Abstract
Purpose
To identify whether the presence of preoperative depression in patients with diabetes mellitus is a risk factor for postoperative cognitive dysfunction after coronary artery bypass graft (CABG) surgery.
Methods
Data from 90 patients with diabetes mellitus undergoing elective CABG were analyzed. Hemodynamic data (arterial and jugular venous blood gas values) were measured during cardiopulmonary bypass. Preoperatively, all patients were given the 21-item Beck depression inventory to identify the presence of depression. In addition, all patients underwent a battery of neurological and neuropsychological tests the day before surgery, 7 days after surgery, and 6 months after surgery.
Results
The rate of cognitive dysfunction was 50% at 7 days and 23% at 6 months after surgery. Age, hypertension, presence of depression, duration of SjvO2 ≤ 50%, ascending aorta atherosclerosis, diabetic retinopathy, and insulin therapy were independent predictors of short-term cognitive dysfunction, whereas HbA1c, diabetic retinopathy, insulin therapy, and presence of depression were independent predictors of long-term cognitive dysfunction.
Conclusions
We found that the presence of depression preoperatively is associated with short-term and long-term postoperative cognitive dysfunction in patients with diabetes mellitus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Central nervous system complications are a major cause of morbidity and mortality after cardiac surgery [1, 2]. In fact, neuropsychological dysfunction after cardiopulmonary bypass (CPB) has been reported in as many as 79% of patients during the early postoperative period [1–5].
However, it remains controversial as to whether the presence of preoperative depression is a risk factor for stroke and cognitive dysfunction after surgery [6–13]. Connerney et al. [11] reported that depression was an important independent risk factor for cardiac events after coronary artery bypass graft (CABG) surgery. Rymaszewska et al. [7] showed that high preoperative depression could be a predictor of postoperative psychological outcomes. In contrast, Monk et al. [9] showed no relationship between preoperative depression and postoperative cognitive decline after non-cardiac surgery. Because the prevalence of depression before CABG surgery is approximately 20–25%, it is clinically important to examine whether preoperative depression is related to postoperative cognitive decline after CABG surgery.
Over the past 20–30 years, the prevalence of diabetes mellitus has been rapidly rising throughout the world, the prediction being that it will increase by 200% over the next several decades. Inevitably, physicians will be confronted with an increasing number of patients with diabetes mellitus undergoing CABG surgery. It is widely known that diabetes mellitus is a risk factor for stroke and cognitive dysfunction after cardiac surgery [1, 3, 4]. We hypothesized that the presence of depression in patients with diabetes mellitus may be an independent risk factor for postoperative cognitive dysfunction after cardiac surgery. However, until now, no study has evaluated the relationship between preoperative depression and postoperative cognitive decline after CABG surgery in patients with diabetes mellitus.
The purpose of this study, therefore, was to identify whether preoperative depression is associated with the risk of postoperative cognitive dysfunction after CABG surgery in patients with diabetes mellitus.
Methods
Study population
Between May 1995 and May 2002, a total of 543 patients were scheduled for elective CABG at our institution. Of these, 103 patients were identified as having diabetes mellitus and 100 agreed to participate in this study. All procedures used in this study were approved by the Ethics Committee of our institution, and written informed consent was obtained from all patients.
A pre-existing diagnosis of diabetes mellitus was determined by chart-diagnosis of type-2 diabetes, and current hyperglycemic therapy, including diet, oral medications, or insulin therapy, was noted. Duration of disease was based on the time of initiation of medical therapy. Renal failure was defined by a creatinine concentration >2.0 mg/dL. Prior stroke was determined by a history of ischemic cerebrovascular disease with symptomatic neurological disorders and confirmed by preoperative brain computed tomography and magnetic resonance imaging (MRI). Presence of moderate to severe atherosclerotic lesions in the ascending aorta and carotid artery stenosis were diagnosed by preoperative ultrasonography and MRI.
Operative procedure
All patients received diazepam (10 mg orally) 1 h before anesthesia. Anesthesia was induced with 0.2 mg/kg midazolam, 20 μg/kg fentanyl, and 0.2 mg/kg vecuronium, and was followed by tracheal intubation. After induction of anesthesia, a pulmonary arterial catheter (VigilanceR, Swan-Ganz CCO Thermodilution Catheter; Baxter, Irvine, CA, USA) was inserted through the right internal jugular vein. For continuous monitoring of jugular venous oxygen saturation (SjvO2), a 4-Fr fiberoptic oximetry catheter (Dual-lumen oximetry catheterR; Baxter) was inserted into the right jugular bulb using a modified Seldinger technique. This catheter was connected to an analysis system (ExplorerTM system; Baxter) and calibrated in vivo by aspirating blood through the catheter. The position of the jugular bulb catheter was verified by standard radiography, and the catheter was repositioned until correctly situated (catheter tip cranial to the line extending from the atlanto-occipital joint space and caudal to the lower margin of the orbit). SjvO2 data were obtained and processed in a monitor-computer interface and displayed and stored every 5 s with an Apple computer (Apple Computer, Cupertino, CA, USA) [14].
The partial pressures of arterial and jugular venous blood gases were analyzed using Stat Profile UltimaR (Nova Biomedical, Boston, MA, USA) and CO-oximeter (OSM3, HemoximeterR, Radiometer, Copenhagen, Denmark). All patients were ventilated with oxygen (50%) and nitrogen (50%). End-tidal CO2 was monitored (UltimaR; Datex, Helsinki, Finland) and maintained between 35 and 40 mmHg. After anesthesia induction, 5–10 mg/kg/h propofol was infused using a syringe pump, this being continued until the patients arrived in the intensive-care unit. Muscular relaxation was achieved by intermittent administration of vecuronium. No volatile anesthetic was administered. The tympanic temperature was continuously monitored by Mon-a-ThermR (Mallinckrodt, St Louis, MO, USA). PaO2 was maintained at 150–250 mmHg during the study.
The CPB was primed with a crystalloid, non-glucose-containing solution, and a nonpulsatile pump flow rate of 2.2–2.5 L/min/m2 was maintained. A membrane oxygenator and a 40-μm arterial line filter were used, and PaCO2, uncorrected for temperature, was adjusted to normocapnic levels (35–40 mmHg) by varying fresh gas flow to the membrane oxygenator (alpha-stat regulation). Target tympanic temperatures were 34.5–36.0°C. The limit of maximum inflow temperature was set at 37.5°C. Hematocrit during CPB was maintained at >20% using blood transfusions as necessary. Phenylephrine infusions were used during CPB to maintain mean arterial pressure at 50–80 mmHg. Insulin infusions were used during CPB to maintain blood sugar between 100 and 200 mg/dL. Distal and proximal coronary anastomoses were performed during a single aortic cross-clamp.
Hemodynamic data and arterial and jugular venous blood gases were measured at different times, as previously described in detail [14, 15]. The presence or absence of carotid artery stenosis, defined as narrowing >50% [4], was confirmed by preoperative ultrasonography and MRI. The presence of atherosclerotic lesions in the ascending aorta, defined as atherosclerotic lesions ≥3.0 mm thick with diffuse irregularities, large, mobile or protruding atheromata, ulcerated plaques, and/or thrombi [4], was confirmed by intraoperative epiaortic ultrasonography.
Assessment of depression
Preoperatively, all patients were given the 21-item Beck depression inventory (BDI) to identify the presence of depression. This test was performed by trained specialists in the division of psychiatry of our institute. Patients with a score of 10 or greater on the inventory were identified as having symptoms of depression. Patients who were on antidepressant medication preoperatively continued their treatment postoperatively. Physicians ordered psychiatric consultations if they were deemed necessary.
Neurological and neuropsychological assessments
All patients underwent a battery of neurological and neuropsychological tests on the day before the operation, and these were repeated at 7 days and at 6 months after the surgery. These tests were administered by trained specialists and intra and interobserver validity was ensured. The neuropsychological portion of the study design followed the consensus statements on the assessment of central nervous system disorders after cardiac surgery [16]. Cognitive function was assessed using the following tests:
-
1
mini-mental state examination;
-
2
Rey auditory verbal learning test;
-
3
trail-making test (part A);
-
4
trail-making test (part B);
-
5
digit span forward; and
-
6
grooved pegboard.
Statistical analysis
All data are expressed as mean ± SD. To obtain an indication of overall outcome, significant impairment was defined as a decline from preoperative testing of more than 1 SD on more than 20% of test measures (at least 2 of 6). Univariate comparisons between subjects with and without cognitive impairment were performed using the unpaired t test or the chi-squared test for dichotomous variables and ANOVA for ordered categorical and continuous variables. The latter analyses were performed nonparametrically when regression residuals suggested that the model fit was poor. Stepwise logistic regression was used to choose a best set of independent predictors of both short-term and long-term cognitive impairment. Variables entered into the initial logistic models were those with a univariate probability value of P < 0.2. The final model included all variables with an independent significance level of P < 0.1. The quality of the fit of the logistic model was tested with the Hosmer and Lemeshow goodness-of-fit test.
Statistical significance was set at P < 0.05. All calculations were performed on Stat View 5.0 software packages (Abacus Concepts, Berkeley, CA, USA).
Results
We were unable to perform neuropsychological assessments for 3 patients 6 months after the surgery, because these patients died within 6 months of the operation. Three patients (excluding patients with major neurological defects) had incomplete cognitive data, and one patient with diabetes mellitus refused follow-up. Major neurological defects (defined as clinical evidence of focal cerebral infarction including hemiparesis, visual or gait disturbances, mental changes (confusion, agitation, inability to make contact with other people), or a combination of these) were observed in 3 patients after the operation. We thus performed neuropsychological assessments on 90 patients with diabetes mellitus. The demographic data of these patients are shown in Table 1.
Twenty-eight patients were diagnosed with depression in the pre-operative period. All patients received anti-depressants, for example selective serotonin reuptake inhibitors (SSRI), serotonin noradrenalin reuptake inhibitors (SNRI), and tricyclic or tetracyclic antidepressants. Other mental diseases, for example schizophrenia, were not found in the 28 depressed patients. None of the depressed patients were under specific psychiatric care.
The raw neurocognitive test scores at baseline, 7 days, and 6 months after the surgery are shown in Table 2. There were no significant differences in mini-mental examination, trail-making test (part A) scores, or digit span forward between the four groups at baseline, 7 days, or 6 months after surgery. Trail-making test (part B) scores and grooved pegboard test scores at 7 days were longer compared with baseline, although these values returned to normal at 6 months after the surgery. Immediate and delayed recall test scores at 7 days were also prolonged compared with baseline, and these prolongations persisted for 6 months postoperatively.
The rate of cognitive dysfunction at 7 days and 6 months after surgery is shown in Table 3. The rate of cognitive dysfunction was 50% at 7 days and 23.3% at 6 months after surgery.
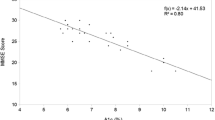
The characteristics of patients with diabetes mellitus with and without cognitive dysfunction at 7 days and 6 months postoperatively are shown in Table 4. There were significant differences in duration of SjvO2 ≤ 50%, presence of ascending aorta atherosclerosis, presence of diabetic retinopathy, HbA1c values, and presence of depression between patients with and without cognitive dysfunction at 7 days after surgery. There were significant differences in the presence of ascending aorta atherosclerosis and diabetic retinopathy, and HbA1c values and the presence of depression between patients with and without cognitive dysfunction at 6 months after the surgery.
The independent predictors of cognitive dysfunction at 7 days and 6 months after cardiac surgery are shown in Table 5. Age, hypertension, presence of depression, duration of SjvO2 ≤ 50%, ascending aorta atherosclerosis, diabetic retinopathy, and insulin therapy were independent predictors of short-term cognitive dysfunction, and HbA1c, diabetic retinopathy, insulin therapy, and presence of depression were independent predictors of long-term cognitive dysfunction. HbA1c, diabetic retinopathy, insulin therapy, and presence of depression were, therefore, associated with both short-term and long-term cognitive dysfunction in patients with diabetes mellitus.
Discussion
This study showed that preoperative depression was associated with both short-term and long-term postoperative cognitive dysfunction in patients with diabetes mellitus.
With the rapid increase in the prevalence of diabetes mellitus throughout the world, physicians are likely to be confronted with an increasing population of patients with diabetes mellitus undergoing CABG surgery. Many studies have shown that diabetes is one of the key factors associated with postoperative cognitive dysfunction in patients undergoing cardiac surgery [1–5]. In fact, Hogue et al. [17] and Newman et al. [3] showed that diabetes is an independent predictor of postoperative neurological complications. Hence, it is clinically important to be aware of factors related to postoperative neurological complications in patients with diabetes mellitus.
The prevalence of preoperative depression is approximately 20–25% before cardiac surgery [7]. In this study, the prevalence of cognitive dysfunction was 50% at 7 days and 23.3% at 6 months after surgery. Newman et al. [2] examined the incidence of neurocognitive decline preoperatively, before discharge, and 6 weeks, 6 months, and 5 years after CABG surgery in 261 patients who underwent CABG, and found that the incidence of cognitive decline was 53% at discharge, 36% at 6 weeks, 24% at 6 months, and 42% at 5 years, findings which are similar to our results. Some reports showed that depression could be associated with postoperative cardiac events. Connerney et al. [11] reported that major depressive disorders (risk ratio 2.3 (95% CI 1.17–4.56)) are associated with adverse cardiac events. Tully et al. [18] demonstrated the relationship between preoperative depression and the occurrence of atrial fibrillation after cardiac surgery. In contrast with the close relationship between the presence of depression and postoperative adverse cardiac events, there have been controversial reports regarding whether the presence of depression would be related to postoperative cognitive dysfunction in patients without diabetes mellitus [9, 10, 13, 19–21]. Paterniti et al. [6] reported that depressive symptoms predicted cognitive decline in an elderly, non-surgical population of 1003 individuals with good cognitive function at recruitment. Monk et al. [9] reported no relationship between preoperative depression and postoperative cognitive dysfunction after CABG surgery. McKhann et al. [10] demonstrated no relationship between depression (measured by the Center for Epidemiological Study of Depression Scale) and cognitive decline after CABG. In contrast, Rothenhäusler et al. [19] showed an association between major depression and short-term cognitive deficits. To date, there are no data examining whether depression is one of the preoperative predictors of postoperative cognitive dysfunction in patients with diabetes mellitus. This study indicates that the presence of depression is likely to be associated with postoperative cognitive dysfunction in patients with diabetes mellitus.
Although the reasons for the association between preoperative depression and the likelihood of postoperative cognitive dysfunction remain uncertain, several possible mechanisms for this could be considered. First, Paterniti et al. [6] speculated that depressive symptoms and cognitive decline are linked to modifications in activity of similar cerebral areas, and that chronic depression causes cognitive decline by release of adrenocorticotrophic hormone and the consequent secretion of glucocorticoids. Prolonged secretion of glucocorticoids may have deleterious effects, for example hippocampal atrophy. Another possibility is that different degrees of inflammatory cytokine responses after surgery may modulate postoperative cognitive dysfunction. In fact, overproduction of inflammatory cytokines has been reported after cardiac surgery [22]. Kudoh et al. [23] reported that the plasma cytokine response to surgical stress in chronically depressed patients is different from that in patients without depression.
Study limitations
There is concern whether neurocognitive tests can be entirely correctly performed in patients with depression. Andrew et al. [21] reported that changes in the mood state do not affect the results of postoperative neurocognitive tests.
We did not identify any relationship between postoperative cognitive dysfunction and ascending aorta atherosclerosis or carotid artery stenosis 6 months after surgery. Several reports have shown that ascending aorta atherosclerosis and carotid artery stenosis are major risk factors for cognitive dysfunction. As described in our previous report, the presence of these diseases may have affected our results.
In this study, we used propofol and fentanyl as anesthetics. Kudoh et al. [24] reported that use of small-dose ketamine could improve the postoperative state of depressed patients. Hence, it is possible that other anesthetic agents may modulate neurocognitive decline.
In this study, we found that trail-making test scores and grooved pegboard scores had recovered within 6 months, whereas Rey auditory verbal learning test scores had not recovered 6 months after surgery. Rudolph et al. [25] demonstrated that the impaired domain could affect the results of tests that predominantly measure other cognitive domains.
There are no standard analytic criteria for postoperative cognitive decline, because of the extensive heterogeneity of its definition, as described in detail by Rudolph et al. [25]. More extensive study is therefore necessary to identify the mechanisms, related factors and rate of postoperative cognitive decline after cardiac surgery.
We could not find a relationship between severity of diabetes and the presence of depression in this study (data not shown). However, it has been reported that the incidence of depression in diabetic patients is higher than that in non-diabetic patients [26], because alterations in monoamines (serotonin and noradrenaline), increases in cortisol by the hypothalamus–pituitary–adrenal axis, and trophic agents, for example the brain-derived neurotrophic factor, through glycogen synthase kinase-3, constitute some of the abnormalities documented in diabetic patients.
We showed a relationship between reduced SjvO2 during CPB and short-term, but not long-term, postoperative cognitive dysfunction. This finding is consistent with our previous reports [4, 14] and another report [27], although the reason for this is not yet proved. A possible mechanism for this is that global hypoperfusion during CPB, indicated by reduced SjvO2, might contribute to short-term postoperative cognitive dysfunction, and microembolism or other unknown factors might contribute to long-term postoperative cognitive dysfunction.
In conclusion, we found that preoperative depression is associated with short-term and long-term postoperative cognitive dysfunction in patients with diabetes mellitus.
References
Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, Aggarwal A, Marschall K, Graham SH, Ley C. Adverse cerebral outcomes after coronary bypass surgery. New Engl J Med. 1996;335:1857–63.
Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, Mark DB, Reves JG, Blumenthal JA. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. New Engl J Med. 2001;344:395–402.
Newman MF, Croughwell ND, Blumenthal JA, Lowry E, White WD, Spillane W, Davis RD Jr, Glower DD, Smith LR, Mahanna EP. Predictors of cognitive decline after cardiac operation. Ann Thorac Surg. 1995;59:1326–30.
Kadoi Y, Saito S, Fujita N, Goto F. Risk factors for cognitive dysfunction after coronary artery bypass graft surgery in patients with type 2 diabetes. J Thorac Cardiovasc Surg. 2005;129:576–83.
Miyoshi S, Morita T, Kadoi Y, Goto F. Analysis of the factors related to a decrease in jugular venous oxygen saturation in patients with diabetes mellitus during normothermic cardiopulmonary bypass. Surg Today. 2005;35:530–4.
Paterniti S, Verdier-Taillefer MH, Dufouil C, Alpérovitch A. Depressive symptoms and cognitive decline in elderly people. Longitudinal study. Br J Psychiatry. 2002;181:406–10.
Rymaszewska J, Kiejna A, Hadryś T. Depression and anxiety in coronary artery bypass grafting patients. Eur Psychiatry. 2003;18:155–60.
Stroobant N, Vingerhoets G. Depression, anxiety, and neuropsychological performance in coronary artery bypass graft patients: a follow-up study. Psychosomatics. 2008;49:326–31.
Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30.
McKhann GM, Borowicz LM, Goldsborough MA, Enger C, Selnes OA. Depression and cognitive decline after coronary artery bypass grafting. Lancet. 1997;349(9061):1282–4.
Connerney I, Shapiro PA, McLaughlin JS, Bagiella E, Sloan RP. Relation between depression after coronary artery bypass surgery and 12-month outcome: a prospective study. Lancet. 2001;358(9295):1766–71.
Detroyer E, Dobbels F, Verfaillie E, Meyfroidt G, Sergeant P, Milisen K. Is preoperative anxiety and depression associated with onset of delirium after cardiac surgery in older patients? A prospective cohort study. J Am Geriatr Soc. 2008;56:2278–84.
Tully PJ, Baker RA, Knight JL, Turnbull DA, Winefield HR. Neuropsychological function 5 years after cardiac surgery and the effect of psychological distress. Arch Clin Neuropsychol. 2009;24:741–51.
Kadoi Y, Saito S, Goto F, Fujita N. Decrease in jugular venous oxygen saturation during normothermic cardiopulmonary bypass predicts short-term postoperative neurologic dysfunction in elderly patients. J Am Coll Cardiol. 2001;38:1450–5.
Kadoi Y, Saito S, Kawahara F, Goto F, Owada R, Fujita N. Jugular venous bulb oxygen saturation in patients with preexisting diabetes mellitus or stroke during normothermic cardiopulmonary bypass. Anesthesiology. 2000;92:1324–9.
Murkin JM, Newman SP, Stump DA, Blumenthal JA. Statement of consensus on assessment of neurobehavioral outcomes after cardiac surgery. Ann Thorac Surg. 1995;59:1289–95.
Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation. 1999;100:642–7.
Tully PJ, Bennetts JS, Baker RA, McGavigan AD, Turnbull DA, Winefield HR. Anxiety, depression, and stress as risk factors for atrial fibrillation after cardiac surgery. Heart Lung. 2010 [Epub ahead of print].
Rothenhäusler HB, Grieser B, Nollert G, Reichart B, Schelling G, Kapfhammer HP. Psychiatric and psychosocial outcome of cardiac surgery with cardiopulmonary bypass: a prospective 12-month follow-up study. Gen Hosp Psychiatry. 2005;27:18–28.
Spezzaferri R, Modica M, Racca V, Ripamonti V, Tavanelli M, Brambilla G, Ferratini M. Psychological disorders after coronary artery by-pass surgery: a one-year prospective study. Monaldi Arch Chest Dis. 2000;72:200–5.
Andrew MJ, Baker RA, Kneebone AC, Knight JL. Mood state as a predictor of neuropsychological deficits following cardiac surgery. J Psychosom Res. 2000;48:537–46.
Kawamura T, Inada K, Nara N, Wakusawa R, Endo S. Influence of methylprednisolone on cytokine balance during cardiac surgery. Crit Care Med. 1999;27:545–8.
Kudoh A, Katagai H, Takazawa T. Plasma inflammatory cytokine response to surgical trauma in chronic depressed patients. Cytokine. 2001;13:104–8.
Kudoh A, Takahira Y, Katagai H, Takazawa T. Small-dose ketamine improves the postoperative state of depressed patients. Anesth Analg. 2002;95:114–8.
Rudolph JL, Schreiber KA, Culley DJ, McGlinchey RE, Crosby G, Levitsky S, Marcantono ER. Measurement of post-operative cognitive dysfunction after cardiac surgery: a systematic review. Acta Anaesthesiol Scand. 2010;54:663–77.
Castillo-Quan JI, Barrera-Buenfil DJ, Pérez-Osorio JM, Alvarez-Cervera FJ. Depression and diabetes: from epidemiology to neurobiology. Rev Neurol. 2010;51:347–59.
Shaaban Ali M, Harmer M, Latto I. Jugular bulb oximetry during cardiac surgery. Anaesthesia. 2000;56:24–37.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kadoi, Y., Kawauchi, C., Ide, M. et al. Preoperative depression is a risk factor for postoperative short-term and long-term cognitive dysfunction in patients with diabetes mellitus. J Anesth 25, 10–17 (2011). https://doi.org/10.1007/s00540-010-1072-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-010-1072-5