Abstract
Low-Ag-based Pb-free solder and its evaluation for electromigration (EM) reliability are research areas of active development. This paper studies the effect of adding Ni and Ge microelements on the EM behavior of low-Ag-based SnAgCu solder. Comparative measurements of the EM resistance of Sn1.0Ag0.5Cu (SAC) and Sn1.0Ag0.5Cu0.07Ni0.01Ge (SACNG) solders (in wt %) were conducted. In these experiments, SACNG showed a higher EM resistance than SAC. It was concluded that adding Ni and Ge enhanced the EM resistance of low-Ag-based SnAgCu solders. This shows the same effect of Ni and Ge on the EM resistance as that which has been reported for Sn3.5Ag0.5Cu solder. An analysis of these observations suggests that grain boundary sliding plays a dominant role in stress relaxation and causes hillock growth. Based on this assumption, a possible mechanism was proposed to explain the effects of Ni and Ge on the EM resistance of SnAgCu solder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Solder joints are ubiquitous in electronic products. In recent decades, to meet the demand of the rapidly increasing input/output interconnection density driven by the miniaturization of microelectronic devices, flip-chip technology has developed. A flip chip utilizes a solder bump array for its electrical interconnections; thus, the packaging size was significantly reduced due to the shortened interconnection length. Currently, the size of a Sn-based solder bump has been reduced to 100 μm or less. As the size of the solder bump decreases, the electromigration (EM) reliability of the solder joints caused by the increasing current density becomes a more serious issue for electronic packaging.
EM is the phenomenon of atomic diffusion caused by electron flow in high density. It is significantly affected by the current density and temperature (part of which is due to Joule heating). With atomic accumulation and depletion, hillocks and voids are formed in the material, respectively. The growth of hillocks and voids deteriorates the reliability of the integrated circuits (Black 1969; Attardo and Rosenberg 1970; Abé et al. 2006). In flip-chip solder joints, EM-induced failure has been attributed to a current density concentration near the cathode corner where the electron flow enters into the solder (Yeh et al. 2002; Choi et al. 2003). The high current density causes the initial formation of voids, which quickly spread across the entire under bump metallization/solder interface and lead to an electrical discontinuity.
Traditionally used eutectic SnPb solder has been banned in electronics since July 2006 due to the harmful impact of Pb on the environment and human health. Consequently, the development of Pb-free solders is a primary goal. Of the various Pb-free solders that are available today, the so-called SnAgCu group has become the most highly favored substitute because of its excellent wetting on Cu and because of its thermo-mechanical properties (Miller et al. 1994; Zribi et al. 2001). Based on this ternary system, high-order solders (such as SnAgCuNiGe) are also viable options for meeting various product requirements (Anderson et al. 2002; Hidaka et al. 2009; Saka et al. 2009). In 2002, Zeng and Tu (2002) summarized the suggested Ag content as being in the range of 3.2–4.7 wt %. Unfortunately, as a noble metal, Ag increases the product cost of solder manufacturing. Thus, the development of low-Ag-based Pb-free solders and their relevant EM reliability evaluation are actively pursued in today’s increasingly competitive economy.
It has been reported that Ni and Ge microelements added to Sn3.5Ag0.5Cu solder (where all of the solder compositions are given in weights in this paper) showed a higher EM resistance compared to Sn3.0Ag0.5Cu and Sn3.5Ag0.5Cu solders (Saka et al. 2009). If this effect of Ni and Ge on the EM resistance is not affected by the amount of Ag, we can expect that adding Ni and Ge would also enhance the EM resistance of low-Ag-based SnAgCu solders. However, the mechanism is still unclear, and thus we must examine the effect of Ni and Ge on low-Ag-based SnAgCu solders directly.
This paper experimentally compares the EM resistance of two typical low-Ag-based Pb-free solders: Sn1.0Ag0.5Cu (SAC) and Sn1.0Ag0.5Cu0.07Ni0.01Ge (SACNG). The effect of adding Ni and Ge on the EM resistance is studied. Following a discussion of the results, a possible mechanism is proposed to explain the observed effect.
2 Experiments
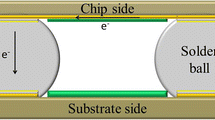
The EM evaluation experiments were conducted based on previous work (Zhao et al. 2010) with a proposed 2D solder joint test structure. Figure 1 depicts the test structure. This structure has the advantages of (1) effectively utilizing the current density concentration near the contact corners to enhance EM, (2) possible accurately analyzing the distribution of the concentrated current density by treating it as a 2D electrical problem, (3) simulating the Cu/solder/Cu joint structure in a flip chip, and (4) enabling the in situ observation of the EM behavior without cross-sectional etching.
a The solder joint test structure and its dimensions (top view) and b the cross-sectional view of A–A in Fig. 1a
2.1 Sample fabrication
The SAC and SACNG solder wires (diameter, 100 μm) were prepared as test materials. All of the samples were fabricated using the following procedures, as illustrated in Fig. 2.
The fabrication of the sample: a a printed board with several uniform Cu electrodes and a resist film; b a magnified view of one pair of Cu electrodes covered with the resist film and highlighted by the black rectangle in a; the schematic diagram of its cross-sectional structure; c microscale soldering; d surface polishing
First, a printed board (FR-4; 15 mm × 10 mm × 1.6 mm) was prepared as the substrate, as shown in Fig. 2a. Several uniform 9-μm-thick Cu electrodes and a resist film were formed on the substrate, as shown in Fig. 2a and b. The resist film covering the substrate and the portions of the Cu electrodes indicated in Fig. 2b served as the corral for shaping a rectangular section (120 μm × 60 μm), where the solder would later fill the black region in Fig. 1. Next, microscale soldering using a soldering iron with a 200 μm-diameter soldering bit was performed. The soldering temperature was 513 K, which is approximately 20 K above the melting point of the solders. The soldering time was several seconds. Fluxes had previously been painted on the Cu electrodes and the soldering section. The role of the fluxes was primarily to remove the initial oxide layers on the Cu and the solder and to prevent further oxidation during soldering. Otherwise, the oxide layer would act as a barrier to retard the interaction between Cu and Sn that forms the intermetallic compound (IMC), rendering the jointing unreliable. With the help of the fluxes, the prepared solder wires were jointed to the Cu, as shown in Fig. 2c. Finally, the sample surface was carefully polished to remove any redundant solder and resist film in the experiment, as shown in Fig. 2d. Figure 3 shows a fabricated sample and a magnified view of the solder joint region.
Because the solder and Cu layers formed on the substrate are thin film materials, the surface roughness of the substrate must be taken into consideration. The roughness was examined using a contact-type surface roughness meter. The measured surface roughness, R max, was about 1.55 μm, where R max is the maximum height calculated by the distance between the line of the profile peaks and the line of the profile valleys measured in the vertical magnification direction of the roughness curve. This maximum value is enough smaller than the thickness of the solder and the Cu. It is concluded that the surface roughness of the substrate affects the calculation of the current density, but the maximum error in the evaluation of the current density due to the roughness is within a permissible range.
2.2 Observation of the solder joints
To achieve bonding in the solder joints, the IMC layer formed by the interaction between the molten solder and the base material is necessary. The terms “reflow” and “wetting reaction” are usually used to discuss this process. In this research, the soldering proceeded at 513 K for several seconds. The wetting reaction temperature approached the common peak temperature of the reflow in the electronic packaging. Nevertheless, the reaction time was much shorter, and no solid-state aging was performed. To confirm the solder joints, the interfacial region was detected using energy-dispersive X-rays (EDXs). As an example, Fig. 4 represents the results of an EDX analysis of a Cu/SACNG solder joint. Three points (one in the Cu, one in the IMC and one in the SACNG solder) were considered. In Point 1, only Cu was detected. Point 2 in the IMC layer showed a combination of Sn, Cu and Ni. Based on the work of Chuang and Lin (2003), the IMC was believed to be (Cu, Ni)6Sn5, and no Cu3Sn was formed. Moreover, the thickness of the IMC layer shown in Fig. 4 was approximately 2 μm. In the SAC solder, the IMC was believed to be only Cu6Sn5 (without solid-state aging) (Chuang and Lin 2003). For both IMCs formed with the SAC and SACNG solders, Ag was not detected, suggesting that Ag was not involved in the IMCs or that the Ag content was below the detection limit of the examination system.
2.3 Experimental procedures
Two testing groups were prepared (defined as Sample Groups 1 and 2) and consisted of SAC and SACNG solders. The EM evaluations of the two groups were conducted under identical experimental conditions to ensure the repeatability of the results. The samples were positioned on a ceramic heater. The temperature of the heater was 333 K. An average current density of 30 kA/cm2 was supplied to the sample for total of 20 h. Here, the average current density was calculated by dividing the applied current by the cross-sectional area of the solder. The current density near the inner corners of the sample was much higher than the average current density due to the effect of the current density concentration. The current flowed from the lower Cu electrode to the upper electrode, as shown in Fig. 3. The anode and cathode ends of the solders were observed every 10 h for current supply, and the EM resistances of the SAC and SACNG solders were compared via the morphological changes in each group.
3 Results and discussion
Figures 5 and 6 represent the morphological changes in Sample Group 1 during the EM evaluation. Voids were initially formed near the left-inner corner (where the electron flow entered into the solders) for both of the cathode ends of the SAC and SACNG solders, as shown in Fig. 5b, c,e and f, respectively. The current density concentration causing void formation near the cathode corner matched the attribution of EM-induced failure in the flip-chip solder joints that was introduced in the previous section. Hillocks were formed at both of the anode ends of the SAC and SACNG solders, as shown in Fig. 6b, c, e and f, respectively. The hillocks were observed near the anode ends where the electron flow left the solder and in some regions farther away from the corners. Moreover, the EM-induced hillocks and voids in Figs. 5 and 6 show growth with an increasing current supply time. The SACNG solder appeared to exhibit higher EM resistance than the SAC solder in Sample Group 1.
A comparison of the cathode ends in Sample Group 1 during the EM evaluation: the morphological changes in the SAC solder a before the current supply, b after 10 h, and c after 20 h of the current supply; the morphological changes in the SACNG solder d before the current supply, e after 10 h, and f after 20 h of the current supply
A comparison of the anode ends in Sample Group 1 during the EM evaluation: the morphological changes in the SAC solder a before the current supply, b after 10 h, and c after 20 h of the current supply; the morphological changes in the SACNG solder d before the current supply, e after 10 h, and f after 20 h of the current supply
In Sample Group 2, the SAC solder showed an obvious morphological change compared to SACNG solder, as shown in Figs. 7 and 8. No clear void formation was observed in the SACNG solder, even after 20 h of the current supply. More and larger hillocks were observed in the SAC solder compared to the SACNG solder, as shown in Fig. 8. As a result, the SACNG solder showed higher EM resistance than the SAC solder in Sample Group 2 (identical to Sample Group 1). In addition, the thicknesses of the SAC and SACNG solders in each group were confirmed to be almost identical via a cross-sectional observation after focused ion beam etching.
A comparison of the cathode ends in Sample Group 2 during the EM evaluation: the morphological changes in the SAC solder a before the current supply, b after 10 h, and c after 20 h of the current supply; the morphological changes in the SACNG solder d before the current supply, e after 10 h, and f after 20 h of the current supply
A comparison of the anode ends in Sample Group 2 during the EM evaluation: the morphological changes in the SAC solder a before the current supply, b after 10 h, and c after 20 h of the current supply; the morphological changes in the SACNG solder d before the current supply, e after 10 h, and f after 20 h of the current supply
Both groups showed that the SACNG solder had higher EM resistance than the SAC solder. It can be concluded that adding Ni and Ge would enhance the EM resistance of SAC solders. In addition, this showed that Ni and Ge have identical effects on the EM resistance, as was reported for Sn3.5Ag0.5Cu solder (Saka et al. 2009).
It is well known that EM builds up compressive stress on the anode side; this stress can be released in the form of material deformation. In this research, grain boundary precipices were observed after the current supply, as shown in Fig. 9, where the grain boundaries sank leading void formation (Fig. 9a) and upheaved leading hillock formation (Fig. 9b–d). This observation indicates that the grain boundaries are good source for stress relaxation. Moreover, the initial top of the hillocks remained unchanged, while the height increased during hillock growth, as shown in Fig. 9b and c. This suggests a most probable mechanism for stress relaxation that is dominated by grain boundary sliding (Lahiri 1970; Chaudhari 1974; Ericson et al. 1991; Gladkikh et al. 1995). It should be mentioned that the compressive stress must reach a critical value to break the bonding of the grain boundaries and enable the boundary to slide along its adjacent boundaries. To discuss the effects of Ni and Ge on the EM resistance of SAC solder, we consider it from the viewpoints of grain boundary sliding and compressive stress.
More hillock growth was observed in the SAC solder. Based on the assumption that grain boundary sliding plays a dominant role in hillock growth, the grain boundaries of the SACNG solder are speculated to be more stable than those of the SAC solder. Hence, the Ni and Ge alloying effect should strengthen the bonding energy of the grain boundaries. In contrast, the Ni in SACNG solder was suggested to be (Cu, Ni)6Sn5 distributed in the Sn phase (Watanabe et al. 2006). Consequently, Ge is the most likely additional element in the grain boundaries. Such a microelement was considered to suppress grain boundary sliding in the SAC solder.
The EM-induced compressive stress is proportional to the atomic density (Korhonen et al. 1993). In other words, the higher the EM rate, the faster the atomic diffusion. Hence, more atoms accumulate in unit time and area, and the compressive stress for maintaining hillock growth increases. In a Sn-rich solder, Sn is the dominant diffusing atom mainly along the grain boundaries. For the SACNG solder, Ge was considered to distribute along the grain boundaries and act as a useful barrier to suppress the diffusion of Sn atoms. Moreover, Ni was suggested to combine with Sn as IMCs in a more stable state in the Sn phase. Following the consideration above, the addition of Ni and Ge suppresses the diffusion of Sn atoms and decreases the EM rate. Thus, the SACNG solder accumulates less compressive stress and slower hillock growth compared to the SAC solder.
4 Conclusions
An EM evaluation of SAC and SACNG solders was conducted. The addition of Ni and Ge microelements was investigated to enhance the EM resistance of low-Ag-based SnAgCu solder. A mechanism was proposed to explain the effects of Ni and Ge. (1) Ge may distribute along grain boundaries to suppress the diffusion of Sn atoms and grain boundary sliding, and (2) Ni also suppresses atomic diffusion by combining with Sn atoms as IMCs in a more stable state in the Sn phase.
References
Abé H, Sasagawa K, Saka M (2006) Electromigration failure of metal lines. Int J Fract 138:219–240. doi:10.1007/s10704-006-0059-6
Anderson IE, Cook BA, Harringa JL, Terpstra RL (2002) Sn-Ag-Cu solders and solder joints: alloy development, microstructure, and properties. JOM 54:26–29. doi:10.1007/BF02701845
Attardo MJ, Rosenberg R (1970) Electromigration damage in aluminum film conductors. J Appl Phys 41:2381–2386. doi:10.1063/1.1659233
Black JR (1969) Electromigration failure modes in aluminum metallization for semiconductor devices. Proc IEEE 57:1587–1594. doi:10.1109/PROC.1969.7340
Chaudhari P (1974) Hillock growth in thin films. J Appl Phys 45:4339–4346. doi:10.1063/1.1663054
Choi WJ, Yeh ECC, Tu KN (2003) Mean-time-to-failure study of flip chip solder joints on Cu/Ni (V)/Al thin-film under-bump-metallization. J Appl Phys 94:5665–5671. doi:10.1063/1.1616993
Chuang C-M, Lin K-L (2003) Effect of microelements addition on the interfacial reaction between Sn-Ag-Cu solders and the Cu substrate. J Electron Mater 32:1426–1431. doi:10.1007/s11664-003-0111-5
Ericson F, Kristensen N, Schweitz J-Å, Smith U (1991) A transmission electron microscopy study of hillocks in thin aluminum films. J Vac Sci Technol B 9:58–63. doi:10.1116/1.585790
Gladkikh A, Lereah Y, Glickman E, Karpovski M, Palevski A, Schubert J (1995) Hillock formation during electromigration in Cu and Al thin films: three-dimensional grain growth. Appl Phys Lett 66:1214–1215. doi:10.1063/1.113240
Hidaka N, Watanabe H, Yoshiba M (2009) Creep behavior of lead-free Sn-Ag-Cu + Ni-Ge solder alloys. J Electron Mater 38:670–677. doi:10.1007/s11664-009-0689-3
Korhonen MA, Børgesen P, Tu KN, Li C-Y (1993) Stress evolution due to electromigration in confined metal lines. J Appl Phys 73:3790–3799. doi:10.1063/1.354073
Lahiri SK (1970) Stress relief and hillock formation in thin lead film. J Appl Phys 41:3172–3176. doi:10.1063/1.1659383
Miller CM, Anderson IE, Smith JF (1994) A viable tin-lead solder substitute: Sn-Ag-Cu. J Electron Mater 23:595–601. doi:10.1007/BF02653344
Saka M, Kohara T, Hasegawa T, Yamashita M (2009) A simple method for testing the electromigration resistance of solders. Microsyst Technol 15:17–25. doi:10.1007/s00542-008-0642-z
Watanabe H, Hidaka N, Shohji I, Ito M (2006) Effect of Ni and Ag on interfacial reaction and microstructure of Sn-Ag-Cu-Ni-Ge lead-free solder. Proc MST 2006:135–146
Yeh ECC, Choi WJ, Tu KN, Elenius P, Balkan H (2002) Current-crowding-induced electromigration failure in flip chip solder joints. Appl Phys Lett 80:580–582. doi:10.1063/1.1432443
Zeng K, Tu KN (2002) Six cases of reliability study of Pb-free solder joints in electronic packaging technology. Mater Sci Eng R 38:55–105. doi:10.1016/S0927-796X(02)00007-4
Zhao X, Saka M, Yamashita M (2010) Electromigration near the corner composed of dissimilar materials in a 2D solder joint structure. In: Proceedings EMAP2010 Conference, Singapore, pp 188–191
Zribi A, Clark A, Zavalij L, Borgesen P, Cotts EJ (2001) The growth of intermetallic compounds at Sn-Ag-Cu solder/Cu and Sn-Ag-Cu solder/Ni interfaces and the associated evolution of the solder microstructure. J Electron Mater 30:1157–1164. doi:10.1007/s11664-001-0144-6
Acknowledgments
The authors wish to acknowledge Mr. H. Watanabe at Fuji Electric Co., Ltd., for his kind help in preparing the samples. We also acknowledge Prof. A.T. Yokobori, Jr., and Mr. T. Matsuzaki at Tohoku University for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, X., Saka, M., Yamashita, M. et al. The effect of adding Ni and Ge microelements on the electromigration resistance of low-Ag based SnAgCu solder. Microsyst Technol 18, 2077–2084 (2012). https://doi.org/10.1007/s00542-012-1577-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-012-1577-y