Abstract
Albumin, dextran, gelatin, and hydroxyethyl starch (HES) solutions are colloids that efficiently expand the circulating blood volume. The administration of colloids restores the intravascular volume with minimal risk of tissue edema in comparison with crystalloid solutions alone. However, colloids are always given for surgical and critically ill patients. The type of the colloid, volumes applied, aggressiveness of fluid resuscitation, and the volume status at the initial phase of administration determine their clinical responses. The outcome after fluid resuscitation with various colloids in critically ill patients seems to be comparable according to systematic reviews. A randomized, adequately powered clinical trial comparing modern nonprotein colloid to albumin is still lacking. Rapidly degradable HES solutions have good hemodynamic effects, and the risk of adverse renal and coagulation effects, as well as allergic reactions, is minimal. The current investigation has also shown the beneficial effect of HES solution (especially HES 130/0.4) on inflammatory response, postoperative nausea and vomiting, and postoperative outcome. The indication of colloids with an assessment of the degree of hypovolemia and safety profiles should thus be taken into consideration before colloid administration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to a systematic review, there is no evidence that any one colloid solution is more efficacious or safer than any other [1]. However, in addition to the type of colloid, the dosing and aggressiveness of colloid administration have important roles in clinical effect. Nonprotein colloids, i.e., hydroxyethyl starches (HES), dextrans, and gelatins, together with the natural colloid albumin, are all of biological origin. Slowly degradable HES is the nonprotein colloid most often used in the United States, whereas rapidly degradable HES is most often used in Europe. However, gelatin, dextran, or albumin solutions are also given to critically ill patients [2].
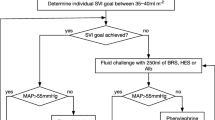
Perioperative fluid optimization has been under discussion during the past few years. Ideally, the goal of fluid administration is to allow adequate tissue perfusion without inducing interstitial edema. Based on the current concept of fluid challenge, circulating plasma deficit may be replaced with an iso-oncotic colloid solution [3, 4]. In critically ill patients or in patients undergoing surgery, colloids are usually given in combination with crystalloids. Therefore, a comparison of the administration of colloids with a crystalloid-based treatment regimen during hypovolemia may not be justified for assessing the possible effects of colloids on morbidity or mortality [5].
The endpoints of fluid therapy should be easily feasible for the clinician. One certain endpoint is the optimization of the hemodynamic response. It is believed that colloids have superior capacity to crystalloids for achieving optimal hemodynamics. However, intravascular volume overload, kidney dysfunction, coagulopathy, extravasation across leaky capillary membranes, and anaphylactic reaction may occur with the administration of any colloid. In this review, we focus on the characteristics of intravenous colloids, colloid choice for hypovolemia, and the safety profile of colloids.
Characteristics of intravenous colloids
Albumin
Albumin is a colloid solution with a homogeneous molecular weight of 69 kDa (Table 1a). Albumin is slightly hypo-oncotic as a 4% solution, iso-oncotic as a 5% solution, and hyper-oncotic as a 20% solution. The solvent is physiological (normal) saline. Albumin has no recommended limit of maximum dose. Iso-oncotic albumin infusion results in plasma volume expansion equal to the volume infused [6]. Hyper-oncotic albumin solution has been given mainly as a volume expander for large-volume paracentesis of ascites [7]. The transfer of infection is not totally excluded, although the method of preparation of the albumin solution minimizes the risk. There are no clinical reports of viral infections transferred by an albumin infusion [8]. Albumin may induce hypersensitivity reactions, and the incidence of anaphylactic reaction is higher than with HES preparations [9]. Twenty-five percent, but not 5%, recombinant albumin is now under clinical trial, and is expected to be used clinically in the near future.
Dextran
Dextran solution is a glucose polymer available with molecular weight of 70 kDa (6% iso-oncotic solution) or 40 kDa (10% hyper-oncotic solution) (Table 1b). The increase in plasma volume is approximately 100% of the administered volume of 6% dextran 70 or 175% of the administered volume of 10% dextran 40 [10]. The plasma-expanding effect lasts 3–5 h.
The incidence of dextran-associated anaphylactic reactions is reported to be 0.273% [9]. However, the incidence of these reactions is lower than those occurring by the use of albumin after introduction of low molecular weight dextran (40 kDa dextran) [11]. Dextran also has dose-dependent negative effects on hemostasis [12]; it may accumulate in tissues and induce hyper-oncotic renal dysfunction [13]. Dextrans are seldom used today for plasma expansion [2]. In addition to their adverse side effects, the reason may be the availability of new rapidly degradable HES solutions with few side effects (as described below). Furthermore, low molecular weight heparins have replaced dextrans as thromboprophylactic agents. Dextran combined with hypertonic saline, with a volume effect of 400% of the administered volume, has been found to be indicated in the immediate treatment of traumatic hemorrhagic shock [14]. The maximum recommended dosage of dextran is 1.5 g/kg/day.
Gelatin
Gelatin solutions are urea- or succinylated cross-linked modifications of bovine collagen (Table 1c). The molecular weight of gelatin solution is relatively low, 30–35 kDa, in comparison with those of other colloids. The carrier consists of NaCl at 110 mmol/l. The immediate plasma expansion effect of gelatin is 80–100% of the administered volume under conditions of normovolemic hemodilution. The plasma-expanding effect lasts 1–2 h. Gelatin has no maximum limit of dose. Gelatin can induce hypersensitivity reactions more often than HES solutions [9]. Although the raw product is from a bovine source, gelatins are believed to be free of the risk of prion transmission [15]. Most of the gelatin is excreted via the kidneys, and no tissue accumulation has been reported.
Hydroxyethyl starch
Hydroxyethyl starch (HES) solutions are high polymeric glucose compounds obtained by hydrolysis and subsequent hydroxyethylation from the maize or potato starch amylopectin [16]. The in vitro molecular weight of HES varies from 70 to 670 kDa (Table 1d). The physicochemical characteristics of hydroxyethyl starches are determined by their concentration (6% or 10% solution), mean molecular weight, degree of substitution, and C2/C6 ratio [17, 18]. Thus, the characteristic of HES is expressed as HES mean molecular weight/degree of substitution/with or without the C2/C6 ratio. The degree of substitution expresses the average number of hydroxyethyl groups per unit of glucose. The C2/C6 ratio refers to the preferential hydroxyethylation site at the carbon atoms of the glucose subunit. Good plasma-expanding capacity of a HES solution with relatively low molecular weight and degree of substitution (i.e., HES 130 kDa/0.38–0.45) is achieved by increasing the C2/C6 ratio (Table 1d). Usually the solvent of HES is normal saline, but presently, HES is also available as a balanced form with a lesser concentration of NaCl than normal saline and other presumably beneficial additives.
The classification of hydroxyethyl starches as rapidly or slowly degradable solutions is based on their physicochemical characteristics. In vivo, the HES molecules of 50–70 kDa are rapidly excreted via the kidneys, and the larger molecules are hydrolyzed by amylase to smaller molecules that can be excreted via the kidneys [17] (Fig. 1). Slowly degradable HES solutions may accumulate in the reticuloendothelial system, whereas rapidly degradable HES, i.e., HES 130/0.4, does not accumulate, even after a repeated dosing regimen [19]. However, the accumulation of HES in the reticuloendothelial system does not seem to depress reticuloendothelial function [20]. Persistent itching, which is also a marker of accumulation of slowly degradable HES preparations, has not been found in patients treated with HES 130/0.4 [18, 21, 22].
The physicochemical characteristics of hydroxyethyl starch. The classification of hydroxyethyl starches as rapidly or slowly degradable solutions (Table 1d) is based on their physicochemical characteristics. In vivo, the hydroxyethyl starch molecules of 50–70 kDa are rapidly excreted via the kidneys, and the larger molecules are hydrolyzed by amylase to smaller ones that can be excreted via the kidneys
The plasma-expanding effect of HES is condition sensitive [3]. In critically ill patients, the immediate plasma-expanding effects of 500 ml 6% HES 200/0.5 have been reported to vary among individuals from 27 to 840 ml [23]. Correspondingly, when HES 200/0.5 was administered to a presumably normovolemic patient preoperatively, the volume effect was approximately 40% of the administered volume, indicating that some HES was leaking outside the intravascular space [24]. In contrast, the immediate volume effect of 6% HES 200/0.5 or 6% HES 130/0.4 is 100% of the administered volume, lasting 3–4 h, as administered to hypovolemic patients under conditions of normovolemic hemodilution [25, 26]. Therefore, the assessment of the degree of hypovolemia is essential as HES or any other colloid is given.
The maximum doses of hydroxyethyl starches range from 20 to 50 ml/kg, depending on the type of HES. A prospective study showed that HES has a low risk of causing anaphylactic reactions (0.058%) compared with gelatins (0.345%) [9]. Hydroxyethyl starch is also available in combination with hypertonic saline, and this combination is indicated in the first-line treatment of hypovolemic shock in trauma patients.
Colloid choice for hypovolemia
Intravascular fluid administration is indicated in hypovolemia. Hypovolemia is associated with poor outcome after surgery, and mortality is increased by sepsis without fluid resuscitation [27, 28]. Early goal-directed therapy including fluid optimization has been proven to significantly improve the outcome of patients with severe sepsis and patients undergoing surgery [28–30].
Colloids are suggested to be indicated when circulation needs additional plasma volume or in the replacement of plasma loss [3]. Experimental models of colloid administration demonstrate the superiority of colloids in rapid resuscitation and restoration of tissue perfusion [31–33]. As expected clinically, colloids produce plasma volume expansion with 25–50% of the volume required for isotonic crystalloid solutions [34–38].
Some authors have suggested that the intensity and timing of the fluid administration might be more important during fluid resuscitation than the type of fluid itself [39]. However, from the clinical point of view, choosing the right kind of intravenous fluid is extremely important, because the efficacy of fluid administration is also significantly dependent on the type of intravenous fluid [3, 40–42]. This idea is supported by the findings in a study of penetrating torso injuries, which showed the adverse effects of excessively intensive fluid resuscitation as increased blood loss and mortality [43].
One clinically useful option in keeping the volume and composition of body fluids as normal as possible is to prevent subsequent hemodynamic compromise by fluid administration. The final aim is to restore adequate oxygen delivery, thus preventing organ failure [44]. The character of the condition sensitivity of the plasma-expanding effects of colloids may also be translated into their hemodynamic responses. In experimental uncontrolled hemorrhage, the response of a colloid or crystalloid on hemodynamics was significantly related to the severity of the injury, i.e., the degree of hypovolemia and the rate of infusion [45].
Clinical trials of the hemodynamic responses of colloids have shown variable findings. The reason may be the differences in study designs, including variability in the timing and dosing of colloids, or in the degree of hypovolemia. The hemodynamic profiles of various colloids have been quite similar because the rate of colloid infusion has been relatively slow. The administration of HES (130, 200 kDa), gelatin, or albumin resulted in comparable hemodynamics during 20–48 h in patients undergoing cardiac or abdominal surgery [46–51]. Similar hemodynamics were also observed with HES 200 kDa or gelatin in patients with sepsis and during normovolemic hemodilution in patients who had undergone hip arthroplasty [52–54]. However, the 5-day hemodynamics in critically ill patients were more stable with HES (200 or 130 kDa) than with albumin [55–57]. The superior immediate effect of HES (200 or 130 kDa) on cardiac output after cardiac surgery compared with albumin and gelatin has recently been reported [58, 59]. The capacity of HES to prevent postoperative nausea and vomiting might also be related to its good maintenance of hemodynamics [60]. According to these studies, a rapidly degradable HES, such as HES 130/0.4, seems to be indicated whenever rapid favorable hemodynamic response is warranted when the circulation is assessed to need volume expansion.
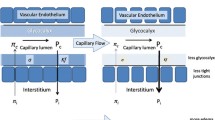
Colloids are also needed for maintaining osmotic pressure during major blood loss. Dilution of serum proteins by the massive administration of crystalloids lowers colloid oncotic pressure (COP) with the risk of progressive expansion of the interstitial space. In some studies, the administration of colloids has resulted in less tissue edema [61–63]. Controversy still exists over whether the choice of fluid for restoration of circulating volume is able to limit the development of tissue edema. It has also been speculated that the weight gain related to perioperative crystalloid administration and increased mortality [64] might be reduced by the administration of colloids [3]. This idea is further supported by the fact that oncotic solutions achieve similar resuscitation goals with less than half the infusion volume of crystalloids [65–67].
Colloids have favorable effects on microcirculation, inflammation, and blood viscosity. Particularly, the rheological effects of dextran, i.e., a decrease in whole blood viscosity, are well known [68]. The capillary leakage can be prevented by administration of HES (pentastarch; HES 200/0.5) in patients with sepsis or trauma [69]. It has also been shown experimentally that pulmonary capillary leakage is prevented to a greater extent by HES than by gelatin [70]. Improvement of microcirculation [71] and decrease of plasma viscosity by HES but not by gelatin [72] may also be translated into good tissue oxygenation not seen during the administration of Ringer’s lactate alone [73, 74] (Fig. 2).
Differences (in percentage from baseline) of tissue oxygen tension in the two volume groups. Data are presented as mean ± SD (n = 21 each). T 0 = baseline (before volume administration and before surgery), T 1 = 60 min later (during surgery), T 2 = 120 min after T 1 (during surgery), T 3 = at the end of surgery, T 4 = on the morning of the first postoperative day in the intensive care unit. HES, hydroxyethyl starch (molecular weight, 130 kDa; degree of substitution, 0.4). † P < 0.05 compared with baseline data, * P < 0.05 compared with the other volume group. (Modified from Lang et al. [73], with permission)
The “balanced concept” of HES means that the electrolytes in HES products consist of modified Ringer’s solution composition but not only NaCl as normal saline. This concept was first introduced with slowly degradable HES (Hextend; HES 670/0.7) solution and recently with rapidly degradable HES (HES 130/0.4). Hydroxyethyl starch was modified to contain less sodium chloride (i.e., Na+, 140 mmol/l; Cl−, 118 mmol/l) than normal saline with additives such as K+, 4.0 mmol/l, Ca2+, 2.5 mmol/l, Mg2+, 1.0 mmol/l, acetate, 24 mmol/l, and malate, 5 mmol/l. A balanced slowly degradable HES solution has less effect on coagulation than the HES in normal saline, but the difference is clinically insignificant [75]. More importantly, the clinical effect of a balanced HES solution is seen in acid–base equilibrium. Balanced rapidly degradable HES 130/0.4 solution induced no disturbances in the acid–base balance, whereas the HES in normal saline showed on average postoperative base-excess decrease of 5 mEq/l in patients who had undergone major noncardiac surgery [76]. Correspondingly, base excess was unchanged with balanced HES 130/0.4 but reduced with the HES in normal solution in elderly patients who had undergone cardiac surgery [77]. Furthermore, less inflammatory endothelial activation and alteration in kidney integrity were observed with the balanced form than with the HES in normal saline [77]. The balanced strategy of fluid administration seems not to harm a patient’s homeostasis and is a promising strategy for correcting hypovolemia. Gelatin, dextran, or albumin solutions are not available in balanced forms. Acidifying an albumin infusion may be an appropriate action under some clinical conditions, such as after cardiac surgery [59].
The possible beneficial effects of colloids on patient outcome or mortality are not mentioned in systematic reviews [5, 78–80]. In trauma patients, the administration of colloids may even increase mortality [79]. However, the characteristics of colloid solutions as well as the fluid composition in controls vary widely in meta-analysis. The patient populations are not always comparable, and the aims of fluid challenge are not indicated. Furthermore, most of the included studies are too underpowered to detect any effect on mortality.
We also still lack an adequately powered study to find the possible effect of modern nonprotein colloid on morbidity and mortality in comparison with albumin. In smaller clinical trials, albumin infusion had no beneficial effects compared with HES or gelatin in critically ill patients [81, 82]. Hypoalbuminemia is associated with increased mortality, but there is no evidence that hypoalbuminemia is an indication for an albumin infusion [83]. Hypoalbuminemia in elderly patients who have undergone cardiac surgery does not improve with albumin infusion in comparison with HES 130/0.4 [84]. It seems also that the administration of albumin is not indicated in patients with burns [85], although it is still the most common colloid used in burn trauma, which usually involves hypoalbuminemia [86].
A previous suggestion of the increased mortality caused by albumin [87] in critically ill patients was not supported by the results of a randomized study with approximately 7,000 patients [88]. The 28-day mortality was 21% and did not differ according to the treatment assignment with either albumin or normal saline. The finding is also in accordance with recent meta-analysis concerning the effects of albumin in critically ill patients [78, 89, 90]. However, subgroup analysis of the Albumin Reviewers’ study [90] suggests that albumin may be related to an unfavorable outcome in trauma patients: the relative risk (95% confidence interval) for death with albumin in trauma patients was 1.36 (0.99–1.86) and that for associated traumatic brain injury was 1.62 (1.12–2.34).
Albumin is also expensive compared to nonprotein colloids, although the exact magnitude of the costs of intravenous fluids in relationship to the total costs of intensive care is difficult to assess [91]. An appropriate indication for albumin might be the replacement of ascites fluid during drainage in patients with cirrhosis and spontaneous bacterial peritonitis [7, 92].
Unfortunately, based on these results of meta-analysis and previous investigations already described, the indication of colloid solution still remains unanswered for mortality outcome, especially in trauma patients.
Safety profile of colloids
Renal function
The reason for renal dysfunction in hypovolemic patients is multifactorial. The reported effects of colloids on renal function are variable but also related to the type of the colloid, according to recent studies [53, 93]. None of these randomized studies comparing various colloids were powered to reveal possible effects on renal replacement therapy or mortality, and no such differences have been observed.
There are several suggestions for the mechanism of renal dysfunction associated with colloids. The decrease of tubular flow during glomerular filtration of colloids (dextrans, 10% HES, or 20% or 25% albumin) may cause renal dysfunction [94]. Accumulation of small molecules in the tubuli may account for acute tubular toxicity. The elevated serum chloride concentrations associated with the carrier of colloid solution may also impair renal blood flow [95].
The slowly degradable HES solutions have been shown to be harmful to kidneys. Osmotic nephrosis-like lesions without any effect on kidney function have been seen in kidney transplant recipients after HES 200/0.62 was administered to brain-dead organ donors [96]. In another nonrandomized study, kidney recipients whose donors were given HES 200/0.62 (n = 15) had a higher creatinine concentration than recipients whose donors were given gelatin (n = 12) [97]. However, there were no differences in the number of renal replacement therapies for recipients when kidney donors were given HES 200/0.5 (n = 20), 450/0.7 (n = 16), or gelatin/albumin (n = 73) [98]. A recent study revealed that the third-generation HES 130/0.4 given to kidney donors seemed to be associated with a better effect on the renal function of recipients than that of HES 200/0.6 [99] (Fig. 3). In that particular study, lower serum creatinine concentrations were observed at 1 month and 1 year after the kidney transplantation, but the difference in delayed graft function was not statistically significant, although it was 11% lower in the HES 130/0.4 group.
Levels of serum creatinine measured in the recipients at 1 month and 1 year posttransplantation who received either hydroxyethyl starch (HES) 200/0.6 or 130/0.4 during the surgery. Data are presented as median (thick line) ± 25/75th percentile (boxes) ± 10/90th percentile (bars) (n = 32 each). The serum creatinine levels at 1 month were lower in the patients treated with HES 130/0.4 than in those treated with HES 200/0.6, and this difference was still observed 1 year after the transplantation. (Modified from Blasco et al. [99], with permission)
In severe sepsis, HES 200/0.62 also showed a slightly worse renal profile than gelatine, and HES was an independent risk factor for acute renal failure [53]. However, no influence of renal failure on renal replacement therapy or mortality was observed. In addition, the serum creatinine concentration seemed to be higher in the HES 200/0.62 group before fluid resuscitation [141 μmol/l (median) in the HES group and 110 μmol/l in the gelatin group]. The hyper-oncotic 10% HES 200/0.5 solution also increased the frequency of renal replacement therapy and mortality in a dose-dependent manner in patients with severe sepsis in comparison with modified Ringer’s lactate (Fig. 4) [93]. The study has been criticized in terms of solely administering hyper-oncotic hyperchloremic solution in the HES group and including patients with serum creatinine concentration as high as 320 μmol/l. Furthermore, in the subgroup analysis, mortality was found to be even lower in the HES group (31%) than in the Ringer’s lactate group (41%) because the dose was limited to less than 22 ml/kg.
Cumulative effect of volume resuscitation on the need for renal replacement therapy (a) and the rate of death at 90 days (b). HES, hydroxyethyl starch. The need for renal replacement therapy and 90-day mortality were significantly correlated with the cumulative dose of HES (P < 0.001 and P = 0.001, respectively) but not with the dose of Ringer’s lactate (P = 0.11 and P = 0.31, respectively). (Modified from Brunkhorst et al. [93], with permission)
A reasonable clinical finding about the risk factors for renal replacement therapy was shown in a cohort study of 3,000 critically ill patients [100]. This study found that sepsis, circulatory failure, hematological malignancy, or renal dysfunction before fluid resuscitation, but not the administration of various hydroxyethyl starches, were independent risk factors for renal replacement therapy.
The results of these studies of slowly degradable HES solutions such as HES 200/0.5 may not be directly applicable to the use of more rapidly degradable HES solutions such as HES 130/0.4. The use of HES 200/0.5 over 5 days in a study of critically ill patients in the intensive care unit was without negative effects on renal function compared to albumin [56]. In a study in elderly patients (>75 years old) undergoing major abdominal or cardiac surgery, administration of 6% HES 200/0.5 [101] or 130/0.4 [76, 84, 102] was not associated with changes in markers of renal dysfunction as compared with albumin or gelatin [102]. In fact, in studies of patients undergoing cardiac surgery [103] or elective aortic aneurysm surgery [104], renal profile according to sensitive kidney-specific markers or creatinine clearance in the group that received gelatin was slightly worse than in the HES 130/0.4 or 200/0.5 groups [103, 104]. Furthermore, HES 130/0.4 did not negatively influence kidney integrity compared with a human albumin-based volume-replacement strategy in patients who had undergone cardiac surgery [97] or aortic aneurysm surgery [105] with preoperative compromised kidney function. In cases of severe renal dysfunction, a single bolus of 500 ml HES 130/0.4 did not impair creatinine clearance either [106].
Any hyper-oncotic colloid may have the potential to produce kidney dysfunction or renal failure because colloids may decrease glomerular filtration [13]. Because the results of colloids regarding renal damage are doubtful and highly probably related to the type and dose of the solution, colloids should always be carefully administered, especially to organ donors and patients with sepsis or renal dysfunction. The logical extrapolation of the results of rapidly degradable HES solutions in surgical patients to critically ill patients with sepsis requires confirmation in clinical trials. The safest colloid today in terms of kidney function seems to be a rapidly degradable HES, such as HES 130/0.4, or gelatin. Insufficiently treated hypovolemia may also induce renal dysfunction and treatment by the administration of crystalloids alone may not achieve restoration of blood volume. Other researchers failed to find any deterioration in renal function associated with the use of various HES preparations: 6% HES 200/0.5 and HES 70/0.5 [101], 6% HES 200/0.5, 6% HES 200/0.62, and 6% HES 670/0.75 [107], even when high doses were used [108].
Coagulation
All colloids induce dilution of red blood cells, platelets, and coagulation factors that may be clinically significant during extreme hemodilution [10]. Slowly degradable HES solutions and dextran especially decrease the activities of coagulation factor VIII and von Willebrand factor and impair clot strength and platelet function in addition to their hemodilutional effects [12, 109, 110] (Fig. 5). However, these detrimental effects of HES on hemostasis were not seen in similar magnitudes by all types of HES in another study [12]. Rapidly degradable HES solution, HES 130/0.4, has been given to patients with severe brain injuries without a negative effect on coagulation factor VIII or von Willebrand factor concentrations [108]. Similarly, in orthopedic patients, HES 130/0.4 did not affect coagulation factor VIII concentration [111].
Percent changes in hematocrit (a), coagulation factor VIIIc (b), and von Willebrand factor (c) during the course of a 10-day volume therapy. Data are presented as mean. VIIIc factor, coagulation factor VIIIc; von Willebrand factor, von Willebrand ristocetin co-factor. Although the hemodilutional effects of hydroxyethyl starch (HES) solutions did not differ among the products tested, the inhibitory effects of HES solutions on VIIIc and von Willebrand factors were significantly different among the types of HES solutions. (Modified from Treib et al. [109], with permission)
In addition to laboratory knowledge about the mechanisms of colloid-induced coagulation disturbance, it is important to evaluate to what extent the shown defect could be translated into increased blood loss in clinical practice. Although albumin may have anticoagulant effects [112], there is no clinical evidence that albumin infusion could increase blood loss in patients undergoing surgery. Gelatin in vitro also decreases the clot strength, but to a lesser degree than rapidly degradable HES solution [113]. Gelatin has also been observed to interfere with platelet aggregation and to impair clot strength in patients undergoing cardiac surgery without an effect on blood loss [114–119].
Increased blood loss has been mainly reported after slowly degradable HES solution or dextran has been given to patients undergoing surgery. In orthopedic and urological surgery, dextran infusions for thromboprophylaxis are associated with an increased postoperative blood loss and the requirement for allogeneic blood [120].
The administration of the slowly degradable HES hetastarch increased cumulative chest tube drainage by an average of 330 ml compared to drainage in patients not receiving hetastarch (HES 670/0.75) in a retrospective analysis of adults undergoing cardiac surgery [121]. It was also found that the hetastarch group received 100% more red blood cells than those not given hetastarch. In an analysis of 14 randomized studies, adult patients undergoing cardiac surgery who were administered HES 200 or 670 kDa had, on average, 96 ml more 24-h cumulative chest tube drainage after cardiopulmonary bypass than those administered albumin [122]. A balanced high molecular weight HES with high molar substitution (HES 670/0.75) given to patients undergoing major abdominal surgery also increased blood loss by an average of 520 ml in comparison to those patients given HES 130/0.4 [123]. In a pooled analysis of randomized trials, intraoperative blood loss could be reduced by an average of 404 ml, and postoperative blood loss could be reduced by an average of 272 ml, with HES 130/0.4 compared to HES 200/0.5 [124]. The volume of red blood cells transfused was also significantly lower, at 137 ml.
Summary
Albumin, dextran, gelatin, and HES solutions are colloids that efficiently expand the circulating blood volume. The administration of colloids restores the intravascular volume with minimal risk of tissue edema in comparison with crystalloid solutions alone. However, colloids are always given for surgical and critically ill patients. The type of the colloid, volumes applied, aggressiveness of fluid resuscitation, and the volume status at the initial phase of administration determine their clinical responses. A randomized, adequately powered clinical trial comparing modern nonprotein colloid to albumin is still lacking. Rapidly degradable HES solutions have good hemodynamic effects and the risk of adverse renal and coagulation effects, as well as allergic reactions, is minimal. The current investigation has also shown the beneficial effect of HES solution (especially HES 130/0.4) on the inflammatory response [125], postoperative nausea and vomiting [60], and postoperative outcome [126].
References
Bunn F, Trivedi D, Ashraf S. Colloid solutions for fluid resuscitation. Cochrane Database Syst Rev. 2008. doi:10.1002/14651858.CD001319.pub.2.
Perner A, Åneman A, Guttormsen AB, Kárason S, Tenhunen J. Preferences for colloid use in Scandinavian intensive care units. Acta Anaesthesiol Scand. 2008;52:750–8.
Chappell D, Jacob M, Hofmann-Kiefer K, Conzen P, Rehm M. A rational approach to perioperative fluid management. Anesthesiology. 2008;109:723–40.
Grocott MP, Mythen MG, Gan TJ. Perioperative fluid management and clinical outcomes in adults. Anesth Analg. 2005;100:1093–106.
Perel P, Roberts I, Pearson M. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2007. doi:10.1002/14651858.CD000567.pub3.
Hiippala S, Linko K, Myllylä G, Lalla M, Hekali R, Makelainen A. Replacement of major surgical blood loss by hypo-oncotic or conventional plasma substitutes. Acta Anaesthesiol Scand. 1995;39:228–35.
Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, Jimenez W, Planas R, Arroyo V. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–66.
Barron ME, Wilkes M, Navickis RJ. A systematic review of the comparative safety of colloids. Arch Surg. 2004;139:552–63.
Laxenaire MC, Charpentier C, Feldman L. Anaphylactoid reactions to colloid plasma substitutes: incidence risk factors mechanisms. A French multicenter prospective study. Ann Fr Anest Reanim. 1994;13:301–10.
Kohler H, Zschiedrich H, Clasen R, Linfante A, Gamm H. The effects of 500 ml 10% hydroxyethyl starch 200/0.5 and 10% dextran 40 on blood volume, colloid osmotic pressure, and renal function in human volunteers. Anaesthetist. 1982;31:61–7.
Ljungström KG. Safety of dextran in relation to other colloids—ten years experience with hapten inhibition. Infus Ther Transfus Med. 1993;20:206–10.
Van der Linden P, Ickx BE. The effects of colloid solutions on hemostasis. Can J Anaesth. 2006;53:S30–9.
Nearman HS, Herman ML. Toxic effects of colloids in the intensive care unit. Crit Care Clin. 1991;7:713–23.
Kramer GC, Wade CE, Prough DS. Hypertonic saline dextran: efficacy and regulatory approval. Acta Anaesthesiol Scand. 1998;42:141–4.
Peano S, Reiner G, Carbonatto M, Bodenbender L, Boland P, Abel KJ. Determination of the clearance factor for transmissible spongiform encephalopathy agents during the manufacturing process of polygeline. Intensive Care Med. 2000;26:608–12.
Treib J, Baron JF, Grauer MT, Strauss RG. An international view of hydroxyethyl starches. Intensive Care Med. 1999;25:258–68.
Jungheinrich C, Neff TA. Pharmacokinetics of hydroxyethyl starch. Clin Pharmacokinet. 2005;44:681–99.
Westphal M, James MFM, Kozek-Langenecker S, Stocker R, Guidet B, van Aken H. Hydroxyethyl starches. Anesthesiology. 2009;111:187–202.
Lehmann GB, Asskali F, Boll M, Burmeister MA, Marx G, Hilgers R, Förster H. HES 130/0.42 shows less alteration of pharmacokinetics than HES 200/0.5 when dosed repeatedly. Br J Anaesth. 2007;98:635–44.
Shatney CH, Chaudry IH. Hydroxyethyl starch administration does not depress reticuloendothelial function or increase mortality from sepsis. Circ Shock. 1994;13:21–6.
Sharland C, Huggett A, Nielson MS, Friedmann PS. Persistent pruritus after pentastarch infusions in intensive care patients. Anaesthesia. 1999;54:500–1.
Morgan PW, Berridge JC. Giving long-persistent starch as volume replacement can cause pruritus after cardiac surgery. Br J Anaesth. 2000;85:696–9.
Christensen P, Andersson J, Rasmussen SE, Andersen PK, Henneberg SW. Changes in circulating blood volume after infusion of hydroxyethyl starch 6% in critically ill patients. Acta Anaesthesiol Scand. 2001;45:414–20.
Rehm M, Haller M, Orth V, Kreimeier U, Jacob M, Dressel H, Mayer S, Brechtelsbauer H, Finsterer U. Changes in blood volume and hematocrit during acute preoperative volume loading with 5% albumin or 6% hetastarch solutions in patients before radical hysterectomy. Anesthesiology. 2001;95:849–56.
Jacob M, Rehm M, Orth V, Lotsch M, Brechtelsbauer H, Weninger E, Finsterer U. Exact measurement of the volume effect of 6% hydroxyethyl starch 130/0.4 (Voluven®) during acute preoperative normovolemic hemodilution. Anaesthesist. 2003;52:896–904.
Rehm M, Orth V, Kreimeier U, Thiel M, Haller M, Brechtelsbauer H, Finsterer U. Changes in intravascular volume during acute normovolemic hemodilution and intraoperative retransfusion in patients with radical hysterectomy. Anesthesiology. 2000;92:657–64.
Carcillo JA, Davis AL, Zaritsky A. Role of early fluid resuscitation in pediatric septic shock. JAMA. 1991;266:1242–5.
Venn R, Steele A, Richardson P, Poloniecki J, Grounds M, Newman P. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth. 2002;88:65–71.
Varpula M, Karlsson S, Parviainen I, Ruokonen E, Pettilä V, Finnsepsis Study Group. Community-acquired septic shock: early management and outcome in a nationwide study in Finland. Acta Anaesthesiol Scand. 2007;51:1320–6.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Kellum JA. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid-base balance with Hextend compared with saline. Crit Care Med. 2002;30:300–5.
Friedman Z, Berkenstadt H, Preisman S, Perel A. A comparison of lactated Ringer’s solution to hydroxyethyl starch 6% in a model of severe hemorrhagic shock and continuous bleeding in dogs. Anesth Analg. 2003;96:39–45.
Boura C, Caron A, Longrois D, Mertes PM, Labrude P, Menu P. Volume expansion with modified hemoglobin solution, colloids, or crystalloid after hemorrhagic shock in rabbits: effects in skeletal muscle oxygen pressure and use versus arterial blood velocity and resistance. Shock. 2003;19:176–82.
Moss GS, Lowe RJ, Jilek J, Levine HD. Colloid or crystalloid in the resuscitation of hemorrhagic shock: a controlled clinical trial. Surgery (St. Louis). 1981;89:434–8.
Nagy KK, Davis J, Duda J, Fildes J, Roberts R, Barrett J. A comparison of pentastarch and lactated Ringer’s solution in the resuscitation of patients with hemorrhagic shock. Circ Shock. 1993;40:289–94.
Younes RN, Yin KC, Amino CJ, Itinoshe M, Rocha e Silva M, Birolini D. Use of pentastarch solution in the treatment of patients with hemorrhagic hypovolemia: randomized phase II study in the emergency room. World J Surg. 1998;22:2–5.
Lowe RJ, Moss GS, Jilek J, Levine HD. Crystalloid vs colloid in the etiology of pulmonary failure after trauma: a randomized trial in man. Surgery (St. Louis). 1977;81:676–83.
Modig J. Advantages of dextran 70 over Ringer acetate solution in shock treatment and in prevention of adult respiratory distress syndrome: a randomized study in man after traumatic-haemorrhagic shock. Resuscitation. 1983;10:219–26.
Vincent J-L. The pros and cons of hydroxyethyl starch solutions. Anesth Analg. 2007;104:484–6.
Boldt J. Volume replacement in the surgical patient–does the type of solution make a difference? Br J Anaesth. 2000;84:783–93.
Tølløfsrud S, Svennevig JL, Breivik H, Kongsgaard U, Øzer M, Hysing E, Mohr B, Seem E, Geiran O, Abdelnour M, Frøysaker T, Noddeland H. Fluid balance and pulmonary functions during and after coronary artery surgery: Ringer’s acetate compared with dextran, polygeline, or albumin. Acta Anaesthesiol Scand. 1995;39:671–7.
Friedman G, Jankowski S, Shahla M, Gomez J, Vincent JL. Hemodynamic effects of 6 and 10% hydroxyethyl starch solutions versus 4% albumin solution in septic patients. J Clin Anesth. 2008;20:528–33.
Bickell WH, Wall MJ Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N Engl J Med. 1994;331:1105–9.
Mythen MG, Webb AR. Preoperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg. 1995;130:423–9.
Krausz MM, Bashenko Y, Hirsh M. Crystalloid and colloid resuscitation of uncontrolled hemorrhagic shock following massive splenic injury. Shock. 2001;16:383–8.
Karanko MS. Effects of three colloid solutions on plasma volume and hemodynamics after coronary bypass surgery. Crit Care Med. 1987;15:1015–22.
Gallandat Huet RC, Siemons AW, Baus D, van Rooyen-Butijn WT, Haagenaars JA, van Oeveren W, Bepperling F. A novel hydroxyethyl starch (Voluven) for effective perioperative plasma volume substitution in cardiac surgery. Can J Anaesth. 2000;47:1207–15.
Haisch G, Boldt J, Krebs C, Suttner S, Lehmann A, Isgro F. Influence of a new hydroxyethyl starch preparation (HES 130/0.4) on coagulation in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2001;15:316–21.
Haisch G, Boldt J, Krebs C, Kumle B, Suttner S, Schulz A. The influence of intravascular volume therapy with a new hydroxyethyl starch preparation (6% HES 130/0.4) on coagulation in patients undergoing major abdominal surgery. Anesth Analg. 2001;92:565–71.
Van der Linden P, De Hert S, Daper A, Trenchant A, Schmartz D, Defrance P, Kimbimbi P. 3.5% Urea-linked gelatin is as effective as 6% HES 200/0.5 for volume management in cardiac surgery patients. Can J Anaesth. 2004;51:236–41.
Van der Linden P, De Hert S, Deraedt D, Deraedt D, Cronheecke S, De Decker K, De Paep R, Rodrigus I, Daper A, Trenchant A. Hydroxyethyl starch 130/0.4 versus modified fluid gelatin for volume expansion in cardiac surgery patients: the effects on perioperative bleeding and transfusion needs. Anesth Analg. 2005;101:629–34.
Mortelmans YJ, Vermaut G, Verbruggen AM, Arnout JF, Vermylen J, Van Aken H, Mortelmans LA. Effects of 6% hydroxyethyl starch and 3% modified fluid gelatin on intravascular volume and coagulation during intraoperative hemodilution. Anesth Analg. 1995;81:1235–42.
Schortgen F, Lacherade JC, Bruneel F, Cattaneo I, Hemery F, Lemaire F, Brochard L. Effects of hydroxyethyl starch and gelatin on renal function in severe sepsis: a multicentre randomised study. Lancet. 2001;357:911–6.
Molnar Z, Mikor A, Leiner T, Szakmany T. Fluid resuscitation with colloids of different molecular weight in septic shock. Intensive Care Med. 2004;30:1356–60.
Boldt J, Heesen M, Müller M, Papsdorf M, Hempelmann G. The effects of albumin versus hydroxyethyl starch solution on cardiorespiratory and circulatory variables in critically ill patients. Anesth Analg. 1996;83:254–61.
Boldt J, Müller M, Mentges D, Papsdorf M, Hempelmann G. Volume therapy in the critically ill: is there a difference? Intensive Care Med. 1998;24:28–36.
Palumbo D, Servillo G, D’Amato L, Volpe ML, Capogrosso G, De Robertis E, Piazza O, Tufano R. The effects of hydroxyethyl starch solution in critically ill patients. Minerva Anestesiol. 2006;72:655–64.
Kuitunen A, Suojaranta-Ylinen R, Kukkonen S, Niemi T. A comparison of the haemodynamic effects of 4% succinylated gelatin, 6% hydroxyethyl starch (200/0.5), and 4% human albumin after cardiac surgery. Scand J Surg. 2007;96:72–8.
Niemi T, Schramko A, Kuitunen A, Kukkonen S, Suojaranta-Ylinen R. Haemodynamics and acid-base equilibrium after cardiac surgery: comparison of rapidly degradable hydroxyethyl starch solutions and albumin. Scand J Surg. 2008;97:259–65.
Moretti EW, Robertson KM, El-Moalem H, Gan TJ. Intraoperative colloid administration reduces postoperative nausea and vomiting and improves postoperative outcomes compared with crystalloid administration. Anesth Analg. 2003;96:611–7.
Haupt MT, Rackow EC. Colloid osmotic pressure and fluid resuscitation with hetastarch, albumin, and saline solutions. Crit Care Med. 1982;10:159–62.
Prien T, Backhaus N, Pelster F, Pircher W, Bunte H, Lawin P. Effect of intraoperative fluid administration and colloid osmotic pressure on the formation of intestinal edema during gastrointestinal surgery. J Clin Anesth. 1990;2:317–23.
Rizoli SB. Crystalloids and colloids in trauma resuscitation: a brief overview of the current debate. J Trauma. 2003;54:S82–8.
Lowell JA, Schifferdecker C, Driscoll DF, Benotti PN, Bistrian BR. Postoperative fluid overload: not a benign problem. Crit Care Med. 1990;18:728–33.
Schertel ER, Allen DA, Muir WW, Hansen BD. Evaluation of a hypertonic sodium chloride/dextran solution for treatment of traumatic shock in dogs. J Am Vet Med Assoc. 1996;208:366–70.
Marx G, Cobas MM, Schuerholz T, Vangerow B, Gratz KF, Hecker H, Sumpelmann R, Rueckoldt H, Leuwer M. Hydroxyethyl starch and modified fluid gelatin maintain plasma volume in a porcine model of septic shock with capillary leakage. Intensive Care Med. 2002;28:629–35.
Zhang H, Voglis S, Kim CH, Slutsky AS. Effects of albumin and Ringer’s lactate on production of lung cytokines and hydrogen peroxide after resuscitated hemorrhage and endotoxemia in rats. Crit Care Med. 2003;31:1515–22.
Menger MD, Sack FU, Hammersen F, Messmer K. Tissue oxygenation after prolonged ischemia in skeletal muscle: therapeutic effect of prophylactic isovolemic hemodilution. Adv Exp Med Biol. 1989;248:387–95.
Webb AR, Moss RF, Tighe D, Mythen MG, al-Saady N, Joseph AE, Bennett ED. A narrow range, medium molecular weight pentastarch reduces structural organ damage in a hyperdynamic porcine model of sepsis. Intensive Care Med. 1992;18:348–55.
Feng X, Yan W, Wang Z, Liu J, Yu M, Zhu S, Xu J. Hydroxyethyl starch, but not modified fluid gelatin, affects inflammatory response in a rat model of polymicrobial sepsis with capillary leakage. Anesth Analg. 2007;104:624–30.
Boldt J, Zickmann B, Rapin J, Hammermann H, Dapper F, Hempelmann G. Influence of volume replacement with different HES-solutions on microcirculatory blood flow in cardiac surgery. Acta Anaesthesiol Scand. 1994;38:432–8.
Neff TA, Fischler L, Mark M, Stocker R, Reinhart WH. The influence of two different hydroxyethyl starch solutions (6% HES 130/0.4 and 200/0.5) on blood viscosity. Anesth Analg. 2005;100:1773–80.
Lang K, Boldt J, Suttner S, Haisch G. Colloids versus crystalloids and tissue oxygen tension in patients undergoing major abdominal surgery. Anesth Analg. 2001;93:405–9.
Lang K, Suttner S, Boldt J, Kumle B, Nagel D. Volume replacement with HES 130/0.4 may reduce the inflammatory response in patients undergoing major abdominal surgery. Can J Anaesth. 2003;50:1009–16.
Martin G, Bennett-Guerrero E, Wakeling H, Kumle B, Nagel D. A prospective, randomized comparison of thromboelastographic coagulation profile in patients receiving lactated Ringer’s solution, 6% hetastarch in a balanced-saline vehicle, or 6% hetastarch in saline during major surgery. J Cardiothorac Vasc Anesth. 2002;16:441–6.
Boldt J, Schöllhorn T, Münchbach J, Pabsdorf M. A total balanced volume replacement strategy using a new balanced hydroxyethyl starch preparation (6% HES 130/0.42) in patients undergoing major abdominal surgery. Eur J Anaesthesiol. 2007;24:267–75.
Boldt J, Suttner S, Brosch C, Lehmann A, Röhm K, Mengistu A. The influence of a balanced volume replacement concept on inflammation, endothelial activation, and kidney integrity in elderly cardiac surgery patients. Intensive Care Med. 2009;35:462–70.
Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ. 1999;316:961–4.
Choi PTL, Yip G, Quinonez LG, Cook DJ. Crystalloids vs. colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999;27:200–10.
Roberts I, Alderson P, Bunn F, Chinnock P, Ker K, Schierhout G. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2004. doi:10.1002/14651858.CD000567.pub3.
Stockwell MA, Scott A, Day A, Riley B, Soni N. Colloid solutions in the critically ill. A randomised comparison of albumin and polygeline 2. Serum albumin concentration and incidences of pulmonary oedema and acute renal failure. Anaesthesia. 1992;47:7–9.
Boldt J, Brosch C, Ducke M, Papsdorf M, Lehmann A. Influence of volume therapy with a modern hydroxyethyl starch preparation on kidney function in cardiac surgery patients with compromised renal function: a comparison with human albumin. Crit Care Med. 2007;35:2740–6.
Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003;237:319–34.
Boldt J, Brosch C, Röhm K, Lehmann A, Mengistu A, Suttner S. Is albumin administration in hypoalbuminemic elderly cardiac surgery patients of benefit with regard to inflammation, endothelial activation, and long-term kidney function? Anesth Analg. 2008;107:1496–503.
Holm C. Resuscitation in shock associated with burns. Tradition or evidence-based medicine? Resuscitation. 2000;44:157–64.
Boldt J, Papsdorf M. Fluid management in burn patients: results from a European survey: more questions than answers. Burns. 2008;34:328–38.
Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ. 1998;317:235–40.
Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–56.
Wilkes MM, Navickis RJ. Patient survival after human albumin administration: a meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;135:149–64.
Alderson P, Bunn F, Lefebvre C, Li Wan Po A, Li L, Roberts I, Schierhout G, Albumin Reviewers. Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev. 2004. doi:10.1002/14651858.CD001208.pub2.
Coughlin MT, Angus DC. Economic evaluation of new therapies in critical illness. Crit Care Med. 2003;31:S7–16.
Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Gines P, Rodes J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–9.
Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K, German Competence Network Sepsis (SepNet). Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–39.
Rozich JD, Paul RV. Acute renal failure precipitated by elevated colloid osmotic pressure. Am J Med. 1989;87:359–60.
Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–35.
Legendre C, Thervet E, Page B, Percheron A, Noël LH, Kreis H. Hydroxyethyl starch and osmotic-nephrosis-like lesions in kidney transplantation. Lancet. 1993;342:248–9.
Cittanova ML, Leblanc I, Legendre CH, Mouquet C, Riou B, Coriat P. Effect of hydroxyethyl starch in brain-dead kidney donors on renal function in kidney-transplant recipients. Lancet. 1996;348:1620–2.
Deman A, Peeters P, Sennesael J. Hydroxyethyl starch does not impair immediate renal function in kidney transplant recipients: a retrospective, multicentre analysis. Nephrol Dial Transplant. 1999;14:1517–20.
Blasco V, Leone M, Antonini F, Geissler A, Albanèse J, Martin C. Comparison of the novel hydroxyethyl starch 130/0.4 and hydroxyethyl starch 200/0.6 in brain-dead donor resuscitation on renal function after transplantation. Br J Anaesth. 2008;100:504–8.
Sakr Y, Payen D, Reinhart K, Sipmann FS, Zavala E, Bewley J, Marx G, Vincent JL. Effects of hydroxyethyl starch administration on renal function in critically ill patients. Br J Anaesth. 2007;98:216–24.
Kumle B, Boldt J, Piper S, Schmidt C, Suttner S, Salopek S. The influence of different intravascular volume replacement regimens on renal function in the elderly. Anesth Analg. 1999;89:1124–30.
Boldt J, Brenner T, Lehmann A, Lang J, Kumle B, Werling C. Influence of two different volume replacement regimens on renal function in elderly patients undergoing cardiac surgery: comparison of new starch preparation with gelatin. Intensive Care Med. 2003;29:763–9.
Boldt J, Brosch C, Röhm K, Papsdorf M, Mengistu A. Comparison of the effects of gelatin and a modern hydroxyethyl starch solution on renal function and inflammatory response in elderly cardiac surgery patients. Br J Anaesth. 2008;100:457–64.
Mahmood A, Gosling P, Vohra RK. Randomized clinical trial comparing the effects on renal function of hydroxyethyl starch or gelatin during aortic aneurysm surgery. Br J Surg. 2007;94:427–33.
Godet G, Lehot JJ, Janvier G, Steib A, De Castro V, Coriat P. Safety of HES 130/0.4 (Voluven®) in patients with preoperative renal dysfunction undergoing abdominal aortic surgery: a prospective, randomized, controlled, parallel-group multicentre trial. Eur J Anaesthesiol. 2008;25:986–94.
Jungheinrich C, Scharpf R, Wargenau M, Bepperling F, Baron JF. The pharmacokinetics and tolerability of an intravenous infusion of the new hydroxyethyl starch 130/0.4 (6%, 500 mL) in mild-to-severe renal impairment. Anesth Analg. 2002;95:544–51.
Dehne M, Muhling J, Sablotzki A, Dehne K, Sucke N, Hempelmann G. Hydroxyethyl starch (HES) does not directly affect renal function in patients with no prior renal impairment. J Clin Anesth. 2001;13:103–11.
Neff TA, Doelberg M, Jungheinrich C, Sauerland A, Spahn DR, Stocker R. Repetitive large-dose infusion of the novel hydroxyethyl starch 130/0.4 in patients with severe head injury. Anesth Analg. 2003;96:1453–9.
Treib J, Haass A, Pindur G. Coagulation disorders caused by hydroxyethyl starch. Thromb Haemost. 1997;78:974–83.
Jonville-Bera AP, Autret-Leca E, Gruel Y. Acquired type I von Willebrand’s disease associated with highly substituted hydroxyethyl starch. N Engl J Med. 2001;345:622–3.
Langeron O, Doelberg M, Ang ET, Bonnet F, Capdevila X, Coriat R. Voluven®, a lower substituted novel hydroxyethyl starch (HES 130/0.4), causes fewer effects on coagulation in major orthopedic surgery than HES 200/0.5. Anesth Analg. 2001;92:855–62.
Joorgensen KA, Stoffersen E. Heparin-like activity of albumin. Thromb Res. 1979;16:69–74.
Niemi T, Kuitunen A. Artificial colloids impair haemostasis. An in vitro study using thromboelastometry coagulation analysis. Acta Anaesthesiol Scand. 2005;49:373–8.
Niemi TT, Suojaranta-Ylinen RT, Kukkonen SI, Kuitunen AH. Gelatin and hydroxyethyl starch, but not albumin, impair hemostasis after cardiac surgery. Anesth Analg. 2006;102:998–1006.
Tabuchi N, de Haan J, Gallandat Huet RC, Boonstra PW, van Oeveren W. Gelatin use impairs platelet adhesion during cardiac surgery. Thromb Haemost. 1995;74:1447–51.
de Jonge E, Levi M, Berends F, van der Ende AE, ten Cate JW, Stoutenbeek CP. Impaired haemostasis by intravenous administration of a gelatin-based plasma expander in human subjects. Thromb Haemost. 1998;79:286–90.
Innerhofer P, Fries D, Margreiter J, Klingler A, Kuhbacher G, Wachter B, Oswald E, Salner E, Frischhut B, Schobersberger W. The effects of perioperatively administered colloids and crystalloids on primary platelet-mediated hemostasis and clot formation. Anesth Analg. 2002;95:858–65.
Beyer R, Harmening U, Rittmeyer O, Zielmann S, Mielck F, Kazmaier S, Kettler D. Use of modified fluid gelatin and hydroxyethyl starch for colloidal volume replacement in major orthopaedic surgery. Br J Anaesth. 1997;78:44–50.
Huttner I, Boldt J, Haisch G, Suttner S, Kumle B, Schulz H. Influence of different colloids on molecular markers of haemostasis and platelet function in patients undergoing major abdominal surgery. Br J Anaesth. 2000;85:417–23.
de Jonge E, Levi M. Effects of different plasma substitutes on blood coagulation: a comparative review. Crit Care Med. 2001;29:1261–7.
Knutson JE, Deering JA, Hall FW, Nuttall GA, Schroeder DR, White RD, Mullany CJ. Does intraoperative hetastarch administration increase blood loss and transfusion requirements after cardiac surgery? Anesth Analg. 2000;90:801–7.
Wilkes MM, Navickis RJ, Sibbald WJ. Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: a meta-analysis of postoperative bleeding. Ann Thorac Surg. 2001;72:527–33.
Boldt J, Haisch G, Suttner S, Kumle B, Schellhaass A. Effects of a new modified, balanced hydroxyethyl starch preparation (Hextend®) on measures of coagulation. Br J Anaesth. 2002;89:722–8.
Kozek-Langenecker SA, Jungheinrich C, Sauermann W. Van der Linden P. The effects of hydroxyethyl starch 130/0.4 (6%) on blood loss and use of blood products in major surgery: a pooled analysis of randomized clinical trials. Anesth Analg. 2008;107:382–90.
Boldt J, Schölhorn T, Mayer J, Piper S, Suttner S. The value of an albumin-based intravascular volume replacement strategy in elderly patients undergoing major abdominal surgery. Anesth Analg. 2006;103:191–9.
Brandstrup B, Tønnesen H, Beier-Holgersen R, Hjortsø E, Ørding H, Lindorff-Larsen K, Rasmussen MS, Lanng C, Wallin L, Iversen LH, Gramkow CS, Okholm M, Blemmer T, Svendsen PE, Rottensten HH, Thage B, Riis J, Jeppesen IS, Teilum D, Christensen AM, Graungaard B, Pott F, Danish Study Group on Perioperative Fluid Therapy. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238:641–8.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Niemi, T.T., Miyashita, R. & Yamakage, M. Colloid solutions: a clinical update. J Anesth 24, 913–925 (2010). https://doi.org/10.1007/s00540-010-1034-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-010-1034-y