Abstract
Background
A diagnostic marker is needed enabling early and specific diagnosis of hepatocellular carcinoma (HCC) associated with non-alcoholic steatohepatitis (NASH). Our recent findings have indicated that circulating apoptosis inhibitor of macrophage (AIM), which usually associates with IgM pentamer in the blood, is activated by its dissociation from IgM. We investigated the serum levels of IgM-free AIM for AIM activation and its possible relationship with development of HCC in NASH.
Methods
Serum levels of IgM-associated and IgM-free AIM were evaluated in patients with non-alcoholic fatty liver, NASH, and NASH-HCC using enzyme-linked immunosorbent assays and immunoblots. Liver biopsy specimens were graded and staged using Brunt’s classification.
Results
Forty-two patients with fatty liver, 141 with NASH, and 26 with NASH-HCC were evaluated. Patients with stage 4 or grade 3 NASH (with or without HCC) exhibited significantly higher levels of both IgM-free and total AIM than those with fatty liver, whereas the ratio of IgM-free-to-total AIM was equivalent in these groups. Among patients with the same fibrosis stage of NASH, those with HCC had significantly higher IgM-free but not total AIM levels, resulting in a proportional increase in the IgM-free/total AIM ratio. Analysis of the areas under the receiver operating characteristic curves indicated the high sensitivity of the IgM-free AIM for NASH-HCC.
Conclusions
Our observations suggest the activation of AIM in blood in the presence of NASH-HCC, with a significant increase in IgM-free AIM levels. IgM-free AIM serum levels appear to be a sensitive diagnostic marker for NASH-HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most common causes of chronic liver disease in the world. NAFLD pathology ranges from non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis (NASH), and NASH-associated hepatocellular carcinoma (NASH-HCC) [1,2,3].
Liver biopsy remains the gold standard for diagnosis and is important to evaluate the grade of liver injury and stage of fibrosis. Clinically, it is very important to identify patients with NASH at high risk for HCC. NASH-HCC is considered to be derived mainly from cirrhotic liver tissue [4, 5] but one fourth to one third of NASH-HCC occurs in non-cirrhotic livers [6,7,8]. Our previous study demonstrated that most patients with NASH-HCC had no significant elevation of serum α-fetoprotein (AFP) level [7]. Therefore, it is very important to develop more useful markers for the early detection of HCC in patients with NASH.
Liver injury, fibrosis, and carcinogenesis are positively and negatively regulated by hepatic macrophages, which include monocyte-derived macrophages and liver-resident Kupffer cells. In the steady state, Kupffer cells in the hepatic sinusoids regulate local immune responses and remove bacteria, toxins, and microbial debris derived from the gastrointestinal tract [9]. Therefore, Kupffer cells have a crucial homeostatic role in protecting the host and are capable of inducing immune responses. In the process of hepatic fibrogenesis and hepatocarcinogenesis, the number of monocyte-derived macrophages markedly increases, while the number of Kupffer cells decreases, suggesting that monocyte-derived macrophages have important functions during this pathologic process [10].
Apoptosis inhibitor of macrophage (AIM), also known as CD5L, is produced by tissue macrophages [11]. AIM associates with the immunoglobulin M (IgM) pentamer in blood, which protects AIM from renal excretion and thus maintains relatively high circulating concentrations [12, 13]. Circulating AIM is incorporated into adipocytes and hepatocytes, resulting in inactivation of cytoplasmic fatty acid synthase via direct binding, thus preventing lipid accumulation [14]. Serum AIM levels are increased in patients with liver damage and HCC [15]. More importantly, AIM accumulates on the HCC cell surface and activates the complement cascade, thereby provoking cellular necrosis in HCC, a mechanism demonstrated to prevent the development of HCC lesions in mice [16]. These findings suggest that AIM plays key roles in protecting liver cells from lipid accumulation and carcinogenesis and that the release of free AIM from IgM is an essential process, allowing it to exert its biological functions. This hypothesis is well-supported by our previous observation that AIM is activated markedly by its dissociation from IgM during acute kidney injury, which facilitates recovery of kidney functions by inducing removal of necrotic cell debris obstructing the proximal renal tubules [17]. These findings lead to consideration of possible therapeutic use of IgM-free AIM if it provides clinical benefits either in preventing or treating HCC.
In this study, we investigated serum AIM levels, particularly IgM-free AIM, in patients with NAFL, NASH, and NASH-HCC. The clinical value of AIM for early diagnosis of NASH-HCC will be discussed.
Methods
Patients
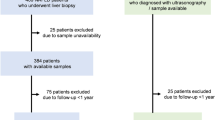
A total of 209 patients, 183 with biopsy-proven NAFLD but no malignancy and 26 with NASH-HCC, were evaluated in this study. All liver biopsies had been performed at Saiseikai Suita Hospital between October 2010 and May 2014. All subjects fulfilled the following criteria for a diagnosis of NAFLD: negative tests for serum HBsAg and anti-HCV antibodies, daily ethanol intake < 20 g and absence of autoimmune liver disease, drug-induced liver injuries, or hereditary liver disease.
The study protocol was approved by the Human Ethics Committee of Saiseikai Suita Hospital. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Clinical and laboratory assessment
Patient characteristics, including age, height, weight, body mass index (BMI), and abdominal circumference, were extracted from the medical records. Blood samples had been obtained in the morning after an overnight fast within 2 weeks prior to liver biopsy. Clinical laboratory tests were conducted in the Department of Clinical Laboratory at Saiseikai Suita Hospital.
Histopathologic examination
After obtaining written informed consent, liver biopsy was performed using a 16-G aspiration needle (Hakko Co., Ltd., Nagano, Japan), yielding specimens at least 2.0 cm in length. Specimens were fixed in formalin, embedded in paraffin, and subjected to hematoxylin–eosin, Masson-trichrome, and Perl’s iron staining. The histologic assessment was made by an expert hepatologist.
Patients with NAFLD were classified into four types according to Matteoni’s classification [18]: type 1, simple steatosis; type 2, steatosis with lobular inflammation; type 3, type 2 plus ballooned hepatocytes; and type 4, presence of either Mallory-Denk bodies or fibrosis. Types 1 and 2 were classified as NAFL and types 3 and 4 as NASH. In addition, patients with NAFLD with fibrosis but without ballooning hepatocytes were classified into type 4.
Patients with NASH were classified by grade and stage according to the classification of Brunt et al. and Kleiner et al. [19, 20]. Grading was semiquantitatively assessed by considering steatosis, lobular, and portal inflammation, and hepatocellular ballooning. Staging was based on the characteristic pattern and evolution of fibrosis in NASH: with initial involvement of perisinusoidal spaces in zone 3 or mild portal/periportal fibrosis (stage 1), pericentral fibrosis associated with portal/periportal fibrosis (stage 2), bridging fibrosis (stage 3), and cirrhosis (stage 4). In this study, the concentration of type IV collagen, a fibrosis marker, was also determined in the serum of all patients [21].
NASH-HCC was diagnosed by histologic examination or by detecting imaging findings [on ultrasound sonography, computed tomography (CT), magnetic resonance imaging (MRI), or hepatic angiography]. Vascular invasion was assessed by dynamic CT, MRI, or angiography. Clinical staging (TNM classification) was determined according to the criteria of the Liver Cancer Study Group of Japan [22]. Des-γ-carboxy prothrombin (DCP) and α-fetoprotein (AFP) levels were measured in all patients with NASH-HCC. We used the optimal cut-off points of DCP and AFP in the analysis of NASH-HCC according to the manufacturer’s instructions.
Analysis of serum IgM-free and IgM-bound AIM
Quantification of serum levels of AIM was performed with ELISA. Total AIM consisting of both IgM-free and IgM-bound AIM was measured by an ELISA system using mouse anti-human AIM monoclonal antibodies (clones #6 and #7) [15]. IgM-free AIM was measured with a CircuLex Human AIM/CD5L/Spα ELISA kit (Medical & Biological Laboratories Co., Ltd, Aichi, Japan) according to the manufacturer’s instructions.
IgM-free AIM and total AIM in sera were also determined by immunoblot analysis [17]. Briefly, sera from the patients were size-fractionated on 5–20% SDS–polyacrylamide gel electrophoresis (PAGE) in a non-reducing condition, blotted on a membrane, and stained by anti-AIM polyclonal antibody. Signals were developed by the ECL system (Millipore Japan). Immunoblots indicated IgM-bound (> 600 kDa) and IgM-free (< 40 kDa) AIM. In addition, sera were applied to SDS-PAGE in a reducing condition, with the resulting immunoblots indicating total AIM (< 40 kDa), that is, IgM-free AIM plus AIM that had been released from IgM by the reducing conditions.
Receiver operating characteristic curve for assessing diagnostic accuracy of AIM
Receiver operating characteristic (ROC) curves were obtained by calculating the sensitivity and specificity of the assay at every possible cut-off point and plotting sensitivity against [1-specificity] in SPSS for Windows (SPSS Japan, Tokyo, Japan). The area under the ROC curve (AUROC) was calculated to determine the diagnostic accuracy of the assay. Appropriate cut-off points were examined for balancing the sensitivity and specificity of the ROC curve, and the optimal cut-off point was identified as that yielding the minimal value for [(1 − sensitivity)2 + (1 − specificity)2] or the maximal value for [sensitivity + specificity − 1] [23].
Statistical analysis
Statistical analysis was performed using SPSS for Windows (SPSS Japan, Tokyo, Japan). Differences in mean values between groups were assessed by the Mann–Whitney U test. Chi square tests were used to compare univariate associations of categorical variables. Correlation between two parameters was analyzed using Spearman’s rank correlation coefficient.
Results
Of the 209 patients with histologically diagnosed NAFLD, 42 had NAFL, 141 had NASH, and 26 had NASH-HCC (Table 1). With each worsening stage of disease, the serum albumin and platelet counts were significantly lower and alanine aminotransferase and type IV collagen levels significantly higher. BMI, abdominal circumference, γ-GTP, and HbA1c were not significantly different among the three groups.
According to the classification of Brunt and Kleiner, the grade and stage of NASH in the non-cancerous liver parenchyma of patients with NASH-HCC were more advanced compared with patients with NASH without HCC (Table 2). It is of note that NASH-HCC occurs in non-cirrhotic livers in many patients because 14 of the 26 (53.8%) patients with NASH-HCC had fibrosis stage 1–3. The tumors in 20 (76.9%) were stage 1 or 2, and the median maximum tumor diameter of all 26 patients with NASH-HCC was 21 mm. These observations indicated that the tumors in the majority of NASH-HCC patients were at an early tumor stage.
First, we determined the differences of IgM-free AIM, total AIM, and the IgM-free/total AIM ratio in patients with NAFL, NASH, and NASH-HCC. These markers did not differ between patients with NAFL and NASH but were significantly higher in NASH-HCC compared with NAFL and NASH (Fig. 1), suggesting that both of increased AIM production and greater release of AIM from IgM occurred in NASH-HCC.
Higher IgM-free AIM and total AIM levels in NASH-HCC in comparison with NAFL and NASH. IgM-free AIM (a) and total AIM (b) were measured in the serum of patients with NAFL (n = 42), NASH (n = 141), and NASH-HCC (n = 26) and the IgM-free/total AIM ratio (c) was calculated. Results are shown as mean with standard deviation. Differences in mean values between each group were assessed with Mann–Whitney U tests. *p < 0.01, NS not significant, AIM apoptosis inhibitor of macrophage, NAFLD non-alcoholic fatty liver disease, NASH non-alcoholic steatohepatitis
Because the group of patients with NASH-HCC included some with a more advanced grade and fibrosis stage of NASH than those with NASH without HCC (Table 2), we assessed the impact of grade and stage on these markers in patients with NASH. The levels of IgM-free AIM and total AIM did not differ between NAFL and NASH at grade 1 or 2 and at stage 0, 1, 2, or 3. However, NASH patients at grade 3 and at stage 4 (cirrhosis) had significantly higher levels of both IgM-free AIM and total AIM (Fig. 2a, b). Importantly, the IgM-free/total AIM ratio did not differ in any grade or stage (Fig. 2c). The higher level of IgM-free AIM in NASH patients at grade 3 and at stage 4 might result from an increased accumulation of total AIM with comparable activation.
Liver cirrhosis-associated increase of IgM-free AIM and total AIM in patients with NASH without HCC. Serum concentrations of IgM-free AIM (a) and total AIM (b) were measured and the IgM-free/total AIM ratio (c) was calculated in the serum of patients with NAFL or NASH without HCC and those with NASH-HCC. Results are shown as mean with standard deviation. Mann–Whitney U tests were used to assess differences in mean values between NAFL and each NASH group. *p < 0.01, NS not significant. The number of patients with NAFL was 42. In NASH groups, the numbers of patients are 76 in grade 1, 50 in grade 2, 15 in grade 3, 29 in stage 0, 42 in stage 1, 30 in stage 2, 32 in stage 3, and 8 in stage 4. AIM apoptosis inhibitor of macrophage; NAFL non-alcoholic fatty liver, NASH non-alcoholic steatohepatitis
Next, we determined HCC-associated differences in IgM-free AIM and total AIM. Serum concentrations of IgM-free AIM and total AIM were compared between patients with NASH and with NASH-HCC who had the same fibrosis stage. IgM-free AIM was significantly higher in patients with NASH-HCC, irrespective of the fibrosis stage in non-cancerous tissues (Fig. 3a). In contrast, total AIM did not differ between NASH and NASH-HCC at any fibrosis stage (Fig. 3b). The IgM-free/total AIM ratio was significantly higher in NASH-HCC at fibrosis stages 3 and 4 (Fig. 3c), suggesting that the increase of IgM-free AIM results from the activation of AIM by dissociation from IgM. The ratio did not differ significantly at fibrosis stages 1 and 2, suggesting the release of free AIM was more prominent in patients at an advanced fibrosis stage.
Increase of IgM-free AIM, but not total AIM, in patients with NASH-HCC. Serum concentrations of IgM-free AIM (a) and total AIM (b) were measured and the IgM-free/total AIM ratio (c) was calculated in the serum of patients with NASH and with NASH-HCC at the same stage of fibrosis. Results are shown as mean with standard deviation. The Mann–Whitney U test was used to assess the differences in mean values between NASH and NASH-HCC at the same stage. *p < 0.01, NS not significant, AIM apoptosis inhibitor of macrophage, NASH non-alcoholic steatohepatitis
Immunoblot analysis in a non-reducing condition revealed both IgM-free AIM and IgM-bound AIM in serum from patients with NASH-HCC, but those with NAFL and NASH without HCC had only IgM-bound AIM (Fig. 4a). An immunoblot of a reducing condition indicated that total AIM was higher in serum from patients with NASH-HCC compared with those with NAFL and NASH without HCC (Fig. 4b). These results were consistent with the data from ELISA showing a significantly higher IgM-free/total AIM ratio in NASH-HCC compared with NASH (Fig. 3).
Higher serum level of IgM-free AIM in patients with NASH-HCC, but not in NAFL or NASH without HCC. Immunoblot analysis demonstrated IgM-bound AIM (> 600 kDa) and IgM-free AIM (< 40 kDa) in non-reducing conditions (a) and total AIM in reducing conditions (b). IgM-free AIM bands were detected only in HCC, and total AIM was significantly higher in HCC compared with NAFL and NASH. AIM apoptosis inhibitor of macrophage, NAFL non-alcoholic fatty liver, NASH non-alcoholic steatohepatitis
We evaluated the correlation between IgM-free AIM and total AIM with Spearman’s correlation analysis in patients with NAFL, NASH, and NASH-HCC (Fig. 5a). NAFL and NASH had low correlations between IgM-free AIM and total AIM (p < 0.001, r = 0.360) with a correlation equation of y = 0.215x. NASH-HCC showed a higher correlation (p < 0.001, r = 0.633) with a correlation equation of y = 0.383x. Based on these equations, IgM-free AIM was 1.8-fold higher in patients with HCC compared with NAFL and NASH at the same concentrations of total AIM. These data suggest that the activation of AIM by dissociation from IgM was induced to a greater extent in patients with NASH-HCC than in those with NAFL and NASH.
HCC-related changes in IgM-free AIM and its diagnostic value. a Serum concentrations of IgM-free and total AIM were measured and the correlation between IgM-free AIM and total AIM was determined with Spearman’s correlation analysis. Results are shown as the correlation index (r) with the p value and the correlation equation. b The ROC curve was obtained by calculating the sensitivity and specificity of IgM-free and total AIM and the IgM-free/total AIM ratio. The AUROC values are shown in the table
We determined the diagnostic value of IgM-free, total AIM, and the IgM-free/total AIM ratio in HCC (Fig. 5b). The ROC curve was obtained by calculating the sensitivity and specificity of these markers. The AUROC indicated that IgM-free AIM was the best discriminating marker for NASH-HCC because AUROC was 0.929 for IgM-free AIM, 0.806 for total AIM, and 0.842 for the IgM-free/total AIM ratio. Balancing sensitivity and specificity of the ROC curve [23] indicated that the optimal cut-off point of IgM-free AIM was 1.6 μg/mL for predicting HCC in patients with NASH. Using this cut-off point, 23 of 26 patients with NASH-HCC had a positive result for IgM-free AIM and the sensitivity was 88.5%. Among 177 patients with NAFL and NASH without HCC, 153 patients tested negative for IgM-free AIM and the specificity was 86.4%. The high sensitivity and specificity suggest that IgM-free AIM has diagnostic value for detecting NASH-HCC.
Patients with NASH-HCC were classified into four groups depending on whether they were positive or negative for the HCC markers DCP and AFP (Table S1). Among the 26 patients with HCC, 14 were positive for DCP at a cut-off of 40 mAU/mL, with a sensitivity of 53.8%. Seven patients were positive for AFP at a cut-off of 20 ng/mL, with a sensitivity of 26.9%. IgM-free AIM, total AIM, and the IgM-free/total ratio AIM did not differ according to positivity or negativity for DCP or AFP. These data indicate that these markers were not correlated with DCP and AFP in NASH-HCC. The sensitivity of IgM-free AIM for HCC was higher than that of DCP and AFP.
Discussion
Macrophages play important roles in various liver diseases [9, 10] and AIM secreted from macrophages may be a key component to regulate liver injuries. In this study, while AIM production is increased in a cirrhotic state, AIM activation by dissociation of AIM from IgM is enhanced in NASH-HCC without increase of AIM production. Therefore, our observation supports the idea that serum IgM-free AIM levels is a novel marker for the early diagnosis of NASH-HCC.
Both IgM-free AIM and total AIM levels were increased in cirrhosis and the increase of these markers may be induced by lipid modulation and inflammatory stimuli [13, 24, 25]. The liver X receptor (LXR), a nuclear receptor that mainly regulates lipid metabolism, forms a heterodimer with retinoid X receptors and is involved in the regulation of AIM gene expression in macrophages [13, 24, 25]. Interestingly, LXR expression in the liver was positive in 50% of patients with NAFL and in 97% of patients with NASH but only 30% of healthy controls [26]. Thus, an increase in LXR expression may also contribute to the increase in AIM levels in patients with severe NASH.
AIM associated with IgM and increase in IgM levels might be associated with total AIM levels. Obesity-induced augmentation of IgM increased blood AIM levels in mice [27]. Liver injury in response to carbon tetrachloride increased serum AIM and IgM levels in mice [15]. And hypergammaglobulinemia in patients is reported to be a marker for histologically advanced types of fibrosis in the progression of liver disease. However, although serum IgA and IgG levels correlated with hepatic fibrosis in patients with HCV infection, serum IgM levels did not [28]. In NASH patients, there was no association between serum IgM levels and fibrosis stage [29], and in HCV-infected alcoholic patients with cirrhosis, there was no correlation between serum IgM levels and HCC progression [30]. Based on these reports, it was unlikely that serum IgM levels predicted fibrosis or carcinogenesis in NASH patients in contrast to AIM levels. Further studies are required for correlation analysis of serum AIM and IgM levels in patients with NASH-associated HCC.
IgM-free AIM, but not total AIM, was significantly higher in patients with NASH-HCC compared with those with NASH without HCC at the same fibrosis stage. The IgM-free/total AIM ratio increased proportionately in NASH-HCC. Activation of AIM through release from IgM in NASH-HCC may be a key step in preventing progression of HCC. Although the mechanism by which AIM dissociates from the IgM pentamer remains to be elucidated, altered glycosylation of either or both AIM and IgM may affect their association, as in the case of the association between complement factor C1q and IgM [27, 31]. Human AIM possesses O-glycosylation sites but no N-glycosylation site, while mouse AIM has N- and O-glycosylation sites, which play important roles in modulating its secretion and its lipolytic function [32]. Two forms of AIM in human serum were observed at 38 and 40 kDa, resulting from differences in sialic acid content [33]. Changes in sialylations on AIM may occur in HCC as altered sialylation of other proteins has been reported by several groups in the serum of patients with HCC [34,35,36,37,38]. Certain enzymatic modifications of glycosylation structure in patients with NASH-HCC may have an impact on AIM association with IgM. For instance, the neuraminidase that cleaves sialic acid mediates the activation of transforming growth factor-β by releasing the active substance from the latent form [39, 40]. Production of neuraminidases was increased in tissues and the serum in patients with HCC [41, 42]. It may be worth evaluating whether neuraminidases may induce AIM release from IgM. Although the present study provided the first evidence of HCC-dependent activation of AIM, further studies are necessary to clarify the precise mechanism of AIM release from IgM.
DCP and AFP have been clinically used as standard biomarkers for HCC. It is widely accepted that the AFP has high sensitivity for the presence of HCV-associated HCC but poor sensitivity for that of NASH-HCC, while the sensitivity of DCP appears to be similar in the diagnosis for HCV-associated and NASH-associated HCC [7, 43]. Importantly, serum AIM levels were not correlated with the levels of DCP or AFP in patients with NASH-HCC. There was no report of the optimal cut-off points of DCP and AFP for NASH-associated HCC. Further studies are needed to determine the optimal cut-off point of these markers and to compare their sensitivity and specificity with those of IgM-free AIM. As DCP and AFP are produced by HCC cells and AIM is secreted by macrophages, the combination of free AIM with DCP or AFP may increase their sensitivity as markers of NASH-HCC.
The fibrotic stage may have different impacts on serum AIM in patients with NASH and patients with HBV and HCV, just as the stage at which HCC develops differs. In HCV infection, HCC mainly develops in patients with chronic hepatitis at advanced stages or cirrhosis, whereas NASH-HCC often develops with mild fibrosis in patients with NASH [7, 44]. The increase of total AIM in serum was reported previously in patients with HBV- and HCV-induced cirrhosis [15, 45, 46]. Total AIM levels increased at stages 2 and 3 in patients with chronic hepatitis C [47]. In the present study, however, we found no significant differences in IgM-free and total AIM levels in stage 0–3 of NASH, compared with NAFL. It may require further studies to determine the production and activation of AIM in patients with HBV- and HCV-induced cirrhosis and HCC.
There was a limitation with respect to the interpretation of results in this study because the sample size of NASH-HCC patients was relatively small. In this study, about 10% of patients with low grade and stage of NASH were positive for serum IgM-free AIM. This finding suggests that an AIM-releasing mechanism was already at work at the very early stages of HCC that was unidentified by conventional diagnostic tools. Furthermore, we did not determine the change in AIM levels after treatment for HCC. Long-term follow-up of these patients may provide evidences to support the clinical relevance of AIM.
In conclusion, AIM activation by dissociation from IgM is enhanced in patients with NASH-HCC but not in NAFL or NASH. IgM-free AIM levels could be a useful diagnostic marker for NASH-HCC. Further studies of this novel tool are warranted to see whether it is beneficial for the early diagnosis of NASH-HCC.
References
EASL-EASD-EASO. Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). J Hepatol. 2016;64:1388–402.
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23.
Okanoue T, Umemura A, Yasui K, Itoh Y. Nonalcoholic fatty liver disease and non-alcoholic steatohepatitis in Japan. J Gastroenterol Hepatol. 2011;26(Suppl 1):153–62.
Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of non-alcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–40.
Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of non-alcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–21.
Bullock RE, Zaitoun AM, Aithal GP, Ryder SD, Beckingham IJ, Lobo DN. Association of non-alcoholic steatohepatitis without significant fibrosis with hepatocellular carcinoma. J Hepatol. 2004;41:685–6.
Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, et al. Characteristics of patients with non-alcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–33.
Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, et al. Carriage of the PNPLA3 rs738409 C > G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81.
Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212–23.
Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–94.
Miyazaki T, Hirokami Y, Matsuhashi N, Takatsuka H, Naito M. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J Exp Med. 1999;189:413–22.
Tissot JD, Sanchez JC, Vuadens F, Scherl A, Schifferli JA, Hochstrasser DF, et al. IgM are associated to Sp alpha (CD5 antigen-like). Electrophoresis. 2002;23:1203–6.
Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, et al. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–13.
Kurokawa J, Arai S, Nakashima K, Nagano H, Nishijima A, Miyata K, et al. Macrophage-derived AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab. 2010;11:479–92.
Yamazaki T, Mori M, Arai S, Tateishi R, Abe M, Ban M, et al. Circulating AIM as an indicator of liver damage and hepatocellular carcinoma in humans. PLoS One. 2014;9:e109123.
Maehara N, Arai S, Mori M, Iwamura Y, Kurokawa J, Kai T, et al. Circulating AIM prevents hepatocellular carcinoma through complement activation. Cell Rep. 2014;9:61–74.
Arai S, Kitada K, Yamazaki T, Takai R, Zhang X, Tsugawa Y, et al. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat Med. 2016;22:183–93.
Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9.
Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74.
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21.
Mizuno M, Shima T, Oya H, Mitsumoto Y, Mizuno C, Isoda S, et al. Classification of patients with non-alcoholic fatty liver disease using rapid immunoassay of serum type IV collagen compared with that using liver histology and other fibrosis markers. Hepatol Res. 2016. doi:10.1111/hepr.12710.
Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245:909–22.
Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96:644–7.
Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309.
Valledor AF, Hsu LC, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc Natl Acad Sci USA D. 2004;101:17813–8.
Ahn SB, Jang K, Jun DW, Lee BH, Shin KJ. Expression of liver X receptor correlates with intrahepatic inflammation and fibrosis in patients with non-alcoholic fatty liver disease. Dig Dis Sci. 2014;59:2975–82.
Arai S, Maehara N, Iwamura Y, Honda S, Nakashima K, Kai T, et al. Obesity-associated autoantibody production requires AIM to retain the immunoglobulin M immune complex on follicular dendritic cells. Cell Rep. 2013;3:1187–98.
Watt K, Uhanova J, Gong Y, et al. Serum immunoglobulins predict the extent of hepatic fibrosis in patients with chronic hepatitis C virus infection. J Viral Hepat. 2004;11:251–6.
McPherson S, Henderson E, Burt AD, et al. Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60(5):1055–62.
Ferrín G, Rodríguez-Perálvarez M, Aguilar-Melero P, et al. Plasma protein biomarkers of hepatocellular carcinoma in HCV-infected alcoholic patients with cirrhosis. PLoS One. 2015;10(3):e0118527.
Smathers RL, Chiang DJ, McMullen MR, Feldstein AE, Roychowdhury S, Nagy LE. Soluble IgM links apoptosis to complement activation in early alcoholic liver disease in mice. Mol Immunol. 2016;72:9–18.
Mori M, Kimura H, Iwamura Y, Arai S, Miyazaki T. Modification of N-glycosylation modulates the secretion and lipolytic function of apoptosis inhibitor of macrophage (AIM). FEBS Lett. 2012;586:3569–74.
Sarrias MR, Padilla O, Monreal Y, Carrascal M, Abian J, Vives J, et al. Biochemical characterisation of recombinant and circulating human Spalpha. Tissue Antigens. 2004;63:335–44.
Dall’Olio F, Chiricolo M, D’Errico A, Gruppioni E, Altimari A, Fiorentino M, et al. Expression of beta-galactoside alpha2,6 sialyltransferase and of alpha2,6-sialylated glycoconjugates in normal human liver, hepatocarcinoma, and cirrhosis. Glycobiology. 2004;14:39–49.
Ang IL, Poon TC, Lai PB, Chan AT, Ngai SM, Hui AY, et al. Study of serum haptoglobin and its glycoforms in the diagnosis of hepatocellular carcinoma: a glycoproteomic approach. J Proteom Res. 2006;5:2691–700.
Chrostek L, Cylwik B, Panasiuk A, Brodowska-Adamusiak D, Gruszewska E. Lipid-bound sialic acid (LSA) in liver diseases of different aetiologies. Ann Hepatol. 2011;10:150–4.
Sun C, Chen P, Chen Q, Sun L, Kang X, Qin X, et al. Serum paraoxonase 1 heteroplasmon, a fucosylated, and sialylated glycoprotein in distinguishing early hepatocellular carcinoma from liver cirrhosis patients. Acta Biochim Biophys Sin (Shanghai). 2012;44:765–73.
Comunale MA, Wang M, Anbarasan N, Betesh L, Karabudak A, Moritz E, et al. Total serum glycan analysis is superior to lectin-FLISA for the early detection of hepatocellular carcinoma. Proteom Clin Appl. 2013;7:690–700.
Carlson CM, Turpin EA, Moser LA, O’Brien KB, Cline TD, Jones JC, et al. Transforming growth factor-β: activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. PLoS Pathog. 2010;6:e1001136.
Namachivayam K, Blanco CL, Frost BL, Reeves AA, Jagadeeswaran R, MohanKumar K, et al. Preterm human milk contains a large pool of latent TGF-β, which can be activated by exogenous neuraminidase. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1055–65.
Miyagi T, Wada T, Yamaguchi K, Shiozaki K, Sato I, Kakugawa Y, et al. Human sialidase as a cancer marker. Proteomics. 2008;8:3303–11.
Hou G, Liu G, Yang Y, Li Y, Yuan S, Zhao L, et al. Neuraminidase 1 (NEU1) promotes proliferation and migration as a diagnostic and prognostic biomarker of hepatocellular carcinoma. Oncotarget. 2016. doi:10.18632/oncotarget.11778.
Tokushige K, Hyogo H, Nakajima T, Ono M, Kawaguchi T, Honda K, et al. Hepatocellular carcinoma in Japanese patients with non-alcoholic fatty liver disease and alcoholic liver disease: multicenter survey. J Gastroenterol. 2016;51:586–96.
Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–9.
Gangadharan B, Antrobus R, Dwek RA, Zitzmann N. Novel serum biomarker candidates for liver fibrosis in hepatitis C patients. Clin Chem. 2007;53:1792–9.
Gray J, Chattopadhyay D, Beale GS, Patman GL, Miele L, King BP, et al. A proteomic strategy to identify novel serum biomarkers for liver cirrhosis and hepatocellular cancer in individuals with fatty liver disease. BMC Cancer. 2009;9:271.
Mera K, Uto H, Mawatari S, Ido A, Yoshimine Y, Nosaki T, et al. Serum levels of apoptosis inhibitor of macrophage are associated with hepatic fibrosis in patients with chronic hepatitis C. BMC Gastroenterol. 2014;14:27.
Acknowledgements
This study was conducted based on the research collaboration contract between Saiseikai Suita Hospital and Eidia Co Ltd. and on the research collaboration contract between Toru Miyazaki in Tokyo University and Eidia Co Ltd. The authors thank the Department of Clinical Laboratory of Saiseikai Suita Hospital for suggestions for this study and carrying out clinical laboratory tests. This work was partly supported by CREST (AMED; to T.M.) and by the Research Program on Hepatitis from AMED (to T.O.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Employment: Noriyuki Koyama/Eisai Co Ltd. Yuka Kanetsuki/Sekisui Medical Co Ltd. Jiro Hirota/Sekisui Medical Co Ltd. Tomohide Asai/Sekisui Medical Co Ltd.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koyama, N., Yamazaki, T., Kanetsuki, Y. et al. Activation of apoptosis inhibitor of macrophage is a sensitive diagnostic marker for NASH-associated hepatocellular carcinoma. J Gastroenterol 53, 770–779 (2018). https://doi.org/10.1007/s00535-017-1398-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1398-y