Abstract
Purpose
The oxygen reserve index (ORi™) is a parameter used for the noninvasive evaluation of arterial partial pressure of oxygen (PaO2), specifically in the 100–200 mmHg range. We aimed to report on the impact of indocyanine green (ICG) on the ORi™.
Methods
In this study, we retrospectively examined patients who underwent neurosurgery between April and July 2019 and assessed the impact of ICG on ORi™. We excluded patients who did not use ICG or who were not examined for ORi™. The dose and timing of ICG administration were determined by a neurosurgeon. The changes in ORi™ were measured for up to 30 min.
Results
We analyzed ten patients and found that the ORi™ increased to 1.00 in all of them. The median time for ORi ™ to rise to 1.00 after ICG administration was 2 min (range 1–4). After rising to 1.00, ORi ™ decreased and took 27 min to return to the pre-dose value.
Conclusion
It is important to consider the initial rapid increase and subsequent slow decrease in ORi™ when using ICG during surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oxygen reserve index (ORi™) is a measurement parameter that noninvasively evaluates the oxygenation state at a continuous range of 100–200 mmHg arterial partial pressure of oxygen (PaO2) [1].
Conventional percutaneous oxygen saturation (SpO2) cannot be used to evaluate a state where the oxygen saturation of hemoglobin is ≥ 100%; a 100% SpO2 value is shown when PaO2 exceeds approximately 100 mmHg. Consequently, the ORi™ has been recently attracting attention as a management parameter that avoids hypoxia and hyperoxia when PaO2 exceeds 200 mmHg, since it allows the evaluation of oxygenation at 100–200 mmHg PaO2. It has been reported that the evaluation of the oxygenation reserve capacity at the time of rapid introduction using ORi™ allows earlier evaluation and correction of oxygenation deterioration than the evaluation using SpO2 [2].
Indocyanine green (ICG) is a widely used drug in liver function evaluation. There has been a recent increase in the number of reports on the effectiveness of using ICG during surgery. In cerebrovascular surgery, it is used for angiographic purposes. In addition, there has been an increase in the applications of ICG, such as evaluation of postoperative liver function during hepatectomy [3] and evaluation of blood flow in grafts transplanted at surgery [4]. Infusion of dyes such as ICG and indigo carmine has been known to result in abnormal pulse oximeter SpO2 values [5]. Isosu et al. [6] reported that indigo carmine, which is used for gynecological urinary tract evaluation, reduces ORi™. However, the impact of ICG on ORi™ has not been previously reported.
Hence, we analyzed the ORi™ measured during surgery of patients administered with ICG.
Methods
This retrospective study was approved by the Higashihiroshima Medical Center Ethics Committee (2019–2021). We enrolled patients who had undergone a scheduled neurosurgery between April, when ORi™ measurements began, and July 2019. Informed consent was obtained in the form of an opt-out option advertised at the Higashihiroshima Medical Center. We excluded patients who did not provide consent, had undergone neurosurgery without ORi™ measurement, or were not administered ICG. Timing, dosage, and other vital data involving the use of ICG were extracted from the ORSYS™ (Philips Electronics Japan, Tokyo, Japan) automated anesthesia records. Indication and time of ICG were determined by a neurosurgeon, and ICG was administrated intravenously via a peripheral venous catheter in the upper limb. The survey items included age, sex, height, weight, surgical procedure, Radical-7® (Masimo Corp., Irvine, CA, USA) ORi™, and Radical-7® SpO2. Radical-7® version 1.5.8.4i was used and there was no version update during the investigation period. The sensor used for measuring ORi™ was an RD rainbow Lite SET™-1 Adt (Masimo Corp., Irvine, CA, USA). We recorded ORi™ in all patients immediately before the administration of ICG at 1-min intervals until a maximum of 30 min after administration.
The changes in ORi™ were evaluated by comparing the pre-administration value with the value at each time point after administration. We performed between-group comparisons using a paired t test (two-sided) by spreadsheet calculations (Microsoft Excel 2019, Microsoft Corp., Redmond, WA, USA) with statistical significance set at P < 0.05.
Results
Among the 27 patients who underwent neurosurgery under general anesthesia during the study period, ten were included in the final analysis. Seventeen patients were excluded from the study because they did not receive ICG during neurosurgery. Table 1 shows the patient background, surgical procedure, and the dose of ICG in the enrolled patients. ICG was administered for angiographic purposes after revascularization or after clipping. The ICG dose ranged from 2.5 to 12.5 mg and was administered at the discretion of the neurosurgeon. Patient no. 8 underwent measurement for 26 min after administration, while the remaining nine patients underwent measurement for 30 min after administration.
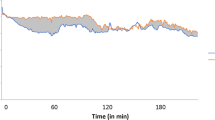
General anesthesia was induced and maintained using propofol, remifentanil, fentanyl, and rocuronium. After anesthesia induction, fresh gas flow was changed to 1 L/min of oxygen and 2 L/min of air in all cases and maintained during surgery. The respiratory minute volume was adjusted appropriately such that the end-tidal CO2 partial pressure was not less than 30 mmHg. Ventilation was volume-controlled with a positive end-expiratory pressure of less than 5 mmHg. In two patients, the respiratory minute volume was changed within 30 min of ICG administration, but SpO2 value did not change near the respiratory minute volume change. No significant changes were observed in the hemodynamic or respiratory values after ICG administration. The ORi™ increased to 1.00 in all patients after ICG administration, at a median time of 2 min (range 1–4 min), and then gradually decreased in the subsequent 26 min; the post-administration value was significantly higher than the pre-administration value (Figs. 1 and 2).
Differences in the pre- and post-administration oxygen reserve index (ORi™) values. Asterisks at each time indicates a significant difference between the pre- and post-administration values. ORi™ increased significantly 2 min after indocyanine green administration and then gradually decreased. For 26 min after indocyanine green administration, the post-administration values were significantly higher than the pre-administration values
Patient no. 10 presented a decrease in the SpO2 to 97% at 2 min after ICG administration. Patient no. 4 presented a decrease in the SpO2 to 94% at 17 min after ICG administration; however, the SpO2 of patient no. 4 increased to 99% at 16 min after ICG administration and was stabilized to 99% after 18 min. Therefore, we assumed that these changes were unrelated to ICG administration.
Discussion
In this study, we observed that the ORi™ rapidly increased to 1.00 after ICG administration in all patients, including patient no. 8 in whom the dose was the least of this study, regardless of the dose, and then, gradually decreased. ICG is not chemically altered in the body and has a half-life of 3.4 min with its level exponentially decreasing for up to 15 min [7, 8]. The observed changes in the ORi™ were consistent with the pharmacokinetics of ICG, indicating that the ORi™ is strongly influenced by ICG. Studies have shown that ORi™ estimates the oxygen saturation in venous blood and that an increase in the ORi™ reflects the apparent increase in the oxygen saturation in venous blood [9]. Furthermore, a study has reported that ICG administration increases the apparent regional cerebral oxygen saturation (rSO2) [10]. The rSO2 reading is used for assessing cerebral blood flow, and reflects both arterial and venous blood oxygen saturation. In many cases, as in the current study, arterial blood oxygen saturation is close to 100%, with little room to increase. Therefore, the increase in the ORi™ reading in the current study reflected an apparent increase in the venous blood oxygen saturation upon ICG administration.
Intravenous administration of ICG has been reported to decrease the pulse oximeter values [11]. In contrast, we observed only two cases with decreases in the SpO2 value after ICG administration. A typical pulse oximeter compares the absorbance of red light (near 650 nm wavelength) and infrared light (near 900 nm wavelength) [12]. ICG, like deoxyhemoglobin, has a lower absorbance at 900 nm than at 650 nm. Therefore, ICG administration is suggested to increase deoxyhemoglobin, leading to a decrease in the SpO2 value. However, Radical-7® uses multi-wavelength light sources to measure oxygen saturation; therefore, it can detect the peak absorbance of ICG at wavelengths near 800 nm [13, 14]. Presumably, Radical-7® discerns ICG through the absorbance ratio, and therefore, SpO2 reduction caused by ICG absorbance occurred less in this study.
ICG administration is accompanied by a simultaneously increased oxygenation for ORi™ and an unchanged or decreased oxygenation for SpO2. In our study, there were no major changes in the respiratory settings and circulatory dynamics after ICG administration. Therefore, there were probably no changes in arterial and venous blood oxygen saturations indicating an effect of ICG. Considering the undisclosed nature of the ORi™ algorithm details, it is impossible to accurately determine the phenomenon that occurs after ICG administration. However, when using Radical-7®, it is necessary to know that in indigo carmine, which has an absorbance peak below red light in Radical-7®, there is a decrease in both SpO2 and ORi™; however, Radical-7® causes opposite changes in SpO2 and ORi™ ofICG.
This study was limited to recordings obtained at 1-min intervals. The peak time of ORi ™ in this study ranged from 1 to 4 min and the wide interval between the recordings presumably caused this wide error. Furthermore, the infusion rate of ICG and the monitoring site for ORi ™ were unknown. Although, ICG was infused using a single shot manner and ORi™ was equipped at any finger of a hand, these factors may have contributed to small errors in the peak time of ORi ™.
In conclusion, we found a rapid increase in the ORi™ approximately 2 min after ICG administration, followed by a gradual decrease. It took 27 min for the values to return to the pre-administration values. Contrastingly, the SpO2 was almost unchanged or slightly decreased. When using Radical-7® as an index of arterial blood oxygenation, it should be noted that ICG administration causes opposite changes in the ORi™ and SpO2.
References
Scheeren TWL, Belda FJ, Perel A. The oxygen reserve index (ORI): a new tool to monitor oxygen therapy. J Clin Monit Comput. 2018;32:379–89.
Yoshida K, Isosu T, Noji Y, Hasegawa M, Iseki Y, Imaizumi T, Sanbe N, Obara S, Murakawa M. Usefulness of oxygen reserve index (ORiTM), a new parameter of oxygenation reserve potential, for rapid sequence induction of general anesthesia. J Clin Monit Comput. 2018;32:687–91.
Wang L, Xie L, Zhang N, Zhu W, Zhou J, Pan Q, Mao A, Lin Z, Wang L, Zhao Y. Predictive value of intraoperative indocyanine green clearance measurement on postoperative liver function after anatomic major liver resection. J Gastrointest Surg. 2019. https://doi.org/10.1007/s11605-019-04262-5.
Malagon-Lopez P, Caeeasco-Lopez C, Garcia-Senosiain O, Del-Rio M, Priego D, Julian-lbanez JF, Higueras-Surie C. When to assess the DIEP flap perfusion by intraoperative indocyanine green angiography in breast reconstruction? Breast. 2019. https://doi.org/10.1016/j.breast.2019.07.009.
Schwller MS, Unger RJ, Kelner MJ. Effects of intravenously administered dyes on pulse oximetry readings. Anesthesiology. 1986;65:550–2.
Isosu T, Yoshida K, Oishi R, Imaizumi T, Iseki Y, Sanbe N, Ikegami Y, Obara S, Kurosawa S, Murakawa M. Effects of indigo carmine intravenous injection on oxygen reserve index (ORiTM) measurement. J Clin Monit Comput. 2018;32:693–7.
Reinhart MB, Huntington CR, Blair LJ, Heniford BT, Augenstein VA. Indocyanine green:historical context, current applications, and future consideration. Surg Innov. 2016;23:166–75.
Weiler M, Kassis T, Dixon JB. Sensitivity analysis of near-infrared functional lymphatic imaging. J Biomed Opt. 2012;17:066019. https://doi.org/10.1117/1.JBO.17.6.066019.
Applegate RL 2nd, Dorotta IL, Wells B, Juma D, Applegate PM. The relationship between oxygen reserve index and arterial partial pressure of oxygen during surgery. Anesth Analg. 2016;123:623–33.
Vinary B, Chidananda Swarmy MN, Sunil Kumar HR, Satish R. Effect of indocyanine green dye administration on cerebral oxygen saturation. Indian J Anaesth. 2016;60:64–5.
Ediriwickrema LS, Francis JH, Arslan-Carlon V, Dalecki PH, Brodie SE, Marr BP, Abramson DH. Intravenous injection of indocyanine green results in an artificial transient desaturation by pulse oximetry. Retin cases Brief Rep. 2015;3:252–5.
Jubran A. Pulse oximetry. Crit Care. 2015;9:272.
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
The authors have no sources of funding to declare for this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kondo, H., Nakamura, R., Kobatake, A. et al. Effects of intravenous injection of indocyanine green on the oxygen reserve index (ORi™). J Anesth 34, 338–341 (2020). https://doi.org/10.1007/s00540-020-02746-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-020-02746-2