Abstract
Purpose
This prospective randomized study compared the coracoid and retroclavicular approaches to ultrasound-guided infraclavicular brachial plexus block (IBPB) in terms of needle tip and shaft visibility and quality of block. We hypothesized that the retroclavicular approach would increase needle tip and shaft visibility and decrease the number of needle passes compared to the coracoid approach.
Methods
A total of 100 adult patients who received IBPB block for upper limb surgery were randomized into two groups: a coracoid approach group (group C) and a retroclavicular approach group (group R). In group C, the needle was inserted 2 cm medial and 2 cm inferior to the coracoid process and directed from ventral to dorsal. In group R, the needle insertion point was posterior to the clavicle and the needle was advanced from cephalad to caudal. All ultrasound images were digitally stored for analysis. The primary aim of the present study was to compare needle tip and shaft visibility between the coracoid approach and retroclavicular approach in patients undergoing upper limb surgery. The secondary aim was to investigate differences between the two groups in the number of needle passes, sensory and motor block success rates, surgical success rate, block performance time, block performance-related pain, patient satisfaction, use of supplemental local anesthetic and analgesic, and complications.
Results
Needle tip visibility and needle shaft visibility were significantly better in group R (p = 0.040, p = 0.032, respectively). Block performance time and anesthesia-related time were significantly shorter in group R (p = 0.022, p = 0.038, respectively). Number of needle passes was significantly lower in group R (p = 0.044). Paresthesia during block performance was significantly higher in group C (p = 0.045). There were no statistically significant differences between the two groups in terms of sensory or motor block success, surgical success, block-related pain, and patient satisfaction.
Conclusion
The retroclavicular approach is associated with better needle tip and shaft visibility, reduced performance time and anesthesia-related time, less paresthesia during block performance, and fewer needle passes than the coracoid approach.
Trıal registry number
Clinicaltrials.gov (no. NCT02673086).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infraclavicular brachial plexus block (IBPB) was described by Bazy in 1914 and modified by Raj in 1973 [1]. Since then, several approaches to IBPB have been described, using various surface landmarks, needle insertions, and recommended needle directions. The three most common approaches are the coracoid approach [2], lateral sagittal approach [3], and vertical approach [4]. Others include the parasagittal [5] and pericoracoid approaches [6]. Among all of these approaches, the coracoid approach is the most widely used [7].
In the coracoid approach, the needle is inserted 2 cm medial and 2 cm inferior to the coracoid process and directed from ventral to dorsal. The plexus is encountered at an average depth of 4.5 cm but may be as deep as 7.5 cm [8]. In the retroclavicular approach, the needle insertion point is posterior to the clavicle instead of inferior to it. The needle is inserted immediately above the clavicle in the space between the coracoid process and the clavicle, and advanced from cephalad to caudal [9].
The ultrasound (US)-guided posterior approach to IBPB was first described by Hebbard and Royse [10]. Charbonneau et al. reported that retroclavicular block was a quick, safe, and reliable alternative for distal arm block [9]. Unlike the coracoid approach, the needle insertion point is posterior to the clavicle in the retroclavicular approach. Therefore, the needle shaft is aligned perpendicular to the ultrasound beam, thereby allowing better needle tip and shaft visibility.
The aim of the study reported in the present paper was to test the hypothesis that the retroclavicular approach increases the visibility of both the needle tip and shaft compared with the coracoid approach (and that this improved visibility influences the course and result of the block). The primary endpoint of this study was the difference in visibility and the secondary endpoints were the number of needle passes, block performance time, procedure-related pain, sensory and motor blockade, use of additional analgesics, complications, and patient satisfaction.
Methods
This study was carried out in accordance with the Declaration of Helsinki, approved by the Ethics Committee of the Training and Research Hospital, Antalya, Turkey (approval number 70/13), and registered in the Clinicaltrials.gov clinical trials registry (no. NCT02673086). Written informed consent was obtained from all patients before they were included in the study. Patients who received IBPB for elective elbow, forearm, wrist, or hand surgery were randomized by means of computer-generated order randomization into two groups: a retroclavicular approach group (group R) and a coracoid approach group (group C). Exclusion criteria included patients <18 years old, body mass index (BMI) <20 or >35 kg/m2, inability to provide written informed consent, refusal of regional anesthesia, or contraindication for regional anesthesia.
On arrival at the operating room, a peripheral venous line was established. Standard monitoring including noninvasive blood pressure, five-lead electrocardiography, and pulse oximetry was used. Patients were premedicated with a 0.05 mg/kg intravenous bolus of midazolam 5 min before the block. All IBPBs were performed by two independent anesthesiologists who were blinded to the study and experienced in these techniques. A 21-gauge 100 mm needle (Stimuplex® A, B. Braun Melsungen AG, Germany) was used for the blocks. Patients were positioned supine, the arm was adducted, and the head was rotated to the contralateral side of the blockade. Before all of the blocks, the skin was cleaned with chlorhexidine and the skin and subcutaneous tissue were anesthetized with 2–4 ml of 1% lidocaine. A Mindray (Shenzhen, China) DC-T6 ultrasound machine with a 10-MHz linear probe with a sterile cover was used to perform the blocks.
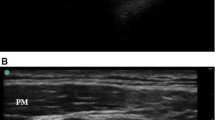
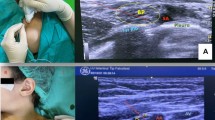
The coracoid approach to the IBPB was performed according to a previously described technique [11]. The US probe was placed parasagittally just medial to the coracoid process and caudal from the clavicle (Fig. 1a). The needle was inserted cephalad to the ultrasound probe using an in-plane technique and advanced in a caudal direction toward the posterior aspect of the axillary artery, in the vicinity of the posterior cord of the brachial plexus (Fig. 1b). The retroclavicular approach to the IBPB was performed as described by Charbonneau et al. [9]. The US probe was placed parasagittally just medial to the coracoid process and caudal from the clavicle. The needle insertion point was located in the supraclavicular fossa, just medial to the shoulder at a point sufficiently posterior to the clavicle and medial to the trapezius muscle insertion point on the clavicle (Fig. 2a). The needle was inserted immediately above the clavicle in the space between the coracoid process and the clavicle and advanced from cephalad to caudal (Fig. 2b).
The blocks were performed by administering 25 ml bupivacaine 0.5% at the 6-o’clock position with respect to the axillary artery with the aim of providing a U-shaped spread around the vessel. The procedures were recorded with a digital camera recorder so that they could be reviewed after study completion by two independent anesthesiologists who were blinded to the study. Needle tip visibility was evaluated using a five-point Likert scale (1: very poor, 2: poor, 3: fair, 4: good, 5: very good). The total block performance time (from skin puncture to needle removal) was recorded. Shaft visibility was scored as follows: (1) none of the shaft was visualized; (2) only a small segment of the shaft was visualized; (3) less than half of the shaft was visualized; (4) almost all of the shaft was visualized; (5) the entire shaft was visualized.
Sensory assessments were performed every 5 min after needle removal for 30 min in the regions of the radial, median, ulnar, and musculocutaneous nerves of the forearm based on a three-point scale with a cold test [0: normal sensation, 1: analgesia (patient can feel touch but not cold), 2: anesthesia (patient cannot feel touch)] carried out by a blinded observer. Motor block was evaluated for flexion of the elbow, opposition of the thumb, abduction of the thumb, and adduction of the thumb based on a three-point scale (0: no block, 1: paresis, 2: paralysis). The maximal composite score was 16 points. Block success was defined as a composite score of 14 at 30 min. The number of needle passes and the block performance time were recorded by an independent observer. Any retraction of a least 10 mm and re-advancement of the needle was counted as an additional needle pass. Block performance time was defined as the time from the first insertion of the blocking needle to its removal. Onset time was defined as the time required for a composite score of 14 points. If the composite score was >14 after 30 min, the patient was transferred to the operating room to begin the surgery, and the onset time was not recorded in this case. If the composite score was less than 14/16, additional intravenous analgesic or general anesthesia was administered [12]. The total anesthesia-related time was the sum of the performance and onset times.
In the case of pain during surgery, the patient was administered intravenous analgesic, general anesthesia, rescue blocks, or local anesthetic infiltration. Surgical success was defined as no requirement for additional local anesthetic, intravenous analgesic, rescue blocks, or general anesthesia during the surgery. The use of supplementary local anesthetic and the use of intravenous analgesic were recorded by the blinded observer. Block performance-related pain was evaluated with a verbal rating scale (VRS) score after the removal of the needle. The VRS was scored from 0 (no pain) to 10 (the worst pain imaginable) to denote increasing pain intensity.
Postoperatively, the patients were asked to grade their satisfaction about the surgery under the block (1: very poor, 2: poor, 3: average, 4: satisfied, 5: very satisfied). Complications such as needle-induced paresthesia, vascular puncture, Horner syndrome, dyspnea, and symptoms of local anesthetic toxicity were recorded by a blinded observer. To rule out pneumothorax, all the patients studied were evaluated by performing bedside US examinations of the chest before discharge.
Study power was based on data from the needle tip visibility study by Jandzinski et al. [13], using an SD of 0.9 on a five-point visibility scale with a difference of one point being clinically significant. A calculated sample size of 39 patients per group was required to provide a statistical power of 0.80 and an alpha of 0.05. Therefore, 50 patients per group were included to replace any dropouts. Statistical analysis was performed using the SPSS version 21 statistical software (SPSS Inc., Chicago, IL, USA). All numerical data were tested for a normal distribution with the Kolmogorov–Smirnov test. Continuous variables are presented as the mean ± standard deviation (SD) and ordinal variables are presented as the median and range. Categorical variables are presented as the number (n) and the percentage (%) of patients. Differences between mean values for normally distributed variables were compared using Student’s t test. Non-normally distributed variables and ordinal variables were compared with the Mann–Whitney U test and the Wilcoxon rank sum test. The chi-squared test and Fisher’s exact test were used for categorical data where appropriate. A value of p < 0.05 was considered to indicate statistical significance.
Results
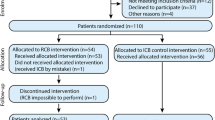
A total of 130 patients were assessed for study eligibility, 30 of whom did not undergo randomization. Eighteen patients did not meet the inclusion criteria for various reasons, seven patients declined to participate in the study, and five patients declined regional anesthesia. Therefore, 100 patients were enrolled, and all were successfully followed up according to the study protocol (Fig. 3).
There was no difference in age, gender, height, weight, body mass index, and American Society of Anesthesiologists (ASA) physical status between the two groups (Table 1).
The results for the primary endpoints are shown in Table 2. Significantly better needle tip visibility was obtained in group R than in group C (p = 0.040). Needle shaft visibility was significantly better in group R than in group C (p = 0.032). Block performance time was statistically shorter in group R (2.8 ± 1.6 vs 6.2 ± 2.2 min; p = 0.022). Similarly, the anesthesia-related time was statistically shorter in group R (17.9 ± 2.1 vs 23.9 ± 2.2 min; p = 0.038). The number of needle passes was significantly lower in group R (p = 0.044). Two patients in group R and five patients in group C felt mild pain after skin incision, so additional intravenous analgesic and 3–5 ml of additional 1% lidocaine infiltration was administered by the surgeon to these patients. Due to the level of pain felt, one patient in group R could not tolerate the surgery despite additional analgesic and local anesthetic, and general anesthesia was necessary. No statistically significant differences were observed between the two groups in sensory block success, motor block success, surgical success, onset time, block-related pain, and patient satisfaction (Table 2).
The times taken to develop complete sensory and motor block in the first 30 min after block performance are shown in Table 3. No differences in these times were found between groups. The spread of sensory block to the four terminal nerves after 30 min is shown in Table 4. The time required for the spread of sensory block to the musculocutaneous nerve in both groups was significantly longer than the times required for the spread of sensory block to the other nerves at 10 min.
There was no significant difference in block-related pain between the two groups based on VRS scores (p = 0.574). Paresthesia during block performance was significantly higher in group C (p = 0.045). No persistent paresthesia was observed in either group. Vascular puncture was observed in one patient in group C, but this situation did not affect block success because the needle was retracted slightly after vascular puncture and repositioned. There were no other adverse effects or complications in either group, and no neurological deficit was diagnosed in any patient 24–36 h postoperatively (Table 2).
Discussion
This randomized prospective study demonstrated that needle tip and shaft visibility was significantly better with the retroclavicular approach to IBPB than with the coracoid approach. Furthermore, block and anesthesia-related times were significantly shorter and the number of needle passes and block-related paresthesia were significantly lower with the retroclavicular approach, though the sensory and motor block success rates were similar in both approaches.
The inability to accurately keep track of the needle tip may be a contributing factor to procedural complications. In the retroclavicular approach, the needle path lies in a plane parallel to the probe and the needle shaft is aligned perpendicular to the ultrasound beam (Fig. 4). This increases needle tip and shaft visibility. Better needle visibility provides guaranteed needle orientation, thereby avoiding injury to several critical neurovascular structures [14]. In their noncomparative study, Charbonneau et al. [9] reported that needle tip and shaft visibility was good in the retroclavicular approach. The current study is the first clinical study to compare the retroclavicular approach to the coracoid approach for US-guided IBPB. In the current study, needle tip and shaft visibility scores were significantly higher in group R than in group C. This is concordant with the results of Charbonneau et al. [19] for the retroclavicular approach, and significantly better needle tip and shaft visibility contributed to significant differences between the groups in block performance time, anesthesia-related time, and number of needle passes.
The cephalic vein and acromial branch of the thoracoacromial artery are exposed to needle trauma during the coracoid approach because these structures are along the needle path [15]. The rate of paresthesia during block performance has been reported as 17.5% by Frederiksen et al. [16] and 7.5% by Tran et al. [17]. In the current study, similar rates were found, with 12% of patients experiencing paresthesia during the coracoid approach. The retroclavicular approach keeps the needle well away from these neurovascular structures. Therefore, rates of needle trauma and paresthesia during block performance may be lower in the retroclavicular approach. In the present study, paresthesia during block performance was less common in group R. Charbonneau et al. [9] reported that in the retroclavicular approach, the needle path avoids the puncture of the pectoralis major and minor, which results in less pain during the procedure. The procedure-related discomfort in that study was recorded as a VRS score of 1.9 ± 1.2. These findings were similar to the results of the current study. Both the retroclavicular and coracoid approaches were also found to be similar in terms of block-related pain. This similarity may be related to local anesthetic use or the sedation provided before block performance in both groups. Due to the less painful procedure, the patient satisfaction scores were high in both groups in the present study.
Abduction of the upper arm at 90° with external rotation of the shoulder decreases the distance between the skin and the brachial plexus, so this position is often preferred to the coracoid approach for IBPB [18]. Although abduction of the arm reduces the depth of the brachial plexus, it does not change the position of the axillary artery relative to the coracoid process or the pleura [19]. Retroclavicular block is performed without the need for abduction of the upper arm. Adduction of the upper arm is advantageous in patients with limited movement or pain of the arm or shoulder. In the current study, the upper arms were positioned in adduction in both groups.
In the present study, anesthesia of the musculocutaneous nerve (MCN) was observed to be delayed in both groups compared to other nerves. The MCN frequently branches from the lateral cord more proximally [7]. Pianezza et al. reported that at least 35% of MCN emerged from the lateral cord proximally to the coracoid process [20]. The delayed anesthesia of MCN may be due to the early branching of this nerve from the lateral cord and because it is difficult for the local anesthetic solution to reach it.
Sutton et al. [21] reported that if the lateral cord was separated from the artery by a small distance, the local anesthetic failed to reach the cord. They proposed that the MCN anesthesia lag is due to the tendency to push the needle more caudally under the axillary artery in the retroclavicular approach. They reported that if the needle was withdrawn slightly and redirected anteriorly to deposit a small aliquot of local anesthetic at the lateral cord, the lag time for a complete block was eliminated. The MCN anesthesia lag may be due to the reasons reported by Sutton et al.
The current study does, however, have several limitations. Firstly, although the sample size was sufficient to evaluate needle visibility, it may not have been sufficient to detect rarer effects and complications of the procedure such as vascular puncture, Horner syndrome, pneumothorax, or postoperative neurological deficits. Secondly, the BMIs of the patients in the study were normal. Different results could be obtained with a study sample of obese patients.
In conclusion, the results of this study demonstrated that the retroclavicular approach for US-guided IBPB is associated with better needle tip and shaft visibility, shorter performance and anesthesia-related times, fewer needle passes, and lower paresthesia during block performance than the coracoid approach. On the other hand, the retroclavicular approach was similar to the coracoid approach in terms of success rate and patient comfort.
References
Raj PP, Montgomery SJ, Nettles D, Jenkins MT. Infraclavicular brachial plexus block—a new approach. Anesth Analg. 1973;52:897–904.
Desroches J. The infraclavicular brachial plexus block by the coracoid approach is clinically effective: an observational study of 150 patients. Can J Anaesth. 2003;50:253–7.
Gürkan Y, Hoşten T, Solak M, Toker K. Lateral sagittal infraclavicular block: clinical experience in 380 patients. Acta Anaesthesiol Scand. 2008;52:262–6.
Mosaffa F, Gharaei B, Rafeeyan M, Gachkar L. Comparing vertical and coracoid approaches for infraclavicular block in orthopedic surgery of the forearm and hand. J Clin Anesth. 2012;24:196–200.
Wilson JL, Brown DL, Wong GY, Ehman RL, Cahill DR. Infraclavicular brachial plexus block: parasagittal anatomy important to the coracoid technique. Anesth Analg. 1998;87:870–3.
Bocquet JD, N’takpe N, Draganescu C, Ridarch A, Jullien YR. The coracoid block: demonstration of a simple approach using the pectoralis minor as landmark. Can J Anaesth. 2005;52:1040–6.
Neal JM, Gerancher JC, Hebl JR, Ilfeld BM, McCartney CJ, Franco CD, Hogan QH. Upper extremity regional anesthesia: essentials of our current understanding, 2008. Reg Anesth Pain Med. 2009;34:134–70.
Macfarlane A, Anderson K. Infraclavicular brachial plexus block. Continuing education in anaesthesia. Crit Care Pain. 2009;9:139–43.
Charbonneau J, Fréchette Y, Sansoucy Y, Echave P. The ultrasound-guided retroclavicular block: a prospective feasibility study. Reg Anesth Pain Med. 2015;40:605–9.
Hebbard P, Royse C. Ultrasound guided posterior approach to the infraclavicular brachial plexus. Anaesthesia. 2007;62:539.
Taboada M, Rodriquez J, Amor M. Is ultrasound guidance superior to conventional nerve stimulation for coracoid infraclavicular brachial plexus block? Reg Anesth Pain Med. 2009;34:357–60.
Yazer MS, Finlayson RJ, Tran DQ. A randomized comparison between infraclavicular block and targeted intracluster injection supraclavicular block. Reg Anesth Pain Med. 2015;40:11–5.
Jandzinski DI, Carson N, Davis D, Rubens DJ, Voci SL, Gottlieb RH. Treated needles: do they facilitate sonographically guided biopsies? J Ultrasound Med. 2003;22:1233–7.
Schwemmer U, Geppert T, Steinfeldt T, Wunder C. Improvement of sonographic visualization of cannula needle tips by alignment of the needle lumen: in vitro investigation of established needle tip forms. Anaesthesist (in press).
Soeding P, Eizenberg N. Review article: anatomical considerations for ultrasound guidance for regional anesthesia of the neck and upper limb. Can J Anaesth. 2009;56:518–33.
Frederiksen BS, Koscielniak-Nielsen ZJ, Jacobsen RB, Rasmussen H, Hesselbjerg L. Procedural pain of an ultrasound-guided brachial plexus block: a comparison of axillary and infraclavicular approaches. Acta Anaesthesiol Scand. 2010;54:408–13.
Tran DQ, Russo G, Muñoz L, Zaouter C, Finlayson RJ. A prospective, randomized comparison between ultrasound-guided supraclavicular, infraclavicular, and axillary brachial plexus blocks. Reg Anesth Pain Med. 2009;34:366–71.
Wang FY, Wu SH, Lu IC, Hsu HT, Soo LY, Tang CS, Chu KS. Ultrasonographic examination to search out the optimal upper arm position for coracoid approach to infraclavicular brachial plexus block—a volunteer study. Acta Anaesthesiol Taiwan. 2007;45:15–20.
Ruíz A, Sala X, Bargalló X, Hurtado P, Arguis MJ, Carrera A. The influence of arm abduction on the anatomic relations of infraclavicular brachial plexus: an ultrasound study. Anesth Analg. 2009;108:364–6.
Pianezza A, Salces y Nedeo A, Chaynes P, Bickler PE, Minville V. The emergence level of the musculocutaneous nerve from the brachial plexus: implications for infraclavicular nerve blocks. Anesth Analg. 2012;114:1131–3.
Sutton EM, Bullock WM, Gadsden J. The retroclavicular brachial plexus block: additional advantages. Reg Anesth Pain Med. 2015;40:733–4.
Author information
Authors and Affiliations
Contributions
N.K.O. and A.S.K. conceived and performed the study, analyzed the data, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors of this manuscript have any conflict of interest to declare.
Funding
Departmental resources were used for the study.
About this article
Cite this article
Kavrut Ozturk, N., Kavakli, A.S. Comparison of the coracoid and retroclavicular approaches for ultrasound-guided infraclavicular brachial plexus block. J Anesth 31, 572–578 (2017). https://doi.org/10.1007/s00540-017-2359-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-017-2359-6