and cured for about 2 h at 60 °C. After curing, the PDMS with microwires was placed into the ethanol pool for 6 h to swell slightly, then the microwires being cured in the PDMS block was drawn out with gentle force, and therefore leading to the formation of circular cross-section microchannel with diameter of 100 µm in the PDMS block. Following, small holes (about 2 mm in diameter) were punched vertically, and PTFE tubes were jointed to the microchannel. Finally, channel ends were plugged by gelatinizing PDMS.
2.2.2 Coating of Teflon AF1600 on inner wall microchannel
Teflon AF1600 film was coated on inner wall of PDMS microchannel by using the method developed by Cho et al. (2009). A solution of 2 % 1H, 1H, 2H, 2H-perfluorodecyltriethoxysilane was filled in PDMS microfluidic channel for 30 min, then heated at 110 °C for 10 min to promote adhesion between PDMS and the Teflon AF1600 solution. A 6 % Teflon AF1600 solution was flowed into the microchannels. Once they were filled, different vacuum forces were applied for 20 min to remove excess Teflon AF1600 solution from the microchannels (see Fig. 1). The Teflon AF1600-coated PDMS devices were heated to 155 °C for 20 min to evaporate fluoroinert solvent, and then heated further to 175 °C (15 °C above the its glass transition temperature) for 20 min to form a smooth Teflon AF1600 film.
2.2.3 Self-assembly nano gold on inner surface of Teflon AF1600 film within microchannel
The freshly prepared modified solution (6 % potassium permanganate: 64 % nitric acid = 10:1 v:v) was added into same microchannels with integrated Teflon AF1600 film, and the beakers were hold in water bath at 40, 60, 80 °C for 1 h. Then the modified solution was pumped from microchannels and extensively rinsed with doubled-distilled water for several times. Afterwards, 0.1 % of the cationic polymer PDDA solution were filled in the microchannels for 30 min, and rinsed with doubled-distilled water. Then the gold colloids were filled in the microchannels for 0.5, 1, 1.5, 2 and 6 h, respectively. Finally, the microchannels were extensively rinsed with deionized water. The fabrication procedure was illustrated schematically in Fig. 2.
2.3 Characterization
The morphology and distribution of integrated nano gold were characterized with field emission scanning electron microscope (FESEM; FEI Nova Nano SEM 400 operated at 10.0 kV). The chemical structure of Teflon AF1600 films were characterized with Fourier Transform Infrared Spectroscopy (FTIR; IRPrestige-21, Shimadzu, Japan). The Raman spectra were collected on Raman microscope (LabRAM HR, HORIBA Jobin–Yvon, Germany) with the 633 nm line of a He–Ne laser as the excitation source. The laser power at the sample was set to 1.7 mW. All the Raman spectra were collected with a 10× objective (NA = 0.25, Olympus) corresponding to a laser spot on the sample of 3 µm in diameter. Exposure time (CCD integration times) of 2 s and accumulated frequency of 2 were used to collect all Raman spectra, and the resolution was 0.35 cm−1. The spectral acquisition time was 2 s. Each measurement was repeated at least three times.
2.4 SERS measurements for R6G/BSA in SERS chip
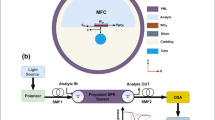
In the SERS experiments, R6G was used as the probe molecule. For the SERS studies, R6G solution was entered the hollow core of microstructures from inlet via applying negative pressure on outlet. Then, the outlet of microchannels were cut and upturned by rotating chip, and was covered with a 1 mm-thickness quartz plate. Finally, beam was coupled via objective len into the core of microstructures by adjusting sample platform. The schematic diagram of experimental setup was shown in Fig. 3. The backscattered Raman signal was collected though the same objective lens into Raman spectrometer. To ensure that collected spectrum was from the core and not the cladding, Raman spectrum of the air-filled LCW were investigated and showed minimal spectral interference from Teflon AF1600.
3 Results and discussion
3.1 Design of microfluidic SERS chip integrated with LCW modified with nano gold
To solve the problem of low detection sensitivity caused by narrow scope of excitation light in conventional microfluidic SERS detection chip, and poor detection repeatability caused by the aggregation of metal colloidal after addition of sample molecules, we designed a novel microfluidic SERS chip with integrated Teflon AF1600 LCW modified with nano gold which had excellent characteristics of surface plasma resonance, biocompatibility and difficult oxidation. It was achieved by coating Teflon AF1600 film on the inner wall of microchannel and in situ immobilization gold nanoparticles on the Teflon AF1600 film within the microchannel. Based on the total reflection principle of light,when the hollow core was filled of sample solution, excitation light could be coupled into the core of Teflon AF1600 LCW from one end and propagated within it, and an accumulative SERS signal could be collected from the both ends of integrated microstructure by using forward/backward Raman scattering. Compared with forward Raman Scattering, backward Raman scattering layout has a simpler configuration for portable SERS applications. So backward Raman scattering was used to measure Raman or SERS signals of the integrated microstructure. In order to obtain excellent optical transmission ability and make the operation more simple and cheaper and without leaking, the microfluidic SERS chip was designed to be 60 × 20 mm and involve one straight PDMS microchannel. A scanning electron micrograph of the cross section of the PDMS-channel coated with Teflon AF1600 film was shown in Fig. 4. The cross-section was circular and the core diameter was about 85 µm. To obtain better couple efficient between objective and waveguide microstructure, 10× objective lens (NA = 0.25) was chosen to focus excitation light and collect the Raman light. Otherwise, to ensure the same coupling loss between exciting/collection light and Teflon AF1600 LCW and maintain an enclosed space to avoid evaporation of solvent in every test, the outlet tube of chip was vertically cut, and a quartz plate with a low absorption coefficient was attached to the open end of the microchannel. The schematic of the integrated SERS detection microstructure was illustrated in the dashed box of Fig. 3.
3.2 Fabrication of on-chip microstructure that integrated Teflon AF1600 LCW modified with nano gold
3.2.1 Optimization of the on-chip Teflon AF1600 LCW microstructure
The Raman enhancement of LCW was achieved by extension optical path of the excitation light to excite more sample molecules. Therefore, the Raman enhancement effect of Teflon AF1600 LCW depended on its optical transmission performance that depended on thickness and smoothness of cladding layer film (Peter et al. 1998; Datta et al. 2003). In our experiment, the Teflon AF1600 films were coated on inner wall
of PDMS microchannels to form waveguide microstructures by using the method developed by Cho et al. (2009), which could protect PDMS from heat damage in a cost-effective, less time-consuming manner. The thickness and smoothness of coated Teflon AF1600 films were affected by vacuum force and response time. In order to obtain Teflon AF1600 LCW with excellent Raman enhancement effect, different waveguide microstructures were prepared by using different magnitude of vacuum force and response time. Through comparing the Raman signal intensity of 10−4 mol/L R6G in different waveguide microstructures (Fig. 5), −90 KPa of vacuum force and 10 min of response time conditions were chosen based on the results. Optimal waveguide microstructure was imaged by SEM (Fig. 4). The observed Raman bands that were assigned to R6G included xanthene ring C–C stretch mode at 1180, 1505, and 1642 cm−1, CH2 wag mode at 1305 cm−1, C–H bend mode at 1357 cm−1 (Wang et al. 2015; Chen et al. 2010). Calculations showed that a 5-µm-thick Teflon AF film was necessary to confine the light to the liquid core (Peter et al. 1998). In our work, the cladding thickness is typically 8–11 µm, the thickness and uniformity were enough to confine and guide light waves very well. The detection sensitivity of 10−4 mol/L R6G was 2 orders of magnitude greater than that measured in an uncoated conventional microchannel.
3.2.2 Immobilization of gold nanoparticles on inner surface of Teflon AF1600 LCW
In order to improve SERS detection sensitivity and repeatability of microfluidic SERS chip by using synergetic Raman enhancement effect of Teflon AF1600 LCW and nano gold, we proposed that nano gold were in situ immobilized on inner surface of the Teflon AF1600 LCW within microchannel using a chemical self-assembly method. However, It was difficult to get satisfactory result due to the fact that surface of Teflon AF1600 film had large numbers of C-F groups as compared to few OH groups (which could bind to silane monomer or cationic polymer such as PDDA). Therefore, the inner surface of Teflon AF1600 LCW should be modified to introduce hydroxyl group first. In the present study, a mixture of potassium permanganate solution and nitric acid was used to treat Teflon AF1600 films by strong oxidation, under heated water bath. To avoid damage the micro featured waveguides and creating cracks on the Teflon AF1600 film surface, we set the heating time to 1 h, and investigated influence of heating temperature on both structure of Teflon AF1600 films and Raman enhancement effect of waveguide microstructures (Fig. 6). It was observed that stretching of hydroxyl group appeared after chemical treatment and continued to increase with increasing of treatment temperature. Meanwhile, the Raman peak intensity of R6G in waveguide microstructure unchanged before and after chemical treatment at 40 °C, but it weakened after chemical treatment at 60 °C and continued to decrease with increasing of treatment temperature. This demonstrated optical transmission performance of the waveguide microstructure weakened with increasing of treatment temperature, probably because much substance deposited on the surface of films after chemical treatment. Furthermore, the deposited compound increased with increasing of chemical treatment temperature (Wang et al. 2010). To maintain good optical transmission performance, water bath at 40 °C was chosen as treatment temperature of waveguide microstructure.
After chemical treatment, nano gold could be immobilized on the inner surface of the Teflon AF1600 LCW which was filled with gold colloidal after precoating with a thin layer of PDDA for different time by using a chemical self-assembly method. It was well-known that electromagnetic enhancement effect of nano gold depended on size and density of nano gold. When gold nanoparticles with same particle size were integrated into Teflon AF1600 LCW, both electromagnetic enhancement effect of nano gold and optical path length of wavegude were affected by coverage density of nano gold (Fan et al. 2011; Han et al. 2010). Therefore, in the case of the same microstructure length, Raman signal gain of integrated microstructure was influenced by coverage densities of nano gold. In order to obtain excellent SERS enhancement effect, the coverage densities of gold nanoparticles were adjusted by controlling the assembly time. The absorption spectra showed absorption peak intensity of assembled gold nanoparticles increased with prolonging assembly time, and it eventually reached stability at 6 h [Fig. S2 (supporting information)]. It demonstrated the coverage densities of gold nanoparticles increased with the extension of assembly time, and it eventually reached maximum at 6 h. By varying the assembly times, assembled gold nanoparticles were imaged by SEM [Fig. S3 (supporting information)]. It showed that the particle size of nano gold was about 20 nm, the coverage density of nano gold increased from 7 particles/µm2 to 15, 21, 37, 70 particles/µm2 as assembly time increased from 0.5 h to 1, 1.5, 2 and 6 h, respectively, which were caused by electrostatic adsorption interaction between the surface of gold nanoparticles and PDDA.
To evaluate relationship between the coverage densities of gold nanoparticles and SERS enhancement effect of the integrated microstructure in the case of same microstructure length, The 5 cm-long integrated microstructures with different coverage densities of nano gold were filled with 10−5 mol/L R6G aqueous solution, and SERS measurements were performed using a backward transmission geometry at an excitation wavelength of 632.8 nm (Fig. 7). It was found that the SERS enhancement effect of the 5 cm-long integrated microstructures were significantly improved after immobilization gold nanoparticles, meanwhile it altered with the change of coverage densities of gold nanoparticles. In the case of gold nanoparticles coverage densities of 0–21 particles/µm2, the SERS enhancement effect of the integrated microstructures increased with increasing coverage densities of gold nanoparticles, because it was achieved by the synergistic Raman enhancement of Teflon AF1600 LCW and gold nanoparticles at lower nanoparticle coverage densities where light attenuation was low. However, when coverage densities of gold nanoparticles increased from 21 to 37 particles/µm2, SERS intensity decreased to values lower than for 15 particles/µm2 coverage, because Raman enhancement effect of the Teflon AF1600 LCW decreased at 37 particles/µm2 coverage where light attenuation increased due to the increased coverage densities of gold nanoparticles. However, when coverage densities of gold nanoparticles were greater than 37 particles/µm2, the SERS enhancement effect of the integrated microstructures increased with increasing coverage densities of gold nanoparticles, but the signal intensities did not still reached the value for 15 particles/µm2 coverage, because the SERS enhancement effect was mainly obtained by the electromagnetic enhancement of gold nanoparticles at higher nanoparticle coverage densities where light attenuation was great, while the enhancement effect of Teflon AF1600 LCW was almost disappeared. By comparison, it was found that the integrated microstructure with nano gold coverage density of 21 particles/µm2 showed the best SERS enhancement effect, because it had the best synergistic Raman enhancement of Teflon AF1600 LCW and gold nanoparticles. The influence of microstructure length on SERS enhancement effect was further evaluated by using cutback method (Marcuse 1981) in which the length of the integrated microstructures were successively reduced through a series of vertical cuts at inlet end of microchannels (Fig. S4). Although it was destructive, the cutback method was adopted because of the difficulty of preparing the exact same integrated microstructures to ensure uniformity between measurements. As shown in Fig. 8, in the cases of coverage density of 21 particles/µm2, SERS signal intensity increased with length of integrated microstructures, and it eventually reached stability at the length of 1.5 cm. It was indicated that the optical path length was 1.5 cm and a SERS signal accumulation along the optical path was occurred.
SERS Spectra of 10−5M R6G were collected from the integrated microstructures with different coverage densities of nano gold, the lengths of integrated microstructure were all 5 cm, and the coverage densities of nano gold were: a 0 particles/µm2; b 7 particles/µm2; c 15 particles/µm2; d 21 particles/µm2; e 37 particles/µm2; f 70 particles/µm2, respectively
3.3 SERS performance of the microfluidic SERS chip integrated Teflon AF1600 LCW modified with nano gold
In order to evaluate SERS performance of the developed microfluidic SERS chip and determine how the integrated microstructure enhance the SERS signal in comparison with a microfluidic SERS chip only with gold nanoparticles, different concentrations of R6G solutions were injected into microfluidic SERS chips. Once they were filled, SERS detections were conducted. The SERS spectra were shown in Fig. 9. As was shown, both microfluidic SERS chips equipped with nano gold and Teflon AF1600 LCW modified with nano gold demonstrated strong SERS enhancement, and the detection limit was 10−7 and 10−11 mol/L respectively. Owing to the synergy Raman enhancement of nano gold and Teflon AF1600 LCW, the microfluidic SERS chip integrated Teflon AF1600 LCW modified with nano gold exhibited better Raman enhancement effect than that integrated only with nano gold. what is more, the minimum detected active R6G concentration of 10−11 mol/L was almost three orders of magnitude lower than previous demonstrations using a microfluidic SERS chip that integrated ARROW LCW and nano silver colloidal (Measor et al. 2007).
To evaluate the enhancement ability of the microfluidic SERS chip, the enhancement factor (EF) was calculate using the following equation (Chen et al. 2014):
where the I SERS and I Raman were the intensity of SERS signal and the intensity of Raman signal without SERS enhancement surstrate, respectively, C SERS and C Raman were the concentration of molecules contributing these intensities, respectively. The calculation results of EF were shown in Table 1.
In addition to the high SERS enhancement, the reproducibility and stability of the SERS signals were the other things that we had to notice in its reliable application. To illustrate the SERS detection reproducibility of the microfluidic SERS chip, the SERS spectra of 10−6mol/L R6G within the different integrated microstructures that were prepared under the same experimental conditions were recorded under consistent test conditions, as shown in Fig. 10a. Obviously, SERS spectra of R6G were enhanced greatly at each integrated microstructure, and the difference among the five SERS signals was small with relative standard deviation (RSD) of 7.5 % at the 1362 cm−1. To evaluate the duration stability of the integrated microstructure, the SERS spectra of 10−6mol/L R6G within five integrated microstructure with different storage time were collected under consistent test conditions, as shown in Fig. 10b. The RSD was 9.08 % for different duration time. The results indicated that the integrated microstructure had excellent reproducibility and duration stability, which was caused by the uinform and stable distribution of gold nanoparticles and accumulation of SERS signals within the integrated microstructure.
3.4 Application of the microfluidic SERS chip for BSA detection
To further demonstrate excellent SERS response of the as-prepared microfluidic SERS chip to biological samples, 10−5mol/L bovine serum albumin (BSA) was chosen for the test sample, and was injected into an uncoated conventional microchannel (UM), a microfluidic SERS chip integrated only with nano gold (GM), and the as-prepared micorfluidic SERS chip integrated Teflon AF1600 LCW modified with nano gold, respectively (IM). SERS signals were measured under the same test conditions (Fig. 11), and the Raman shift of BSA were listed in Table S1 (supporting information) (Chen and Lord 1976; Iosin et al. 2009; Synytsya et al. 2007). It was found that no Raman peaks of BSA were found in the UM. In the GM, several weaker Raman peaks of BSA were found, but the number of Raman peaks was less. It indicated that nano gold had Raman enhancement effect on BSA, but the enhancement effect was weaker. In the IM, more Raman peaks of BSA were observed, and the intensity of Raman peaks was significantly improved. It also illustrated that the designed microfluidic SERS chip had obvious synergetic Raman enhancement effect for BSA, which could provide a promising application in biosensor and quantitative analysis of spectroscopy.
4 Conclusions
In summary, a microfluidic SERS chip with integrated surface liquid core optical waveguide modified with nano gold was proposed and fabricated. The prepared microfluidic SERS chip showed great synergetic Raman enhancement effect for R6G and BSA. By controlling magnitude of vacuum force and response time, treatment temperature of inner surface of Teflon AF1600 LCW, and assembly time of nano gold, Raman enhancement effect of the waveguide microstructure was optimized, and SERS enhancement effect of the integrated microstructure was optimal for the 1.5 cm-long integrated microstructure with coverage density of nano gold about 21 particles/µm2, and detectable concentration for R6G was lowered to 10−11 mol/L, and had great detection repeatability and duration stability. More importantly, production of the microfluidic SERS chip could be easily and economically realized by the proposed fabrication strategy. The detection sensitivy can be further improved by utilization of resonance SERS detection (Jernshøj and Hassing 2010; Lin et al. 2014) or use of Ag or Au nanostructures (Ding et al. 2010; Cui et al. 2015; Khurana et al. 2016) which result in larger enhancements of the Raman cross section. Otherwise, the operating flexibility can be further improved by inserting optical fiber into the microchannel (Dochow et al. 2013; Ashok et al. 2010). The as-prepared microfluidic SERS chip would have great potential applications in high sensitivity and portable SERS detection for chemistry and biomolecules.
References
Altkorn R, Koev I, Van Duyne RP, Litorja M (1997) Low-loss liquid core optical fiber for low-refractive-index liquids: fabrication, characterization, and application in Raman spectroscopy. Appl Opt 36:8992–8998
Altkorn R, Koev I, Pelletier MJ (1999) Raman performance characteristics of Teflon–AF 2400 liquid-core optical-fiber sample cells. Appl Spectrosc 53:1169–1176
Anne Marz TH, Dana Cialla, Michael Schmitta (2011) Droplet formation via flow-through microdevices in Raman and surface enhanced Raman spectroscopy—concepts and applications. Lab Chip 11:3584–3592
Ashok PC, Praveen BB, Dholakia K (2013) Optofluidic Raman sensor for simultaneous detection of the toxicity and quality of alcoholic beverages. J Raman Spectrosc 44:795–797
Ashok PC, Singh GP, Tan KM, Dholakia K (2010) Fiber probe based microfluidic raman spectroscopy. Opt Express 8:7642–7649
Chen MC, Lord RC (1976) Laser-excited Raman spectroscopy of biomolecules. VIII. Conformational study of bovine serum albumin. J Am Chem Soc 98:990–992
Chen L, Luo L, Chen Z, Zhang M, Zapien JA, Lee CS, Lee ST (2010) ZnO/Au composite nanoarrays as substrates for surface-enhanced Raman scattering detection. J Phys Chem C 114:93–100
Chen J, Su H, You X, Gao J, Lau WM, Zhang D (2014) 3D TiO2 submicrostructures decorated by silver nanoparticles as SERS substrate for organic pollutants detection and degradation. Mater Res Bull 49:560–565
Cho SH, Godin J, Lo YH (2009) Optofluidic waveguides in Teflon AF-coated PDMS microfluidic channels. IEEE Photonics Technol Lett 21:1057–1059
Cui Q, Xia B, Mitzscherling S, Masic A, Li L, Bargheer M, Möhwald H (2015) Preparation of gold nanostars and their study in selective catalytic reactions. Colloids Surf A 465:20–25
Datta A, Eom IY, Dhar A (2003) Microfabrication and characterization of teflon AF-coated liquid core waveguide channels in silicon. IEEE Sens J 3:788–795
Ding HP, Xin GQ, Chen KC, Zhang M, Liu Q, Hao J, Liu HG (2010) Silver dendritic nanostructures formed at the solid/liquid interface via electroless deposition. Colloids Surf A Physicochem Eng Asp 353:166–171
Dochow S, Becker M, Spittel R et al (2013) Raman-on-chip device and detection fibres with fibre Bragg grating for analysis of solutions and particles. Lab Chip 13:1109–1113
Eftekhari F, Irizar J, Hulbert L, Helmy AS (2011) A comparative study of Raman enhancement in capillaries. J Appl Phys 109:113104
Eftekhari F, Lee A, Kumacheva E, Helmy AS (2012) Examining metal nanoparticle surface chemistry using hollow-core, photonic-crystal, fiber-assisted SERS. Opt Lett 37:680–682
Fan M, Andrade GFS, Brolod AG (2011) A review on the fabrication of substrates for surface enhanced Raman spectroscopy and their applications in analytical chemistry. Anal Chim Acta 693:7–25
Fleischmann M, Hendra PJ, McQuillan AJ (1974) Raman-spectra of pyridine adsorbed at a silver electrode. Chem Phys Lett 26:163–166
Frens G (1973) Controlled nucleation for regulation of particle-size in monodisperse gold suspensions. Nat Phys Sci 241:20–22
Fu C, Wang Y, Chen G (2015) Aptamer-based surface-enhanced Raman scattering microfluidic sensor for sensitive and selective polychlorinated biphenyls detection. Anal Chem 87:9555–9558
Han Y, Oo MKK, Sukhishvili S, Du H (2010) Photonic crystal fiber as an optofluidic platform for surface enhanced Raman scattering. Proc SPIE 7839:783908
Hoonejani MR, Pallaoro A, Braun GB (2015) Quantitative multiplexed simulated-cell identification by SERS in microfluidic devices. Nanoscale 7:16834–16840
Iosin M, Toderas F, Baldeck PL, Astilean S (2009) Study of protein–gold nanoparticle conjugates by fluorescence and surface-enhanced Raman scattering. J Mol Struct 196:924–926
Jernshøj KD, Hassing S (2010) Survival of molecular information under surfaced-enhanced resonance Raman (SERRS) conditions. J Raman Spectrosc 41:727–738
Jia Y, Jiang J, Ma X, Li Y, Huang H, Cai K, Cai S, Wu Y (2008) PDMS microchannel fabrication technique based on microwire-molding. Chin Sci Bull 53:3928–3936
Khurana P, Thatai S, Sa Prasad, Soni S, Kumar D (2016) Agcore–Aushell bimetallic nanocomposites: Gold shell thickness dependent study for SERS enhancement. Microchem J 1248:19–823
Lee H, Xu L, Koh D, Nyayapathi N, Oh KW (2014) Various on-chip sensors with microfluidics for biological applications. Sensors 14:17008–17036
Li QL, Li BW, Wang YQ (2013) Surface-enhanced Raman scattering microfluidic sensor. Rsc Adv 3:13015–13026
Lin CC, Chang CW (2014) AuNPs@mesoSiO2 composites for SERS detection of DTNB molecule. Biosens Bioelectron 51:297–303
Lin J, Zhang Y, Qian J, He S (2014) A nano-plasmonic chip for simultaneous sensing with dual-resonance surface-enhanced Raman scattering and localized surface plasmon resonance. Laser Photonics Rev 8:610–616
Manor R, Datta A, Ahmad I, Holtz M, Gangopadhyay S, Dallas T (2003) Microfabrication and characterization of liquid core waveguide glass channels coated with Teflon AF. IEEE Sens J 3:687–692
Mao H, Wu W, She D, Sun G, Lv P, Xu J (2014) Microfluidic surface-enhanced Raman scattering sensors based on nanopillar forests realized by an oxygen-plasma-stripping-of-photoresist technique. Small 10:127–134
Marcuse D (1981) Principles of optical fiber measeurements. Academic, New York
Measor P, Seballos L, Yin D, Zhang JZ (2007) On-chip surface-enhanced Raman scattering detection using integrated liquid-core waveguides. Appl Phys Lett 90:211107
Novara C, Lamberti A, Chiadò A, Virga A, Rivolo P, Geobaldo F, Giorgis F (2016) Surface-enhanced Raman spectroscopy on porous silicon membranes decorated with Ag nanoparticles integrated in elastomeric microfluidic chips. RSC Adv 6:21865–21870
Peter D, Ms Belz, Klein KF, Grattan KTV, Frankea H (1998) Physical analysis of teflon coated capillary waveguides. Sens Actuators, B 51:278–284
Qi D, Berger AJ (2004) Quantitative analysis of Raman signal enhancement from aqueous samples in liquid core optical fibers. Appl Spectrosc 58:1165–1171
Synytsya A, Alexa P, de Boer J, Loewe M, Moosburger M, Wurkner M, Volka K (2007) Raman spectroscopic study of serum albumins: an effect of proton- and γ-irradiation. J Raman Spectrosc 38:1646–1655
Talian I, Huebner J (2013) Separation followed by direct SERS detection of explosives on a novel black silicon multifunctional nanostructured surface prepared in a microfluidic channel. J Raman Spectrosc 44:536–539
Tian Y, Zhang L, Zuo J, Li Z, Gao S, Lu G (2007) Raman sensitivity enhancement for aqueous absorbing sample using Teflon-AF 2400 liquid core optical fibre cell. Anal Chim Acta 581:154–158
Wang S, Li J, Suo J, Luo T (2010) Surface modification of porous poly (tetrafluoraethylene) film by a simple chemical oxidation treatment. Appl Surf Sci 256:2293–2298
Wang C, Xu Y, Zhao H, Gang C, Lai C, Liao X, Wang R (2015) Detection and analysis of SERS effect of nano gold by self-assembly chemical plating composite method. Appl Surf Sci 353:750–756
Wu CW, Gong GC (2008) Fabrication of PDMS-based nitrite sensors using Teflon AF coating microchannels. IEEE Sens J 8:465–469
Yang X, Shi C, Wheeler D et al (2010) High-sensitivity molecular sensing using hollow-core photonic crystal fiber and surface enhanced Raman scattering. J Opt Soc Am A 27:977–984
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (NFSC: 21375156) and National High Technology Research and Development Program of China (863 Projects) (No. 2015AA021104); Frontier Research Key Projects of Chongqing Science and Technology Committee,[cstc2015jcyjBX0010], Scientific and Technical Innovation Projects for People’s Livelihood of Chongqing Science and Technology Committee,[cstc2015shms zx00014] and Key Project of Central University Basic Scientific Research Business Expenses (No. 106112015CDJZR225512), Benefit Projects for People’s Livelihood by Science and Technology, Chongqing Science and Technology Committee [cstc2015jcsf8001].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, C., Xu, Y., Wang, R. et al. Fabrication and effect study of microfluidic SERS chip with integrated surface liquid core optical waveguide modified with nano gold. Microsyst Technol 23, 3059–3068 (2017). https://doi.org/10.1007/s00542-016-3138-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-016-3138-2