Abstract
Background
Exercise, particularly resistance exercise, is beneficial for sarcopenia in patients with liver cirrhosis. However, the effects of exercise on events remain unclear. We aimed to examine the effects of exercise on serious events in patients with liver cirrhosis using a meta-analysis of randomized controlled trials (RCTs).
Methods
A literature search was conducted in 2022. Eleven RCTs were selected for the meta-analysis (exercise group, n = 232; control group, n = 193). Serious events were defined as death or serious complications according to the original articles. A meta-analysis was performed using a random-effects model. The primary outcome was the incidence of serious events.
Results
In the 11 RCTs, the incidence of serious events was 5.6% (13/232) and 12.3% (24/193) in the exercise and control groups, respectively. However, a meta-analysis demonstrated no significant difference in the incidence of serious events between the two groups (risk difference [RD] − 0.03, 95% confidence intervals (CI) − 0.07 to 0.02). In a stratification analysis based on a combination of aerobic and resistance exercise, five RCTs (n = 185) were enrolled. The incidence of serious events was 6.25% (7/112) and 24.7% (18/73) in the combination exercise and control groups, respectively. A meta-analysis demonstrated a significant reduction in the incidence of serious events in the combination exercise group compared with the control group (RD − 0.12; 95% CI − 0.21 to − 0.03).
Conclusions
Resistance exercise in combination with aerobic exercise reduces serious events in patients with liver cirrhosis. A combination of aerobic and resistance exercise may be beneficial to improve the prognosis of patients with liver cirrhosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sarcopenia and frailty are highly prevalent in patients with liver cirrhosis [1,2,3,4]. Sarcopenia and frailty are associated with an increased risk of serious events, including hepatic encephalopathy, liver failure, hepatocellular carcinoma, and infection [1,2,3,4]. A recent meta-analysis further demonstrated that sarcopenia is independently associated with a higher risk of mortality in patients with liver cirrhosis [5]. Moreover, accumulating evidence suggests that the 6-min walking distance (6MWD), a frailty index that measures the distance covered over a period of 6 min, is an independent prognostic factor in patients with liver cirrhosis [6,7,8]. Thus, several major clinical practice guidelines have focused on sarcopenia and frailty as important factors in the management of patients with liver cirrhosis [1,2,3,4, 9]. In particular, sarcopenia is proposed as an initial assessment item for the management of patients with liver cirrhosis in the Evidence-Based Clinical Practice Guidelines for Liver Cirrhosis 2020 [1, 2] and the usefulness of the guideline has been validated [10].
Exercise is fundamental for the prevention and improvement of sarcopenia and frailty in patients with liver cirrhosis [11]. Exercise improves aerobic endurance, muscle mass, and strength in patients with liver cirrhosis [12,13,14,15]. Exercise has also been reported to improve health-related quality of life, such as fatigue [12]. Resistance exercise, in particular, has a prominent effect on sarcopenia [12]. Resistance exercise reportedly increases muscle strength and size and has beneficial effects on general performance measures in patients with liver cirrhosis [13, 14, 16, 17].
Generally, the beneficial effects of exercise on sarcopenia have been established; however, opposing results have been reported regarding the effects of exercise on serious events in patients with liver cirrhosis. A previous study reported that exercise increases portal pressure and the risk of variceal bleeding in patients with liver cirrhosis [18]. Another study demonstrated that exercise causes marked impairment of renal function in patients with ascites [19]. These previous studies have highlighted the potential risks of exercise for serious events and subsequent poor prognosis. In contrast, several studies have reported that the incidence of serious events was equal between the exercise and control groups [12, 14, 15, 20, 21]. Moreover, a few studies demonstrated the beneficial effects of exercise on prognostic factors, including nutritional status, hepatic venous pressure gradient, and insulin resistance [21, 22]. Thus, the effect of exercise on serious events remains controversial, and no meta-analysis has addressed this clinical question.
This study aimed to investigate the effects of exercise, particularly resistance exercise, on the incidence of serious events in patients with liver cirrhosis through a meta-analysis of randomized controlled trials (RCTs).
Methods
Study design
This was a systematic review and meta-analysis of RCTs. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement [23].
Data sources
Published literature up to January 30, 2022, was searched using PubMed, Ovid MEDLINE, Scopus, and Cochrane Library literature databases.
Search terms
Potential articles were identified by search terms and Medical Subject Headings terms relevant to “exercise” OR “training” OR “physical activity”; “liver cirrhosis” OR “cirrhosis” OR “hepatic cirrhosis.” Database searches were organized according to the PICOS model (Population, Intervention, Control, Outcome, Study design) [24].
Six investigators (T.K., S.K., K.H., M.T., J.T., and H.N.) independently reviewed the titles and abstracts of the identified studies. References in each report that met the selection criteria were manually searched to identify other potentially relevant studies. All relevant abstracts and full-text peer-reviewed articles published in English were analyzed.
Inclusion and exclusion criteria
Studies were selected if they met the following inclusion criteria: (1) RCT design; (2) evaluated the effects of aerobic exercise, resistance exercise, or a combination of aerobic and resistance exercises on any outcome in patients with liver cirrhosis; and (3) included information on events, including death and serious complications, during the study period. Studies were excluded if they (1) were not RCTs (non-randomized controlled clinical trials, before-and-after clinical trials, or observational cohort studies); (2) were not original research (systematic reviews, narrative reviews, commentaries, or editorials), were case reports or conference abstracts; (3) included no information about events; (5) were animal studies; or (6) were not published in English.
Primary and secondary outcomes
The primary outcome was the incidence of serious events, defined as death or any serious complications, including hepatic failure, ascites, infection, fracture, hepatocellular carcinoma, and extrahepatic cancer according to original articles reported. We also assessed the following variables as secondary outcomes: incidence of non-serious events, changes in Child–Pugh score, the model for end-stage liver disease (MELD) score, chronic liver disease questionnaire (CLDQ) [20], 6MWD, peak O2 uptake, and maximum heart rate.
Data extraction
Ten investigators (T.K., R.H., D.N., T.T., M.K., S.K., K.H., M.T., J.T., and H.N.) individually screened the records and extracted the data. We extracted the following data from each study: first author's name, publication year, study design, number of subjects, age, sex, sample size, type of exercise (aerobic exercise/ combination of aerobic and resistance exercises), and exercise intervention (exercise time per session, frequency, and period). We also collected data on the incidence of serious events, including death and any serious events; the incidence of non-serious events; and the mean and standard deviation (SD) of the following outcomes at the baseline and end of the study: Child–Pugh score, MELD score, CLDQ, 6MWD, peak O2 uptake, and maximum heart rate.
Quality assessment of the included studies
Two investigators (T.K. and A.K.) independently assessed the quality of the included studies. Randomized controlled trials were assessed using the criteria formulated by the Cochrane Effective Practice and Organization of Care group [25].
Data synthesis
The mean and standard deviation of the net changes in Child–Pugh score, MELD score, CLDQ, 6MWD, peak O2 uptake, and maximum heart rate were calculated for each study. When the outcomes were reported as quartile measures, the mean and SD were calculated from the quartiles using the formula described by Wan et al. [26]. For the SD of the change from baseline to end point, we used the correlation coefficient r = 0.7 as a conservative estimate, as previously described [27].
Statistical analysis
All statistical analyses were performed by biostatisticians (A.K., M.K., K.E., and S.I.). We used risk differences as a summary statistic for the incidence of serious and non-serious events. The standard mean difference (SMD) and 95% confidence intervals (CI) were used as summary statistics for changes in the Child–Pugh score, MELD score, CLDQ, 6MWD, peak O2 uptake, and maximum heart rate [28].
A meta-analysis was performed using the Review Manager Software (Review Manager 5.3; Cochrane Collaboration, Oxford, UK). A random-effects model was applied when the heterogeneity test was P < 0.10. Heterogeneity between studies was evaluated using Cochran’s Q test, I2 index, and t2 test. Publication bias was assessed using the visual assessment of funnel plots, Begg’s test, and Egger’s regression asymmetry test. Statistical significance was set at P < 0.05.
Results
Search results
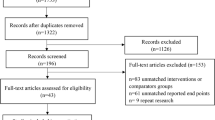
We identified 3004 articles using the pre-specified search criteria. Six additional reports were identified in the references (Fig. 1). After removing duplicates (n = 1154), 1856 articles were screened. We removed 1842 articles for the following reasons: studies unrelated to cirrhosis (n = 213), review articles (n = 195), non-RCTs (n = 144), editorials or letters (n = 73), and unrelated research (n = 1217). The remaining 11 studies were included in the meta-analysis (Analysis 1).
To investigate the impact of a combination of aerobic and resistance exercise on serious and non-serious events, we excluded studies on aerobic exercise alone (n = 5) and self-managed training (n = 1) from the 11 articles. The remaining 5 studies that used resistance exercise were included in the meta-analysis (Analysis 2).
Characteristics of included studies for meta-analysis
All the included studies were RCTs conducted between 2013 and 2020 (Table 1). A total of 425 patients with liver cirrhosis were included; 232 and 193 patients were classified into the exercise and control groups, respectively. The mean age of the participants in the exercise group ranged from 41.6 to 68.0 years. The study durations ranged from 4 to 27 weeks. The types of exercise included aerobic exercise (n = 5), a combination of aerobic and resistance exercises (n = 5), and self-managed training (n = 1).
Quality assessment
The quality of the included studies is summarized in Supplementary Table 1. All studies had a low risk of random sequence generation (Supplementary Table 1). As patients in the exercise group could not be blinded, the quality of blinding of participants and researchers, and the blinding of outcome assessments were at moderate risk. All studies had a low risk of incomplete outcome data and selective reporting.
Heterogeneity among the studies and power analysis for meta-analysis
The I2 and t2 statistics did not show heterogeneity among the studies in the analysis of the incidence of serious events (Figs. 2A, 5A), incidence of non-serious events (Figs. 2B, 5B), changes in Child–Pugh score (Fig. 3A), MELD score (Fig. 3B), CLDQ (Fig. 3C), 6MWD (Fig. 4A), and peak O2 uptake (Fig. 4B). Heterogeneity among the studies was observed only in the analysis of the maximum heart rate (Fig. 4C).
Publication bias
Publication bias was examined using funnel plots (Supplementary Figs. 1A–H, Fig. 2A, B). In Analysis 1, Begg’s test showed no publication bias for the incidence of serious events (Supplementary Fig. 1A). Publication bias was observed for the incidence of non-serious events and maximum heart rate (Supplementary Fig. 1B and H). However, no publication bias was observed in the changes in Child–Pugh score, MELD score, CLDQ, 6MWD, or peak O2 uptake (Supplementary Figs. 1A–G).
In the Egger's regression asymmetry test, there was no publication bias for the incidence of serious events, Child–Pugh score, MELD score, CLDQ, 6MWD, peak O2 uptake, or maximum heart rate (Supplementary Figs. 1A, C–H). Publication bias was observed only for the incidence of non-serious events (Supplementary Fig. 1B).
In Analysis 2, Begg’s test showed no publication bias for the incidence of serious or non-serious events (Supplementary Figs. 2A, B). In Egger's regression asymmetry test, no publication bias was observed for the incidence of serious or non-serious events (Supplementary Figs. 2A, B).
Analysis 1: meta-analysis of the effect of exercise on outcomes in patients with liver cirrhosis
Serious events
In all 11 analyzed studies, no falls or bone fractures were observed in the exercise group, whereas one patient in the control group fractured a bone in the foot (Table 2). Moreover, no patients died in the exercise group, whereas four patients died in the control group (Table 2).
The incidence of serious events was 5.6% (13/232) and 12.3% (24/193) in the exercise and control groups, respectively (Fig. 2A). The exercise group showed an approximately 7% lower incidence of serious events. However, no significant difference was observed in the incidence of serious events between the exercise and control groups (Fig. 2A).
Non-serious events
The incidence of non-serious events was 2.6% (6/232) and 1.0% (2/193) in the exercise and control groups, respectively. There was no significant difference in the incidence of nonserious events between the exercise and control groups (Fig. 2B).
Child–Pugh and MELD scores
No significant difference was observed in the changes in Child–Pugh and MELD scores between the exercise and control groups (Figs. 3A, B).
CLDQ
There were no significant differences in the changes in CLDQ scores between the exercise and control groups (Fig. 3C).
6MWD, peak O2 uptake, and maximum heart rate
Five studies examined the 6MWD, and all studies demonstrated an improvement in the 6MWD in the exercise group compared with the control group. Overall, the 6MWD significantly improved in the exercise group compared with the control group (Fig. 4A).
There were no significant differences in peak O2 uptake or maximum heart rate between the exercise and control groups (Fig. 4B, C).
Analysis 2: meta-analysis of the effect of exercise, including resistance exercise, on outcomes in patients with liver cirrhosis
Serious events
In all five analyzed studies using a combination of aerobic and resistance exercise, the incidence of serious events was 6.25% (7/112) and 24.7% (18/73) in the combination exercise and control groups, respectively. The combination exercise group showed an approximately 18% lower incidence of serious events. Overall, the incidence of serious events was significantly lower in the combination exercise group than in the control group (Fig. 5A).
Non-serious events
The incidence of non-serious events was 4.5% (5/112) and 2.7% (2/73) in the combination exercise and control groups, respectively. There was no significant difference in the incidence of nonserious events between the combination exercise and control groups (Fig. 5B).
Discussion
This meta-analysis demonstrated that exercise did not negatively affect the incidence of serious or non-serious events, liver function, or patient-reported outcomes. We further found that exercise significantly improved the 6MWD. Moreover, our study is the first to reveal that a combination of aerobic and resistance exercise significantly reduces the incidence of serious events in patients with liver cirrhosis.
Our meta-analysis demonstrated that exercise did not negatively affect the incidence of serious/non-serious events or liver function, as evaluated by Child–Pugh and MELD scores. On the other hand, previous studies reported that exercise causes an increase in portal pressure, a reduction of hepatic blood flow [18], and an impairment in renal function in patients with cirrhosis [19]. These studies suggested a risk of serious adverse events associated with exercise. However, the previous studies were single-arm before-and-after studies conducted in the late 1990s, and the predominant etiology of liver cirrhosis was alcohol consumption, hepatitis B virus, and hepatitis C virus. Our meta-analysis evaluated only RCTs conducted after 2013 and included patients with NASH that exercise is a fundamental therapy. Thus, differences in the study design and etiology of liver cirrhosis may be possible reasons for the discrepancy between previous studies and our meta-analysis. None of the RCTs reported harmful effects of exercise. For aerobic exercise, the median protocol was 60 min/session and 3 times/week for 10 weeks. For a combination of aerobic and resistance exercise, the median protocol was 30 min/session and 3 times/week for 12 weeks.
Exercise therapy is not recommended in cirrhotic patients with Child–Pugh class C in the Evidence-Based Clinical Practice Guidelines for Liver Cirrhosis 2020 co-edited by The Japanese Society of Gastroenterology and The Japan Society of Hepatology [1, 2]. However, after the publication of the guidelines, three RCTs demonstrated no harmful effects of exercise even in patients with cirrhosis, of whom over 50% were Child–Pugh class B/C [17, 20, 29]. The combined data from the three RCTs also showed the incidence of serious events was lower in the exercise group compared to the control group (2.8% [n = 2/71] vs. 13.5% [n = 5/37]). Although the duration of exercise was short (8–12 weeks) in the three RCTs, these findings may suggest that suitable exercises tailored for each individual may be feasible and safe for patients with liver cirrhosis, regardless of disease severity.
We demonstrated that exercise significantly improved the 6MWD. The 6MWD has been reported to correlate with Child–Pugh and MELD scores [7, 8]. The 6MWD has also been reported to be a predictor of clinical decompensation in patients with liver cirrhosis [30]. Moreover, the 6MWD was an independent prognostic factor for patients with liver cirrhosis [6,7,8]. It remains unclear why 6MWD is associated with various liver-related outcomes. Recently, Duarte-Rojo et al. reported that the 6MWD correlates with the liver frailty index and can be used as a frailty metric in patients with liver cirrhosis [31]. Frailty is associated with an increased risk for serious events in patients with liver cirrhosis [1,2,3,4]. Accordingly, the 6MWD may be associated with various liver-related outcomes by reflecting frailty status. In this study, all five RCTs demonstrated a favorable effect of exercise on 6MWD, which may be interpreted as the 6MWD being a useful index for evaluating the effects of exercise in patients with liver cirrhosis. The 6MWD is generally measured by physiotherapists, and collaboration between gastroenterologists and rehabilitation is becoming increasingly important in the management of patients with liver cirrhosis.
To the best of our knowledge, this is the first study to demonstrate that a combination of aerobic and resistance exercise significantly reduces the incidence of serious events in patients with liver cirrhosis. In contrast to aerobic exercise, resistance exercise causes the muscles to contract against external resistance and promotes skeletal muscle hypertrophy [32]. Therefore, resistance exercise is used to improve sarcopenia in patients with various chronic diseases [33]. Sarcopenia is highly prevalent in patients with liver cirrhosis and is a risk factor for various severe events, including liver failure, hepatocellular carcinoma, and infection [34, 35]. Tandon et al. reported that resistance exercise improved sarcopenia in patients with liver cirrhosis [36]. Accordingly, resistance exercise may reduce the incidence of serious events by reducing sarcopenia. Moreover, Tantai et al. performed a meta-analysis and demonstrated that sarcopenia is an independent risk factor for mortality in patients with cirrhosis [5]. In our meta-analysis, three patients died in the control group, while no patients died in the combination exercise group. We could not examine the impact of a combination of aerobic and resistance exercise on mortality owing to the small number of deaths. We also have to be cautious in the interpretation of the results, because of an asymmetrical pattern in the funnel plots. However, these data suggest that a combination of aerobic and resistance exercise may be beneficial in suppressing serious events, leading to improved survival in patients with liver cirrhosis.
This study has several limitations. First, the number of deaths was small owing to the short duration of the study period. Second, exercise therapy was not uniform, and we could not determine the suitable intensity and duration of exercise for patients with liver cirrhosis. Third, we were unable to examine other factors associated with serious events, including malnutrition [37], nutritional therapy, myosteatosis [38], and medications due to the heterogeneity of interventions across the studies. Fourth, no studies provided information about the follow-up period after the intervention, which could affect the onset of events. Thus, further studies should focus on the long-term effects of exercise on prognosis using identical exercise protocols, along with information on malnutrition, nutritional therapy, quality of skeletal muscle, medications, and follow-up periods after the intervention in patients with liver cirrhosis.
This meta-analysis demonstrated no negative impact of exercise on the incidence of serious or non-serious events, liver function, or patient-reported outcomes. In addition, we found that exercise significantly improved the 6MWD, a metric of frailty. Furthermore, we first revealed that resistance exercise in combination with aerobic exercise significantly reduced the incidence of serious events in patients with liver cirrhosis. These results suggest that exercise therapy can be safely administered and may improve frailty in patients with cirrhosis. In particular, a combination of aerobic and resistance exercise may be beneficial in suppressing serious events and improving prognosis in patients with liver cirrhosis.
References
Yoshiji H, Nagoshi S, Akahane T, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. J Gastroenterol. 2021;56:593–619.
Yoshiji H, Nagoshi S, Akahane T, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2020. Hepatol Res. 2021;51:725–49.
Lai JC, Tandon P, Bernal W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the american association for the study of liver diseases. Hepatology. 2021;74:1611–44.
European Association for the Study of the Liver, Electronic address eee. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–60.
Tantai X, Liu Y, Yeo YH, et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;76:588–99.
Alameri HF, Sanai FM, Al Dukhayil M, et al. Six minute walk test to assess functional capacity in chronic liver disease patients. World J Gastroenterol. 2007;13:3996–4001.
Carey EJ, Steidley DE, Aqel BA, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16:1373–8.
Pimentel C, Amaral ACC, Gonzalez AM, et al. Six-minute walking test performance is associated with survival in cirrhotic patients. World J Hepatol. 2021;13:1791–801.
Korean Association for the Study of the L. KASL clinical practice guidelines for liver cirrhosis: varices, hepatic encephalopathy, and related complications. Clin Mol Hepatol. 2020;26:83–127.
Hanai T, Nishimura K, Miwa T, et al. Usefulness of nutritional therapy recommended in the Japanese Society of Gastroenterology/Japan Society of Hepatology evidence-based clinical practice guidelines for liver cirrhosis 2020. J Gastroenterol. 2021;56:928–37.
Tandon P, Ismond KP, Riess K, et al. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol. 2018;69:1164–77.
Zenith L, Meena N, Ramadi A, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol. 2014;12(1920–6): e2.
Roman E, Garcia-Galceran C, Torrades T, et al. Effects of an exercise programme on functional capacity, body composition and risk of falls in patients with cirrhosis: a randomized clinical trial. PLoS One. 2016;11: e0151652.
Aamann L, Dam G, Borre M, et al. Resistance training increases muscle strength and muscle size in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2020;18(1179–87): e6.
Roman E, Torrades MT, Nadal MJ, et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci. 2014;59:1966–75.
Williams FR, Vallance A, Faulkner T, et al. Home-based exercise in patients awaiting liver transplantation: a feasibility study. Liver Transpl. 2019;25:995–1006.
Chen HW, Ferrando A, White MG, et al. Home-based physical activity and diet intervention to improve physical function in advanced liver disease: a randomized pilot trial. Dig Dis Sci. 2020;65:3350–9.
Garcia-Pagan JC, Santos C, Barbera JA, et al. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology. 1996;111:1300–6.
Salo J, Guevara M, Fernandez-Esparrach G, et al. Impairment of renal function during moderate physical exercise in cirrhotic patients with ascites: relationship with the activity of neurohormonal systems. Hepatology. 1997;25:1338–42.
Lai JC, Dodge JL, Kappus MR, et al. A multicenter pilot randomized clinical trial of a home-based exercise program for patients with cirrhosis: the strength training intervention (STRIVE). Am J Gastroenterol. 2021;116:717–22.
Macias-Rodriguez RU, Ilarraza-Lomeli H, Ruiz-Margain A, et al. Changes in hepatic venous pressure gradient induced by physical exercise in cirrhosis: results of a pilot randomized open clinical trial. Clin Transl Gastroenterol. 2016;7: e180.
Kaibori M, Ishizaki M, Matsui K, et al. Perioperative exercise for chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. Am J Surg. 2013;206:202–9.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Saaiq M, Ashraf B. Modifying, “Pico” Question into “Picos” model for more robust and reproducible presentation of the methodology employed in a scientific study. World J Plast Surg. 2017;6:390–2.
Effective Practice and Organisation of Care (EPOC). EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services; 2015. http://www.epoccochraneorg/epoc-specific-resources-review-authors.
Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135.
Kawaguchi T, Charlton M, Kawaguchi A, et al. Effects of Mediterranean diet in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression analysis of randomized controlled trials. Semin Liver Dis. 2021;41:225–34.
Izuhara K, Nunomura S, Nanri Y, et al. Periostin: an emerging biomarker for allergic diseases. Allergy. 2019;74:2116–28.
Wallen MP, Keating SE, Hall A, et al. Exercise training is safe and feasible in patients awaiting liver transplantation: a pilot randomized controlled trial. Liver Transpl. 2019;25:1576–80.
Henrique DMN, Malaguti C, Limonge TM, et al. Six-minute walking test as a predictor of clinical decompensation in patients with cirrhosis. J Gastrointestin Liver Dis. 2021;30:103–9.
Duarte-Rojo A, Brown RA, Bloomer PM, et al. A telemedicine alternative to the 6-minute walk test using personal activity trackers in liver transplant candidates. Transplant Direct. 2022;8: e1347.
Grgic J, McLlvenna LC, Fyfe JJ, et al. Does aerobic training promote the same skeletal muscle hypertrophy as resistance training? A systematic review and meta-analysis. Sports Med. 2019;49:233–54.
Hurst C, Robinson SM, Witham MD, et al. Resistance exercise as a treatment for sarcopenia: prescription and delivery. Age Ageing. 2022. https://doi.org/10.1093/ageing/afac003.
Praktiknjo M, Clees C, Pigliacelli A, et al. Sarcopenia is associated with development of acute-on-chronic liver failure in decompensated liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. Clin Transl Gastroenterol. 2019;10: e00025.
Topan MM, Sporea I, Danila M, et al. Impact of sarcopenia on survival and clinical outcomes in patients with liver cirrhosis. Front Nutr. 2021;8: 766451.
Tandon P, Dunn MA, Duarte-Rojo A. Resistance training reduces risk of sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol. 2020;18:1036–9.
Miwa T, Hanai T, Nishimura K, et al. Usefulness of the global leadership initiative on malnutrition criteria to predict sarcopenia and mortality in patients with chronic liver disease. Hepatol Res. 2022;52:928–36.
Sugiyama Y, Ishizu Y, Ando Y, et al. An improved method to assess skeletal muscle mass in patients with liver cirrhosis based on computed tomography images. Hepatol Res. 2022;52:937–46.
Mansouri PM, Ghadami MM, Najafi SSM, et al. The effect of self-management training on self-efficacy of cirrhotic patients referring to transplantation center of nemazee hospital: a randomized controlled clinical trial. Int J Community Based Nurs Midwifery. 2017;5:256–63.
Kruger C, McNeely ML, Bailey RJ, et al. Home exercise training improves exercise capacity in cirrhosis patients: role of exercise adherence. Sci Rep. 2018;8:99.
Acknowledgements
This work was supported by the Research Program on Hepatitis from the Japan Agency for Medical Research and Development, AMED, Grant Number 22fk0210094, and by MHLW Health Labour Sciences Research Grant Number JPMH23HC2002.
Author information
Authors and Affiliations
Contributions
TK and SY participated in the study conception, design, and funding acquisition. TK, SK, KHirota, MT, JT, and HN performed the data searches. TK, RH, DN, TT, MK, SK, KHirota, MT, JT, and HN participated in the data extraction. TK, AK, HM, and KHiraoka performed the quality assessment of the studies. AK and MK performed the analysis. TK, SK, KHirota, MT, JT, HN, HM, KHiraoka, KE, SI, and SY interpreted the data. TK, AK, RH, DN, TT, and MK drafted the manuscript. HM, KHiraoka, KE, SI, and SY participated in the critical revision.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
535_2023_2060_MOESM2_ESM.pptx
Supplementary Fig. 1 Funnel plots for the effect of exercise on outcomes. Incidence of (A) serious and (B) non-serious events, (C) Child–Pugh score, (D) MELD score, (E) CLDQ, (F) 6-minute walking distance, (G) peak O2 uptake, and (H) maximum heart rate. Abbreviations: MELD, the model for end-stage liver disease; CLDQ, chronic liver disease questionnaire. Supplementary Fig. 2 Funnel plots for the effect of a combination of aerobic and resistance exercise on outcomes. Incidence of (A) serious and (B) non-serious events (PPTX 351 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kawaguchi, T., Kawaguchi, A., Hashida, R. et al. Resistance exercise in combination with aerobic exercise reduces the incidence of serious events in patients with liver cirrhosis: a meta-analysis of randomized controlled trials. J Gastroenterol 59, 216–228 (2024). https://doi.org/10.1007/s00535-023-02060-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-023-02060-0