Abstract
A microfluidic chip for the chemiluminescence detection of cobalt (II) in water samples, based on the measurement of light emitted from the cobalt (II) catalysed oxidation of luminol by hydrogen peroxide in basic aqueous solution, is presented. The microfluidic chip was designed and fabricated from polydimethylsiloxane using micro-molding method. Optimized reagents conditions were found to be 5.0 × 10−4 mol/L luminol, 1.0 × 10−2 mol/L hydrogen peroxide, and 8.0 × 10−2 mol/L sodium hydroxide. The system can perform fully automated detection with a reagent consumption of only 2.4 μL each time. The linear range of the cobalt (II) ions concentration was 1.0 × 10−10–1.0 × 10−3 mol/L and the detection limit was 5.6 × 10−11 mol/L with the S/N ratio of 3. The relative standard deviation was 4.6 % for 1.0 × 10−5 mol/L cobalt (II) ions (n = 10).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Many heavy metal ions are harmful to human health. Chemiluminescence (CL) of heavy metal ions has many advantages including high detection sensitivity with simple instruments configuration, wide linear range of signal response, rapid measurement, and convenient setup. CL detection was used in the detection of the chromium (III)-catalysed oxidation of luminol by hydrogen peroxide for the first time (Seitz et al. 1972). Further studies on this reaction showed its high selectivity and sensitivity towards Cr(III) in the presence other metal ions in the aqueous environment (Gammelgaarda et al. 1997).
In recent years, the microfluidic chip has gained great attention. The microfluidic chip has many advantages, such as its short time for hybrid reaction, high separation efficiency, potential portability and low reagent consumption. It has been used in chemical reaction and biochemical analyses due to its inherent advantages of requiring low reagents consumption, however, the extremely small sample size makes achieving high detection sensitivity a challenge.
Due to the characteristics of CL detection, it is well matched to the concept of microfluidic chips, and is uniquely suited to on-line detection. Several reports have shown that CL detection on microfluidic chips is a promising alternative method for the determination of dissolved trace metals. A microchip with an integrated passive micromixer based on chaotic advection was reported, and used in the luminol-peroxide chemiluminescence detection of cobalt (II) ions (Lok et al. 2011). Cobalt (II) and Cu (II) were determined using the luminol–hydrogen peroxide CL reaction catalyzed by metal ions on a microfluidic chip (Huang et al. 2001). The reaction of 1,10-phenanthroline and hydrogen peroxide in the presence of a cationic surfactant was researched for the flow-injection analysis of Cu(II), Ru(III), and Os(VIII) with a microchip (Nogami et al. 2009). A CL detection system that fabricated in poly(dimethylsiloxane) for separation of Cr(III) and other metal ions was reported (Liu et al. 2003). CL has been used in the determination of chromium(III) and total chromium using dual channels on glass chip (Waraporn et al. 2007). For solid-state electrochemiluminescence of ru (bpy) 2+3 /TPA system, three-dimensionally ordered macroporous gold structure has been presented (Gao et al. 2007).
In this paper, a simple microfluidic chip fabricated on PDMS with CL detection has been presented to determine the concentration of cobalt (II) in water. The microfluidic chip has simple structure and can be easily fabricated. The procedures of reagents mixing and detection are both processed on the microfluidic chip. In summary, the CL microfluidic chip has several advantages: It makes use of both the high sensitivity of CL detection and on-line characteristics of microfluidic chips. It has simple structure with length of 65 mm and width of 40 mm. It can be easily fabricated with conventional methods. It can finish CL detection in less than 2 min. Only fairly simple devices were used in the experiment.
2 Materials and methods
2.1 Chemicals
All reagents used were commercially available and of analytical grade, and deionized water was used throughout. Luminol was obtained from Sangon Biotech Co., Ltd. (Shanghai). 30 % (v/v) hydrogen peroxide (H2O2) was obtained from Shanghai Caoxing Zhongli Chemical Regent Co. (Shanghai). Cobalt (II) chloride, hexahydrate (CoCl2·6H2O) and sodium hydroxide (NaOH) was obtained from Xi’an Chemical Reagent Co. (Xi’an).
Alkaline buffer solution was obtained by dissolving NaOH powder in deionised water. A stock solution of luminol with concentration 0.01 mol/L was obtained by dissolving 0.1772 g luminol in 0.1 L NaOH buffer solution and was used after storing at room temperature for a week. 1 × 10−3 mol/L cobalt (II) chloride in deionised water was prepared as stock solution. All working solutions were prepared freshly from the stock solution by dilution with deionized water before the experiments.
2.2 Design of PDMS microfluidic chips
The photo of the microfluidic chip and schematic structure of the ribs in it are shown in Fig. 1. External dimensions of the microfluidic chip are length of 65 mm and width of 40 mm. The microfluidic chip has a cross-channel as the main channel, and with ribs on the channel floor for improving mixing. The microfluidic chip has two groups of ribs with 65 ribs for every group. A group of ribs is named a mixing unit. The length of every mixing unit is 9 mm. When the flow rate is less than 90 μL/min, the mixing can be finished at 5 mm of the second mixing unit. There are four reservoirs on the microfluidic chip, named P1, P2, P3, and P4. The channel length is 40.0 mm from P1 to P2, and 14.0 mm from P3 to P4. The depth and the width of the main channel are 300 and 100 μm, respectively. The height and the width of the rib are 50 and 70 μm, respectively. The space between two adjacent ribs is 70 μm. The ribs are patterned at a 45° angle to the flow direction along the channel length since it was concluded as the optimal angle (Stroock et al. 2002).
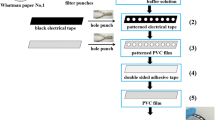
2.3 Fabrication of PDMS microfluidic chips
The PDMS microfluidic chip was fabricated with two steps lithography method. Sylgard 184 silicone elastomer and curing agent were uniformly mixed according to 10:1 as the chip material. Chip layout made by AutoCAD was printed onto a film mask for UV photolithography. A glass wafer was used as a substrate of UV-cured polymer mold for the replication of the channel pattern to PDMS. The fabricating steps were briefly described as follows. Firstly, the wafer was spincoated with SU8 negative photoresist. Secondly, after prebaking at 85 °C for 1 h, the wafer was exposed to UV light through the first photomask. Thirdly, the postbaking at 85 °C for 2 min was done to bridge epoxy polymer. Lastly, the wafer was developed for 3 min in γ-butyrolactone, subsequently rinsed with isopropyl alcohol, and hard baked for 0.2 h to remove organic solution. The second lithography was done by repeating the above steps, and the second photomask was used in the lithography step. Finally, another flat PDMS layer was bonded to the patterned PDMS layer to form the microfluidic chip.
2.4 Experimental setup
The schematic diagram of experimental setup is shown in Fig. 2. Hydrogen peroxide, luminol and cobalt (II) solution in alkaline buffer were respectively delivered from a 1 mL disposable syringe through the syringe pump via 1.0 mm I.D. PTFE tubing into the microfluidic chip. The CL signal was detected with a photomultiplier tube (PMT) (Model H5784-02, Hamamatsu, Japan). The microfluidic chip was positioned directly on top of the PMT, and the centers of the detection area and PMT window were close to each other as near as possible. The PMT was interfaced directly to a personal computer via a serial connection and data was acquired with BPCL software at a sampling rate of 40 Hz. The chip and PMT were housed in a light-tight black box to eliminate background readings during entire experiments. A complete analysis could be finished within 2 min with a reagent consumption of 2.4 μL. The concentration of cobalt (II) was quantified by the relative CL intensity.
3 Results and discussion
3.1 Effect of flow rate on CL intensity
Because the luminol chemiluminescence is a rapid reaction with the maximum of the transient CL signal being reached in approximately 50 s, the flow rate of reagents applied is critical which contributed to the mixing of the reagents and producing the signal. 5.0 × 10−4 mol/L Luminol, 1.0 × 10−5 mol/L cobalt (II) chloride, and 1.0 × 10−2 mol/L hydrogen peroxide were pumped at inlet P1, P3, and P4 at the same velocity by using the syringe pump, respectively. The Effect of the flow rate of reagents on the CL response was investigated in the range 10–100 μL/min by changing the speed of the syringe pump. Figure 3 shows that CL intensity increased rapidly with increasing of flow rate up to 60 μL/min, above which it increased slowly. So, the CL intensity increased with the flow rate increasing. However, if the flow rate was greater, the solvents could not mix sufficiently, and the increased pressure in the microfluidic chip would lead to leakage. When the flow rate was greater than 90 μL/min, the microfluidic chip would leak. Hence, a flow rate of 60 μL/min was selected as a compromise between high signal magnitude and preventing the chip leakage.
3.2 Effect of luminol concentration on CL intensity
The concentration of luminol was varied from 1.0 × 10−6 to 0.7 × 10−3 mol/L with the concentration of hydrogen peroxide keeping at 1.0 × 10−2 mol/L and the concentration of cobalt (II) ions keeping at 1.0 × 10−5 mol/L. Figure 4 shows that the CL intensity increased with increasing luminol concentration. When the luminol concentration was higher than 5.0 × 10−4 mol/L, the CL intensity increased slowly, and almost reached a plateau, therefore 5.0 × 10−4 mol/L luminol was chosen as the optimal.
3.3 Effect of hydrogen peroxide concentration on CL intensity
The concentration of hydrogen peroxide was varied from 1.0 × 10−6 to 1.0 × 10−1 mol/L with the concentration of luminol keeping at 5.0 × 10−4 mol/L and the concentration of cobalt (II) ions keeping at 1.0 × 10−5 mol/L. Figure 5 shows that the CL intensity increased with increasing concentration of hydrogen peroxide. When the concentration of hydrogen peroxide was higher than 1.0 × 10−2 mol/L, the CL intensity increased slowly, and the higher concentration of hydrogen peroxide easily produced bubbles. So, 1.0 × 10−2 mol/L hydrogen peroxide was chosen as the optimal.
3.4 Effect of NaOH concentration on CL intensity
The CL reaction of hydrogen peroxide with luminol must be in alkaline medium. Preliminary assays showed that concentration of the alkaline buffer solution had an important effect on the CL intensity for the determination of cobalt (II) ions. NaOH was chosen as buffer solution. The results in Fig. 6 showed that the CL intensity increased with increasing NaOH concentration in the range 0.01–0.15 mol/L. But the reproducibility was poor when the concentration of NaOH was too high. So the NaOH concentration of 0.08 mol/L was chosen as optimum.
3.5 Effect of cobalt (II) ions concentration on CL intensity
The concentration of cobalt (II) ions ranges from 1.0 × 10−10 to 1.0 × 10−3 mol/L. The cobalt (II) ions, 1.0 × 10−2 mol/L H2O2, and 5.0 × 10−4 mol/L luminol in alkaline buffer solution were introduced into the microfluidic chip. The flow rate was set to 60 μL min−1. The minimum analytical time for each sample was 2 min. The CL intensity increased with increasing concentration of cobalt (II) ions.
3.6 Linearity, repeatability and limit of detection
With 5.0 × 10−4 mol/L luminol and 1.0 × 10−2 mol/L H2O2, the linearity, repeatability and limit of detection of cobalt (II) standard solutions were measured. The response to cobalt (II) concentration was linear over the range 10−10–10−3 mol/L. The regression equation was y = 82C + 15, where y and C represented the CL intensity in counts and the concentration of cobalt (II) ions in mmol/L. Figure 7 shows that the CL intensity versus concentration of cobalt (II) ions. The detection limit for cobalt (II) was found to be 5.6 × 10−11 mol/L with the S/N ratio of 3. The relative standard deviation of the system was examined by making ten consecutive runs with cobalt (II) concentration of 1.0 × 10−5 mol/L and was found to be 4.6 %.
4 Conclusions
We have described the effective use of a simple CL microfluidic chip fabricated in PDMS. The results have shown that the linear range of the cobalt (II) ions concentration was 1.0 × 10−10–1.0 × 10−3 mol/L and the detection limit was 5.6 × 10−11 mol/L (S/N = 3). There are various advantages of the CL microfluidic chip format. It makes use of both the high selectivity and sensitivity of CL detection and the simplicity and on-line characteristics of microfluidic chips. It has simple structure and can be easily fabricated with conventional methods. It can be used repeatedly with little reagent consumption. It is not limited for only cobalt (II) determination, and offers possibility for the determination of other similar chemistries. Consequently, the CL microfluidic chip has great potential for metal ions monitoring in water.
References
Gammelgaarda B, Liao YP, Jøns O (1997) Improvement on simultaneous determination of chromium species in aqueous solution by ion chromatography and chemiluminescence detection. Anal Chim Acta 354(2):107–113
Gao W, Xia XH, Xu JJ, Chen HY (2007) Three-dimensionally ordered macroporous gold structure as an efficient matrix for solid-state electrochemiluminescence of Ru (bpy) 32+/TPA system with high sensitivity. J Phys Chem C 111(33):12213–12219
Huang XJ, Puand QS, Fang ZL (2001) Capillary electrophoresis system with flow injection sample introduction and chemiluminescence detection on a chip platform. Analyst 126(6):281–284
Liu BF, Ozaki M, Utsumi Y, Hattori T, Terabe S (2003) Chemiluminescence detection for a microchip capillary electrophoresis system fabricated in poly(dimethylsiloxane). Anal Chem 75(1):36–41
Lok KS, Kwok YC, Nguyen NT (2011) Passive micromixer for luminol-peroxide chemiluminescence detection. Analyst 136(12):2586–2591
Nogami T, Hashimoto M, Tsukagoshi K (2009) Meta ion analysis using microchip CE with chemiluminescence detection based on 1,10-phenanthroline–hydrogen peroxide reaction. J Sep Sci 32(3):408–412
Seitz WR, Suydam WW, Hercules DM (1972) Determination of trace amounts of chromium(III) using chemiluminescence analysis. Anal Chem 44(6):957–963
Stroock AD, Dertinger SK, Ajdari A, Mezic I, Stone HA, Whitesides GM (2002) Chaotic mixer for microchannels. Science 295(3):647–651
Waraporn SA, Threeprom J, Li H, Lin JM (2007) Determination of chromium(III) and total chromium using dual channels on glass chip with chemiluminescence detection. Talanta 71:2062–2068
Acknowledgments
The authors are grateful for National Natural Science Foundation of China (No: 20890024, No: 91023046, No: 91023017), and Special Funds of Basic Scientific Research Expenses for the Central University (No: DUT11SM11). We would also like to thank Li Qi and Juan Qiao (ICCAS), Xiuzhong Wang (QAU), Mei Yang (LNNU), Hong Shen (ZJU) for useful discussion on CL detection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X., Liu, C., Xu, Z. et al. An effective PDMS microfluidic chip for chemiluminescence detection of cobalt (II) in water. Microsyst Technol 19, 99–103 (2013). https://doi.org/10.1007/s00542-012-1551-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00542-012-1551-8