Abstract
Production of metal nanoparticles using fungi provides a safe and eco-friendly method superior to the traditional physical and chemical methods. Metal nanoparticles can be produced biosynthetically by intracellularly, extracellularly, or both. Extracellular biosynthesis is superior, as it is void of unnecessary adjoining cell components. A commonly used fungus Penicillium chrysogenum (the penicillin producer) has been reported previously to produce gold nanoparticles intracellularly. In the current work, P. chrysogenum was used to produce extracellular gold nanoparticles when gold chloride ion solution (HAuCl4∙3H2O) was added to the fungal filtrate. These nanoparticles were characterized to determine the composition, shape, structure, and particle size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
A nanoparticle is defined as an aggregate of atoms bonded together with a radius between 1 and 100 nm (Bhushan 2010). The material properties of nanoparticles are changed from their bulk properties. When the particle dimension is reduced from a large size, the properties at first remain the same. Then, they slowly start to change until the size drops below 100 nm and great changes occur quickly (Edelstein and Cammarata 1996; Gogotsi 2006; Bhushan 2010; Hosokawa et al. 2012; Bhushan et al. 2014). Nanoparticles can be classified into metal and non-metal nanoparticles. They have different shapes and sizes (Hosokawa et al. 2012; Bhushan et al. 2014).
The toxic metals, such as Cd, Hg, Pb, and Tl, always produce toxic nanoparticles, which may produce adverse effects in both plants and animals (Husen and Siddiqi 2014). Many useful metal nanoparticles have been practically proven. Ag nanoparticles are known to have antibacterial, antifungal, and antioxidant properties (Kim et al. 2007; Perera et al. 2013). Au nanoparticles have potential applications in various fields, such as catalysis, sensor technology, electronics, biological labeling photonics, biomedicine, and optics (Shipway and Willner 2001; Biswas et al. 2004; Wang et al. 2004; Singaravelu et al. 2007; Brüggemann et al. 2014). Au nanoparticles also have applications in many fields such as cancer diagnosis and therapy, drug and gene delivery, and DNA and protein determination (Li et al. 2007; Jain 2012; Perrault 2014). Ag nanoparticles are widely used as antimicrobial agents in a diverse range of products such as air sanitizer sprays, pillows, slippers, respirators, wet wipes, detergents, soaps, shampoos, toothpastes, air filters, coatings of refrigerators, vacuum cleaners, washing machines, food storage containers, and cellular phones (Khaydarov et al. 2011).
Nanoparticles are produced by chemical, physical, and biological techniques of fabrication. Figure 1 shows the three major techniques of fabrication of nanoparticles. Chemical techniques are carried out by precipitation from solution (Meulenkamp 1998; Chae et al. 2010), reduction process, which includes the reduction of metal particles to nanoparticles using chemical reducing agents, such as sodium borohydride or sodium citrate (Jana et al. 2000; Cao and Hu 2009), and chemical vapor deposition (Kong et al. 1998; Kumar and Ando 2010). Physical techniques used for the synthesis of nanoparticles include condensation (Gonzalez et al. 2008), thermal decomposition (Yang and Aoki 2005), laser irradiation (Dolgaev et al. 2002), electrolysis (Huang et al. 2011), and diffusion (Murakami et al. 1999). Biological techniques for the synthesis (biosynthesis) of metal nanoparticles are carried out extracellularly, intracellularly, or both using bacteria, actinomycetes, yeast, fungi, and plants.
Table 1 provides a literature review of biosynthesis of Au-NPs carried out either extracellularly, intracellularly, or both using bacteria and fungi. Table 1a shows examples using bacteria. Bacillus subtilis produces extracellular irregular and intracellular spherical Au-NPs with a size range of 5–50 nm (Southam and Beveridge 1994). Escherichia coli was found to produce extracellular spherical Au-NPs with a size range of less than 10 nm, and intracellular irregular Au-NPs with a size range of 20–50 nm (Deplanche and Macaskie 2008). Pseudomonas aeruginosa produces extracellular spherical Au-NPs with a size range of 10–20 nm (Husseiny et al. 2007). Bacillus licheniformis produces extracellular cubic Au-NPs with a size range of 10–100 nm (Kalishwaralal et al. 2009).
Table 1b shows examples of Au-NPs produced using fungi. Saccharomyces cerevisiae produces extracellular Au-NPs with a size range of 20–60 nm (Lin et al. 2005). Penicillium chrysogenum produces intracellular spherical, triangular, and rod-shaped Au-NPs with a size range of 5–100 nm (Sheikhloo and Salouti 2011). Aspergillus clavatus produces intracellular triangular Au-NPs with a size range of 20–35 nm (Verma et al. 2011). Trichoderma asperellum produces extracellular spherical Au-NPs 15 nm in size, and triangular Au-NPs 30 nm in size (Mukherjee et al. 2012). Aspergillus flavus produces extracellular Au-NPs 18 nm in size, and intracellular Au-NPs 22 nm in size with diverse shapes (Gupta and Bector 2013).
Fungi are believed to be superior to bacteria for biosynthesis as they can be separated easily from their broth media, and are richer in the proteins used in the production of nanoparticles. Both extracellular and intracellular biosynthesis of metal nanoparticles is illustrated schematically in Fig. 2. For intracellular biosynthesis, positive metal (Me+) ions enter the cell of the microorganism, where they become reduced by reductases or reducing substances, giving rise to Me0 atoms that aggregate and deposit as metal nanoparticles (Me-NPs) inside the cell. For extracellular biosynthesis, reductase enzymes or reducing substances pass out of the living cell of a microorganism into the solution of metal ions, where they reduce Me+ ions to Me0 atoms that aggregate to form Me-NPs. Extracellular biosynthesis of nanoparticles has been exploited in many applications and is preferable to intracellular biosynthesis as it has no unnecessary cell components. The ability of fungi to produce extracellular nanoparticles is due to their enormous secretory components which are involved in reduction and biosynthesis (Shankar et al. 2003).
Schematic diagram of intracellular and extracellular biosynthesis of metal nanoparticles. Intracellular Me-NPs are formed inside the living cell. Extracellular Me-NPs are produced outside of the cell. Extracellular biosynthesis has the advantage of producing more pure nanoparticles that are void of cellular components
Fungi are a relatively recent addition to the other microorganisms used to produce nanoparticles, as they have the ability to secrete large amounts of enzymes (Navazi et al. 2010; Verma et al. 2011; Mukerjee et al. 2012). Du et al. (2011) reported that the fungus Penicillium sp. 1–208, when placed in a concentrated aqueous solution of HAuCl4, gave rapid extra- and intracellular Au-NPs. Mukherjee et al. (2001) reported the bioreduction of gold chloride ions to form Au-NPs using the fungus Verticillium sp. Gold ions were reduced by enzymes present in the cell wall leading to the aggregation of Au-NPs. Also, the reduced nicotinamide adenine dinucleotide-dependent reductase, released by Fusarium oxysporum, is responsible for the reduction of gold ions to Au-NPs (Mukherjee et al. 2002).
Extracellular biosynthesis of Au-NPs had been reported with Fusarium semitectum (Sawle et al. 2008). Extracellular biosynthesis of Au-NPs has also been reported with the thermophilic actinomycete Thermomonospora sp. (Ahmad et al. 2003). These microorganisms can produce nanoparticles through the remediation of toxic metals by reducing them under certain conditions (Fortin and Beveridge 2000). The widely available fungus Penicillium chrysogenum, the penicillin producer, occurs in nature and is often found in foods and in the environment (Samson et al. 1977).
Penicillium chrysogenum has been reported to produce intracellular Au-NPs when gold ion solution is added (Sheikhloo and Salouti 2011). In the present work, extracellular Au-NPs were produced to provide an easier technique for gaining more pure Au-NPs free from cellular components.
2 Experimental procedure
2.1 Production of gold nanoparticles
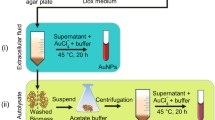
The lyophilized fungus P. chrysogenum (ATCC 10106, Fischer Scientific), first was sub-cultured on plates of malt extract agar medium. This medium contained 20 g malt extract (61001-518, VWR Intl), 20 g glucose (G8270, Sigma Aldrich), 1.0 g peptone (J636, Bioexpress), and 15 g agar (A1296, Sigma Aldrich), all in 1 L sterile distilled water, and incubated for growth at 28 °C for 3–7 days. After fungal growth, the fungal biomass was grown aerobically in Erlenmeyer flasks of malt yeast peptone glucose broth medium (MYPG) containing 0.3 % malt extract, 0.3 % yeast extract (23547, Affymetrix Inc), 0.5 % peptone, and 1 % glucose (Sheikhloo and Salouti 2011). The flasks were incubated at a temperature of 28 °C for 7 days. The fungal biomass was then separated from the culture broth by filtration using Whatman No. 1 filter paper (pore size 11 µm) and washed thoroughly with distilled water to remove medium components. Five grams of fungal biomass fresh weight were suspended in 50 ml distilled water and incubated for 3 days at 28 °C at a stationary condition.
After incubation, the fungal biomass was separated by filtration with a Whatman No. 1 filter paper and the filtrate was collected. Added to 45 ml of the fungal filtrate were 5 ml of 10 mM gold chloride ion solution (G541, Fischer Scientific), in order to reach a final concentration of 1 mM and incubated for 4 days at 28 °C at a stationary condition. The method is illustrated schematically in Fig. 3.
2.2 Characterization of gold nanoparticles
Au-NPs were characterized using UV–Visible spectrophotometer, transmission electron microscopy (TEM), and X-ray Diffraction (XRD). The Cary 100 Bio UV–Visible spectrophotometer was used to run the sample solution in the range of 400–800 nm, which is the range of visible radiation with 1 nm resolution. TEM micrographs were captured on a carbon coated copper grid using FEI Tecnai F20 S/TEM operating at 200 kV. XRD of gold nanoparticles was carried out using a Bruker D8 advanced diffractometer (40 kV, 50 mA, CuKa1 radiation) equipped with a Lynx Eye position sensitive detector, and a Ge 111 incident beam monochromator. A scan range of 30–90 2θ and a step size of 0.015 were used.
3 Results and discussion
In this research, P. chrysogenum was grown aerobically in MYPG broth medium to obtain the fungal biomass. Suspending the fungal biomass in distilled water for 3 days under stationary conditions allowed the diffusion of some cellular substances, such as reductase enzymes or other reducing substances, outside the cell. These cellular substances can then interact with gold chloride ions, reducing them to gold atoms, forming nuclei that aggregate to give Au-NPs. Ahmad et al. (2003) have conducted a same work to produce Au-NPs by using the thermophilic actinomycete Thermomonospora sp. The addition of gold chloride solution to the fungal filtrate resulted in the abrupt change in the color of the filtrate from pale yellow to a faint purple color. This color then intensified gradually to become dark purple after 4 days of incubation in the dark. The appearance of the dark purple color observed in Fig. 4 indicates the reduction of gold ions and formation of Au-NPs. The dark purple color is due to the collective coherent oscillation of conduction electrons at the surface of Au-NPs that interact with the electric field of the incident light, a phenomenon called surface plasmon resonance (SPR). The intense colors and interesting optical properties of metal are due to surface plasmon oscillation when the light strikes the metal electrons (Mie 1908; Mulvaney 1996).
The UV–visible absorption spectrum of Au-NPs shown in Fig. 5 reveals a peak of absorption in the visible range of light, which starts at ~513 nm and increases to reach a maximum at ~570 nm, corresponding to the surface plasmon band of crystalline Au-NPs (Mukherjee et al. 2012). Mie (1908) was the first to explain this phenomenon theoretically by solving the Maxwell equation for the absorption and scattering of electromagnetic radiation by spherical particles. The data agree with the spectroscopic data of gold (Sansonetti and Martin 2005). The UV–visible spectrum in lower wavelength region (˂400 nm), showing absorption of UV light, suggests the presence of protein in solution having aromatic amino acids responsible for the absorption (Bhambure et al. 2009). The TEM micrographs shown in Fig. 6 reveal the presence of a large number of spherical Au-NPs with the majority having the size range of 1–3 nm with a standard deviation of 0.2 nm, and a minority of the nanoparticles having a size range of 6–20 nm. The smaller-sized nanoparticles obtained (1–3 nm) are approximately the same as that recorded by Jamal et al. (2012) for the chemical synthesis of Au-NPs. The particle size distribution reveals the large number of smaller-sized nanoparticles (1–3 nm) with respect to the larger ones (6–20 nm).
UV-Visible absorption spectrum of Au-NPs showing a surface plasmon band characteristic of gold particles, with a maximum absorption at 570 nm, corresponding to gold based on the spectroscopic data of gold in the handbook of basic atomic spectroscopic data (Sansonetti and Martin 2005)
The recorded XRD shown in Fig. 7 revealed four prominent peaks at 2θ angles: 38.3°, 44.4°, 64.8°, and 77.6°, and Brag reflections corresponding to (111), (200), (220), and (311). These values are in agreement with the reported data for gold nanocrystals with face-centered cubic (fcc) structure (Leff et al. 1996; Ahmad et al. 2005; Du et al. 2007; Deplanche and Macaskie 2008), and consistent with the database (Wang et al. 1994). These results confirm the presence of gold nanocrystals.
XRD pattern of Au-NPs. Labeled peaks correspond to the characteristic diffraction peaks of elemental gold based on database information (Wang et al. 1994)
Penicillium chrysogenum was recorded to produce intracellular Au-NPs when exposed to the HAuCl4 solution, as shown by Sheikhloo and Salouti (2011). However, in the present work, P. chrysogenum was found to produce extracellular Au-NPs as well.
4 Conclusion
The use of bacteria and fungi as sources of reducing enzymes that catalyze reactions leading to the formation of nanoparticles is a relatively new strategy. Fungi were found to be superior to bacteria in this field, as they can be easily separated from their broth media and they are richer in the proteins used in the production of nanoparticles. Extracellular enzymes can be easily separated from fungi and used as pure enzymes for production of nanoparticles on a large scale.
Penicillium chrysogenum was used to produce extracellular gold nanoparticles when gold chloride ion solution (HAuCl4∙3H2O) was added to the fungal filtrate. Extracellular biosynthesis was revealed by the change in the color of the supernatant from pale yellow to dark purple. Gold nanoparticles were produced and characterized by using UV–Visible Absorption Spectroscopy, Transmission Electron Microscopy (TEM) and X-Ray Diffraction (XRD) to determine the composition, shape, structure, and particle size.
This study provides a simple bioprocess using the widely distributed P. chrysogenum as a biological system for production of gold nanoparticles. The extracellular biosynthesis of gold nanoparticles conducted in this research can be applied on a large scale to serve in industrial and medical fields. Also, the shape and size of these nanoparticles can be controlled by controlling the conditions of the production process.
References
Ahmad A, Senapati S, Khan MI, Kumar R, Sastry M (2003) Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir 19:3550–3553
Ahmad A, Senapati S, Khan MI, Kumar R, Sastry M (2005) Extra-/intracellular biosynthesis of gold nanoparticles by an alkalotolerant fungus, Trichothecium sp. J Biomed Nanotechnol 1:47–53
Bhambure R, Bule M, Shaligram N, Kamat M, Singhal R (2009) Extracellular biosynthesis of gold nanoparticles using Aspergillus niger—its characterization and stability. Chem Eng Technol 32:1036–1041
Bhushan B (2010) Springer handbook of nanotechnology, 3rd edn. Springer, Heidelberg
Bhushan B, Luo D, Schricker SR, Sigmund W, Zauscher S (2014) Handbook of nanomaterials Properties. Springer, Heidelberg
Biswas A, Aktas O, Schürmann U, Saeed U, Zaporojtchenko V, Faupel F, Strunskus T (2004) Tunable multiple plasmon resonance wavelengths response from multicomponent polymer-metal nanocomposite systems. Appl Phys Lett 84:2655–2657
Brüggemann D, Wolfrum B, de Silva JP (2014) Fabrication, properties and applications of gold nanopillars. In: Bhushan B, Luo D, Schricker SR, Sigmund W, Zauscher S (eds) Handbook of nanomaterials properties. Springer, Heidelberg, pp 317–354
Cao J, Hu X (2009) Synthesis of gold nanoparticles using halloysites. e-J Surf Sci Nanotechnol 7:813–815
Chae KW, Zhang Q, Kim JS, Jeong YH, Cao G (2010) Low-temperature solution growth of ZnO nanotube arrays. Beilstein J Nanotechnol 1:128–134
Deplanche K, Macaskie L (2008) Biorecovery of gold by Escherichia coli and Desulfovibrio desulfuricans. Biotechnol Bioeng 99:1055–1064
Dolgaev S, Simakin A, Voronov V, Shafeev G, Bozon-Verduraz F (2002) Nanoparticles produced by laser ablation of solids in liquid environment. Appl Surf Sci 186:546–551
Du L, Jiang H, Liu X, Wang E (2007) Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5α and its application on direct electrochemistry of hemoglobin. Electrochem Commun 9:1165–1170
Du L, Xian L, Feng JX (2011) Rapid extra-/intracellular biosynthesis of gold nanoparticles by the fungus Penicillium sp. J Nanopart Res 13:921–930
Edelstein AS, Cammarata RC (1996) Nanomaterials: synthesis, properties, and applications. Taylor and Francis, New York
Fortin D, Beveridge TJ (2000) From biology to biotechnology and medical applications. Biomineralisation, Wiley-VCH, Weinheim, pp 7–22
Gogotsi Y (2006) Nanomaterials handbook (advanced materials and technologies). CRC Press, Boca Raton
Gonzalez NYM, El Morsli M, Proulx P (2008) Production of nanoparticles in thermal plasmas: a model including evaporation, nucleation, condensation, and fractal aggregation. J Therm Spray Technol 17:533–550
Gupta S, Bector S (2013) Biosynthesis of extracellular and intracellular gold nanoparticles by Aspergillus fumigatus and Aspergillus flavus. Anton. Leeuw. 103:1113–1123
Hosokawa M, Nogi K, Naito M, Yokoyama T (2012) Nanoparticle technology handbook, 2nd edn. Elsevier, Netherlands
Huang Y-X, Liu X-W, Sun X-F, Sheng G-P, Zhang Y-Y, Yan G-M, Wang S-G, Xu A-W, Yu H-Q (2011) A new cathodic electrode deposit with palladium nanoparticles for cost-effective hydrogen production in a microbial electrolysis cell. Int J Hydrogen Energy 36:2773–2776
Husen A, Siddiqi KS (2014) Phytosynthesis of nanoparticles: controversy and application. Nanoscale Res Lett 9:229–252
Husseiny MI, El-Aziz MA, Badr Y, Mahmoud MA (2007) “Biosynthesis of gold nanoparticles using pseudomonas aeruginosa. Spectrochim Acta A 67:1003–1006
Jain KK (2012) Nanopharmaceuticals. In: Jain KK (ed) Handbook of nanomedicine, 2nd edn. Springer, New York, pp 171–234
Jamal F, Jean-Sébastien G, Maël P, Edmond P, Christian R (2012) Gold nanoparticle synthesis in microfluidic systems and immobilisation in microreactors designed for the catalysis of fine organic reactions. Microsyst Technol 18:151–158
Jana NR, Pal T, Sau TK, Wang ZL (2000) Seed- mediated growth method to prepare cubic copper nanoparticles. Curr Sci 79:1367–1370
Kalishwaralal K, Deepak V, Pandian SRK, Gurunathan S (2009) Biological synthesis of gold nanocubes from Bacillus licheniformis. Biosource Technol 100:5356–5358
Khaydarov RR, Khaydarov RA, Evgrafova S, Wagner S, Cho SY (2011) Environmental and human health issues of silver nanoparticles applications. In: Alpas H, Berkowiz SM, Ermakova I (eds) Environmental security and ecoterrorism. Springer, Netherlands, pp 117–127
Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang CY (2007) Antimicrobial effects of silver nanoparticles. Nanomed Nanotechnol Biol Med 3:95–101
Kong J, Cassell AM, Dai H (1998) Chemical vapor deposition of methane for single-walled carbon nanotubes. Chem Phys Lett 292:567–574
Kumar M, Ando Y (2010) Chemical vapor deposition of carbon nanotubes: areview on growth mechanism and mass production. J Nanosci Nanotechnol 10:3739–3758
Leff DV, Brandt L, Heath JR (1996) Synthesis and characterization of hydrophobic, organically-soluble gold nanocrystals functionalized with primary amines. Langmuir 12:4723–4730
Li J, Wang X, Wang C, Chen B, Dai Y, Zhang R, Song M, Lv G, Fu D (2007) The enhancement effect of gold nanoparticles in drug delivery and as biomarkers of drug-resistant cancer cells. Chem Med Chem 2:374–378
Lin Z, Wu J, Xue R, Yang Y (2005) Spectroscopic characterization of Au3+ biosorption by waste biomass of saccharomyces cerevisiae. Spectrochim Acta A 61:761–765
Meulenkamp EA (1998) Synthesis and growth of ZnO nanoparticles. J Phys Chem B 102:5566–5572
Mie G (1908) Contributions to the optics of turbid media, particularly of colloidal metal solutions. Ann Phys 25:377–445
Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, Parishcha R, Ajaykumar PV, Alam M, Kumar R, Sastry M (2001) Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett 1:515–519
Mukherjee P, Senapati S, Mandal D, Ahmad A, Khan MI, Kumar R, Sastry M (2002) Extracellular synthesis of gold nanoparticles by the fungus Fusarium oxysporum. Chem Bio Chem 3:461–463
Mukherjee P, Mainak Roy, Mandal BP, Choudhury Sipra, Tewari R, Tyagi AK, Kale SP (2012) Synthesis of uniform gold nanoparticles using non-pathogenic bio-control agent: evolution of morphology from nano-spheres to triangular nanoprisms. J Colloid Interf Sci. 367:148–152
Mulvaney P (1996) Surface plasmon spectroscopy of nanosized metal particles. Langmuir 12:788–800
Murakami H, Kobayashi M, Takeuchi H, Kawashima Y (1999) Preparation of poly (DL-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. Int J Pharm 187:143–152
Navazi ZR, Pazouki M, Halek FS (2010) Investigation of culture conditions for biosynthesis of silver nanoparticles using Aspergillus fumigatus. Iran J Biotechnol 8:56–60
Perera S, Bhushan B, Bandara R, Rajapakse G, Rajapakse S, Bandara C (2013) Morphological, antimicrobial, durability, and physical properties of untreated and treated textiles using silver-nanoparticles. Colloids Surf A 436:975–989
Perrault SD (2014) Medical nanomaterials. In: Ge Y, Li S, Wang S, Moore R (eds) Nanomedicine. Springer, New York, pp 83–99
Samson RA, Hadlok R, Stolk AC (1977) A taxonomic study of the Penicillium chrysogenum series. Anton Leeuw Int J Gen Molec Microbiol 43:169–175
Sansonetti JE, Martin WC (2005) Handbook of basic atomic spectroscopic data. American Institute of Physics, Maryland
Sawle BD, Salimath B, Deshpande R, Bedre MD, Prabhakar BK, Venkataraman A (2008) Biosynthesis and stabilization of Au and Au–Ag Alloy nanoparticles by fungus, Fusarium semitectum. Sci Technol Adv Mater 9:035012–035017
Shankar SS, Ahmad A, Pasricha R, Sastry M (2003) Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. MJ Mater Chem 13:1822–1826
Sheikhloo Z, Salouti M (2011) Intracellular biosynthesis of gold nanoparticles by the fungus Penicillium chrysogenum. Int J Nanosci Nanotechnol 7:102–105
Shipway AN, Willner I (2001) Nanoparticles as structural and functional units in surface-confined architectures. Chem Commun 20:2035–2045
Singaravelu G, Arockiamary J, Kumar VG, Govindaraju K (2007) A novel extracellular synthesis of monodisperse Gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf B 57:97–101
Southam G, Beveridge TJ (1994) The in vitro formation of placer gold by bacteria. Geochim Cosmochim Acta 58:4527–4530
Verma VC, Singh SK, Solanki R, Prakash S (2011) Biofabrication of anisotropic gold nanotriangles using extract of endophytic aspergillus clavatus as a dual functional reductant and stabilizer. Nanoscale Res Lett 6:16–22
Wang A, Han J, Guo L, Yu J, Zeng P (1994) Database of standard raman spectra of minerals and related inorganic crystals. Appl Spectrosc 48:959–968
Wang C, Flynn NT, Langer R (2004) Controlled structure and properties of thermoresponsive nanoparticle-hydrogel composites. Adv Mater 16:1074–1079
Yang N, Aoki K (2005) Voltametry of the silver alkylcarboxylate nanoparticles in suspension. Electrochim Acta 50:4868–4872
Acknowledgments
The first author is grateful for a scholarship to conduct research at The Ohio State University, funded by the United States Agency for International Development (USAID)(Program # G-2-00263), administered by the Egyptian Ministry for Higher Education. He is also thankful to Prof. Bharat Bhushan at the Department of Mechanical and Aerospace Engineering, Ohio State University for hosting and providing invaluable advice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magdi, H.M., Bhushan, B. Extracellular biosynthesis and characterization of gold nanoparticles using the fungus Penicillium chrysogenum . Microsyst Technol 21, 2279–2285 (2015). https://doi.org/10.1007/s00542-015-2666-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-015-2666-5